SUMMARY
Ninety-seven isolates of Shigella flexneri from children seeking medical care from three sites in Egypt were characterized. Overall, 46·4% of children (median age 17 months) were febrile or reported blood in their stools, 25·8% were dehydrated and 16·5% were admitted to hospital. Serotypes 2a (37·1%), 1b (18·6%), 1c (17·5%), and 6 (15·5%) comprised over 88·7% of the total isolates. We observed marked resistance to ampicillin (87·6%), tetracycline (84·5%) and trimethoprim–sulfamethoxazole (63·9%). Pulsed-field electrophoresis grouped the majority of isolates within a serotype together, separately from isolates of an alternative serotype. The set gene was present in all serogroup 2a isolates, however, the sen gene was detected in every isolate. Our results show S. flexneri 1c has emerged as a dominant S. flexneri serotype in Egypt. Development and application of a Shigella vaccine should consider the diversity of Shigella serotypes within a geographical region prior to administration.
INTRODUCTION
The World Health Organization (WHO) estimates that more than 5 million children in developing countries die from diarrhoeal diseases and 1·1 million of these deaths are due to Shigella [1]. Shigella flexneri is the most common Shigella spp. found in economically developing areas [1]. In these areas of the world children are often exposed to S. flexneri as a result of substandard hygiene and inadequately treated drinking water. The WHO has called for the development of vaccines to prevent the morbidity and mortality resulting from Shigella infection [2].
Isolates of S. flexneri are identified from other enterobacteria primarily based on the structure of the O antigen component of the lipopolysaccharide [3]. Serotyping methods exploit structural differences in the O antigen and these differences can be used to group Shigella isolates into genera and subgenera [4]. There are eight serotypes of S. flexneri and serotypes 1–5 subdivided into subserotypes [5]. In endemic regions, the distribution of serotypes may change over time [6, 7]. A recently described serotype, S. flexneri 1c, has only been described in two geographic locations, Bangladesh and Egypt [4, 8, 9]. In Bangladesh, serotype 1c has emerged as a significant cause of shigellosis [9]. After initially being observed in the village of Abees 10 km south of Alexandria, Egypt in the early 1990s [8], this serotype has not since been reported in Egyptian studies.
Antibiotic therapy can limit the duration of shigellosis and shedding of the organism. However, resistance has been increasing to the most often administered antibiotics, trimethoprim–sulfamethoxazole, ampicillin and chloramphenicol [10–13]. Quinolones and fluoroquinolones are effective in controlling the bacillus, although resistant isolates have been described [10]. While quinolone usage in paediatric populations has been discouraged [14], available data suggests that this population could effectively be treated with these drugs [14, 15]. Accordingly, the WHO recommends the use of ciprofloxacin as the drug of choice for patients with bloody diarrhoea, irrespective of age [16].
Multi-drug resistant (MDR) enteric bacterial pathogens such as S. flexneri have been documented in Egypt [13, 17–19]. Recently, a correlation between S. flexneri serotype and antimicrobial resistance has been described [20]. However, there is little data describing the distribution of resistant strains of S. flexneri in Egypt.
Isolates of S. flexneri may express a variety of virulence factors. Virulence factors are contained on the 140 kDa invasion plasmid such as the sen gene, encoding the Shigella enterotoxin 2 (Shet-2) [21, 22] as well chromosomally [e.g. set, encoding the Shigella enterotoxin 1 (Shet-1)] [23]. Some virulence factors are found in multiple copies on both the plasmids and the chromosome such as the invasion plasmid antigen gene H (ipaH) [24]. There is evidence to support the idea that the set gene is serotype-specific for S. flexneri 2a isolates [25, 26].
Given the lack of detailed knowledge regarding Egyptian isolates of S. flexneri and the significance of this organism as a cause of paediatric diarrhoea in the region, we sought to describe the clinical characteristics of children seeking medical care for shigellosis caused specifically by S. flexneri from three different populations in lower Egypt. We used phenotypic and genetic approaches to characterize the cultured S. flexneri isolates.
METHODS
Study design and population
The analysis reported here is based on a multi-site outpatient paediatric clinic-based surveillance study. The methodology concerning the study sites, enrolment, data collection, specimen collection, and microbiology have all been previously reported [27–29]. This study utilizes prospective clinic-based surveillance among paediatric populations residing in three regions within Egypt. Study sites included the outpatient paediatric clinic-associated Benha (BH) Fever Hospital, the paediatric clinic-associated Abu Homos (AH) District Hospital, and two paediatric clinics in Mokattum Hills (MH), BH, located north of Cairo is a periurban community of 455 000 persons, where 13% are <6 years of age and 29% are illiterate. In urban areas of BH, nearly 90% of households and in rural areas, 10% of households have water and sewage disposal provided by the municipality. AH is an agricultural community of 348 000 persons, located southeast of Alexandria, Egypt, where 17% is <6 years of age and 56% of the population is illiterate. In AH, ∼90% of urban households have municipal water and sewage disposal compared with 55% of rural residents receiving municipal water and none have sewage disposal. MH, located in the eastern quarter of Cairo in an area called Mansheyet Nasser has a population of ∼30 000, 20% of which is <6 years old, and 40% of which is illiterate. This population is among the lowest socio-economic class, primarily employed as garbage collectors or labourers with low-paying menial jobs.
Children aged <5 years presenting at one of the study clinics with diarrhoea between 20 May 2000 and 31 October 2004 were eligible for enrolment. All children presenting for care at one of the treatment facilities with a primary complaint of diarrhoea were listed sequentially on a study register along with the time and date of the visit and age of the patient. Due to large incidence of disease and limited laboratory and clinical capacity, every fifth child from the study register was given the opportunity to enrol. If a guardian did not wish their child to participate in the study, a parent of the next registered child was asked to enrol their child as a replacement for the refusal.
After obtaining informed consent from the parent/guardian, information on the child's clinical history was recorded and a physical examination performed. The clinical history questionnaire included the child's demographic information, pattern and frequency of diarrhoea, and guardian reported presence or absence of signs and symptoms such as loose stools, vomiting, rectal prolapse, fever, and visible blood in stool. To assess the degree of dehydration, a physical examination recorded information on general appearance, mucus membranes, tearing, skin turgor, radial pulse, rectal temperature and status of anterior fontanelle for infants.
Time from disease onset until evaluation was termed ‘illness length’. Stooling patterns were described by the ‘mean number of stools’ in the 24 h before evaluation and ‘mean maximal stools’ on any day since the illness began. Dehydration was assessed by a physical examination according to criteria provided by the WHO and classified into ‘some dehydration’ and ‘severe dehydration’ [30]. An episode lasting <14 days without visible blood was termed ‘acute watery diarrhoea,’ while ‘dysentery’ was defined as an illness lasting <14 days with visible blood or mucus in the stool. A repeat visit was considered as a continuation of the previous episode if the end of the first episode and the onset of the second were three or fewer days apart. The warm or summer season occurred from 1 May to 31 October, and the cool season from 1 November to 30 April.
Identification of S. flexneri isolates
Shigella spp. were identified using standard microbiological and biochemical procedures. Confirmation of Shigella spp. was made using API-20E biochemical test strips (bioMérieux SA, Marcy l'Etoile, France). Species designation of individual colonies was determined after visual examination of slide agglutination assays using a commercially available serotyping kit (Denka-Seiken, Tokyo, Japan) as per the manufacturer's instructions.
Serotyping
Serotyping was performed as previously described [8] using a commercially available serotyping kit (Denka-Seiken). In addition, the serotyping of S. flexneri 1c was identified using a S. flexneri monoclonal antibody specific for serotype 1c (MASF) [4] kindly provided by Dr Nils Carlin, Göteborg, Sweden.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing (AST) was performed using commercially available discs (Becton Dickinson, Sparks, MD, USA). The antibiotic discs used in this study were ampicillin (AP, 10 μg), tetracycline (TE, 30 μg), nalidixic acid (NA, 30 μg), trimethoprim–sulfamethoxazole (SXT, 25 μg), chloramphenicol (C, 30 μg), ciprofloxacin (CIP, 5 μg), and erythromycin (E, 15 μg). After incubation on Mueller–Hinton agar plates at 37°C for 48 h, zone of inhibition diameters were recorded. Results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [31] as susceptible (S), intermediate (I) or resistant (R). For the purposes of reporting, we refer to both intermediate and susceptible isolates as ‘non-resistant’. Multi-drug resistance was defined as resistance to at least three classes of antibiotics. Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as control strains for susceptibility studies as recommended [31].
Macrorestriction profiling and pulsed-field gel electrophoresis (PFGE)
Genomic DNA from clinical isolates of S. flexneri were prepared and subjected to PFGE using a contour-clamped homogeneous electric field apparatus (CHEF-DRII; Bio-Rad Laboratories, Richmond, CA, USA) as previously described [32] with minor changes in the pulse times; the initial switch time was 1·79 s and the final switch time was 18·7 s with an electrophoresis time of 22 h. Genomic DNA embedded within the plugs was digested with the restriction enzyme XbaI for macrorestriction profiling (mrp) (Fermentas, GmbH, St Loen-Rot, Germany) for 16 h at 37°C. Salmonella enterica serovar Braenderup H9812 digested with XbaI, the PulseNet standard strain for PFGE analysis [32] was used as a molecular weight marker. Gels were analysed using the BioNumerics 4.0 software package (Applied Maths Inc., Austin, TX, USA). Genetic similarities were inferred from dendrograms created using the unweighted pair-group method with averages (UPGMA) analysis of the XbaI mrp–PFGE patterns using the Dice coefficient with a 1·5% tolerance for the band migration distance.
Detection of Shigella enterotoxin genes set and sen
Detection of the set gene, encoding the Shigella enterotoxin 1 product ShET-1 (A and B subunits) was performed by amplifying both set1A and set1B genes by PCR [26]. Detection of the sen gene, encoding the ShET-2 toxin, used previously published the primers and conditions [33]. The presence of ipaH was verified using the primers H8 and H15 [34]. PCR was performed in a thermal cycler 9700 gene amp (Applied Biosystems, Foster City, CA, USA). A 10 μl aliquot of S. flexneri growth obtained from a MacConkey agar plate was suspended in sterile water, boiled for 10 min and 5 μl of the boiled suspension was used as a template in the PCR. Primers were commercially purchased from Sigma-Genosys (The Woodlands, TX, USA). PCR amplicons were separated in 1·5% agarose and visualized by staining with ethidium bromide.
Statistical analysis
Demographic and clinical data were described and compared across regions and predominate serotypes. For analysing an association between dimensional variables and categorical variables (e.g. age and serotype), ANOVA was used, unless normality assumptions were not met, in which case Kruskal–Wallis was performed. Associations between categorical variables were assessed using χ2 or Fisher's exact test. Analysis was conducted using stata version 9 (Stata Corp., College Station, TX, USA), and statistical significance was set at a P value of ⩽0·05.
The study protocol (DoD# NAMRU3.2000.0002 was approved by the United States Naval Medical Research Unit No. 3 in compliance with all applicable Federal regulations governing the protection of human subjects.
This study (Protocol no. 30969) was reviewed and approved by the Institutional Review Boards of the United States Naval Medical Research Unit No. 3, and the Egyptian Ministry of Health and Population in compliance with all Federal regulations governing the protection of human subjects. Informed consents were obtained from all adult participants and from parents or legal guardians of minors.
RESULTS
Phenotypic testing and serotyping
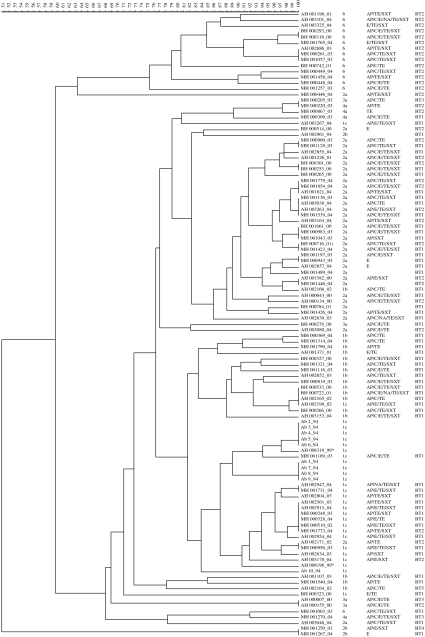
We recovered 171 Shigella spp. from 4384 children with acute diarrhoea (3·9%) living in three separate locations in Egypt from January 2000 to November 2004. These were speciated as S. flexneri (n=103, 60·2%), S. sonnei (n=35, 20·5%), S. dysenteriae (n=16, 9·3%), S. boydii (n=15, 8·8%) and two unconfirmed Shigella spp. After storage we were able to recover 97 S. flexneri isolates for further analysis. Forty-six isolates were from MH, 35 isolates were from the AH region, and the remaining 16 isolates were from BH. All of the S. flexneri isolates were further grouped by serological analysis (Fig.). Seven serotypes were identified and S. flexneri 2a was the most common (36/97, 37·1%). Serotype 1 subgroups 1b (18/97, 18·6%) and 1c (17/97, 17·5%) were next most prominently encountered followed by serotype 6 (15/97, 15·5%). Serotypes 3a and 2b were recovered from all three locations at a low frequency (4/97, 4·1% and 3/97, 3·1% respectively), and serotype 4a (4/97, 4·1%) was exclusive to MH. There were no differences in distribution of the four predominant subserotypes based on study site.
Fig.
Dendrogram of Shigella flexneri isolates from three paediatric populations in Egypt, 2000–4. Isolate names are annotated as follows: the source of the isolate is provided by a two-letter code followed by a unique identifying number; an underscore separates the year of isolation from the identifying number. Source abbreviations: AB, Abees; AH, Abu Homos; BH, Benha; MH, Mokattum Hills. Antibiotic-resistance profile abbreviations are as follows: AP, ampicillin resistance; C, chloramphenicol resistance; E, erythromycin resistance; TE, tetracycline resistance; SXT, trimethoprim–sulfamethoxazole resistance. Biotype abbreviations are BT1, glucose, mannitol fermentation; BT2, glucose, mannitol fermentation, utilization of arabinose; BT3, production of indole oxidase, glucose and mannitol fermentation; BT4, glucose, mannitol fermentation, melibiose, arabinose utilization; BT5, glucose fermentation.
Five different phenotypes were recognized by biochemical testing using API-20E (Fig.). The majority of isolates fermented glucose and mannitol (n=59, BT1) or fermented glucose and mannitol and utilized arabinose (n=33, BT2). Three isolates (two serotype 3a isolates and one serotype 4a) produced indole oxidase and fermented glucose and mannitol (BT3). A solitary serotype 2b isolate was able to ferment glucose and mannitol and utilized melibiose and arabinose (BT4) and a lone serotype 1b isolate only fermented glucose (BT5).
Of the 97 children presenting with culturable S. flexneri, the median age was 17 months [interquartile range (IQR) 10–27 months] and 54·6% were male. Nearly 84·0% of the cases reported in the summer months and 25·8% met WHO criteria for dehydration. Thirty-four percent of patients presented with fever and 21·5% reported having noticed blood in their stool. There were differences in demographic characteristics, clinical presentation and hospitalization rates between the three study sites. Children presenting the MH clinics were older [median age (months) – MH 21·5, BH 12, AH 13; Kruskal–Wallis, P=0·006] and had lower rates of indicators of severe disease. The paediatric clinic associated with BH Fever Hospital had higher rates of fever at presentation (BH 56%, AH 31%, MH 28%; χ2, P=0·12), dysentery (BH 36%, AH 29%, MH 11%; χ2, P=0·055), any dehydration (BH 50%, AH 17%, MH 0; χ2, P<0·001). Vomiting was more frequently associated in AH (69%) and BH (63%) compared to MH (36%) (χ2, P=0·009) In addition, 10/16 (63%) subjects were admitted from the BH Fever Hospital clinic, compared to 17% from the AH clinic and none from MH (χ2, P<0·001). There were no statistically significant differences in clinical characteristics, symptomatology or hospitalization between serotypes (Table 1). However, there appeared to be twice the rate of rectal prolapse in serotypes 1b and 1c (each 18%) compared to 2a (6%) and 6 (7%) (P=0·10), and serotype 1b had relatively higher rates of fever identified (47%) compared to the other predominate serotypes (24–36%) (P=0·23). Of the 97 children, 16 (16·5%) were admitted and seven (43·8%) were attributed to serotype 2a, five were serotype 1b and two admissions were due to serotype 6.
Table 1.
Identification and clinical characteristics of pediatric patients with Shigella flexneri isolated among predominate serotypes (n=86)
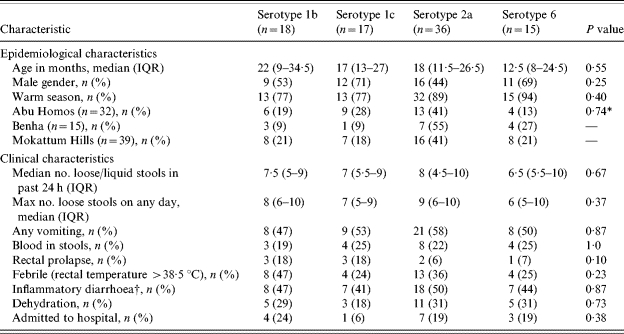
IQR, Inter-quartile range.
Fisher's exact test of site by predominate serotype.
Febrile and/or blood reported in stools.
Correlation between antibiotic resistance and serotype
We investigated whether any relationship was evident between serotype and antimicrobial resistance phenotype as previously reported [35]. All 97 isolates were tested for susceptibility to a panel of seven antimicrobial agents (Fig. 1 and Table 2). The majority of isolates were resistant to ampicillin (87·6%) and tetracycline (84·5%). More variation was observed with SXT (63·9%), chloramphenicol (55·7%) and erythromycin (53·6%). Overt nalidixic acid resistance was only 4·1%; however, intermediate resistance to nalidixic acid was observed in an additional 5·2% of the isolates. None of the isolates were resistant to ciprofloxacin.
Table 2.
Correlation between prevalent serotypes and resistance to individual antimicrobials and phenotypes
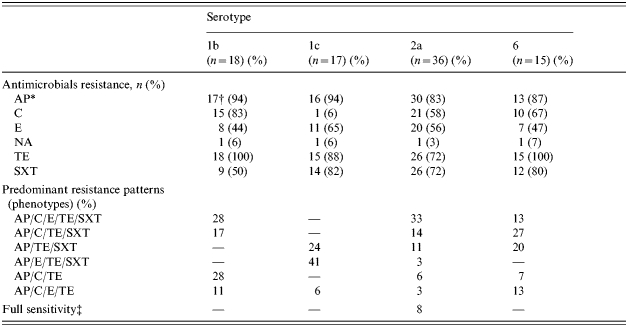
Abbreviations: AP, ampicillin; C, chloramphenicol; E, erythromycin; NA, nalidixic acid; TE, tetracycline; SXT, trimethoprim–sulfamethoxazole.
Numbers outside the parentheses refer to resistant isolates as determined by Kirby–Bauer disc diffusion and numbers inside the parentheses ( ) are the percentage of resistant isolates within a given serotype.
Full sensitivity is defined as an isolate that was sensitive to all of the antibiotics tested in this study.
The resistance to individual antimicrobials between serotypes differed between the predominate serotypes (1b, 1c, 2a, 6). There was higher resistance to chloramphenicol among all serotypes except 1c, where resistance in only 1/17 isolates was found (P<0·0001). Serotype 1b tended to demonstrate less resistance to SXT (50% vs. 72–82%, P=0·14) when compared to the other predominate serotypes. Tetracycline resistance was found in all serotype 1b and 6 isolates, 88% of serotype 1c isolates and 72% of serotype 2a isolates (P=0·01).
There were also notable differences in multi-drug-resistance phenotypes between the serotypes (Table 2). Resistance to three or more antimicrobials occurred in 83% of isolates. For all serotypes (predominate and sporadic), six different phenotypes accounted for 71% of all resistance patterns. For predominant serotypes, these six phenotypes accounted for 65% of all resistance phenotypes. AP/C/E/TE/SXT was the most common phenotype pattern overall (20%) and for 2a (33%). AP/C/TE and AP/C/E/TE/SXT were the most common phenotypes for serotype 1b (28%). The most common phenotype for serotype 1c was AP/E/TE/SXT (41%), and AP/C/TE/SXT was found in 27% of serotype 6 isolates. Two isolates, a serotype 1b and a serotype 6, were resistant to all antimicrobial agents tested with the exception of ciprofloxacin. Among the resistance phenotypes, AP/C/E/TE/SXT was associated with eight out of 15 hospital admissions and eight out of 12 (P=0·12) admissions among the six most common resistance phenotypes (P=0·01).
While a majority of cases occurred in the summer months, there were no differences in serotype distribution and season. Among all isolates, erythromycin resistance tended to occur more often in the winter months (winter 73%, summer 50%; P=0·12) and SXT resistance tended to occur more often in the summer months (summer 68%, winter 53%). Small numbers precluded any further evaluation of season and serotype.
Diversity within S. flexneri 1c revealed by mrp–PFGE
In order to determine the phylogenetic diversity of S. flexneri between the three study sites, mrp using the restriction enzyme XbaI and PFGE (XbaI mrp–PFGE) was performed on all 97 isolates (Fig. 1). Given the recent identification of 1c isolates in Egypt and their recent increase in Bangladesh [9], we were particularly interested in these isolates. Ten previously identified isolates of S. flexneri 1c from Abees, were included to facilitate comparisons Serotyping results of S. flexneri isolates from AH spanning 1995–1999 were reviewed and four previously untypable isolates were subsequently tested using anti-1c antisera. Two of these isolates were shown to be serotype 1c isolates (both from 1999) and were also included in this analysis.
At a similarity level of 80%, 15 clusters were observed with eight of the 15 clusters containing at least two isolates. At this similarity level, the majority of isolates within any given serotype grouped together and separately from isolates of an alternative serotype.
Ten of the 15 S. flexneri 6 isolates formed a single cluster, with four of the five remaining isolates forming closely related branches. Isolates from all three locations with multiple AST profiles were found within this main cluster. Of note, all of these isolates had an identical biochemical profile. The two outlying isolates were both from MH and in addition to possessing a unique XbaI mrp–PFGE fingerprint, had a unique biochemical profile.
Thirty-two of the 36 S. flexneri 2a isolates formed a single cluster, with two of the four remaining isolates located in closely related branches. The main cluster consisted of isolates from all locations and a mixture of biochemical profiles. A single S. flexneri 1b and 2b isolate were also located in this cluster. The two remaining isolates, one from AH and one from MH, each formed a unique XbaI mrp–PFGE group.
At the 80% similarity level, S. flexneri isolates of serotype 1 (either 1b or 1c) could not be distinguished and formed a broad cluster. However, at a similarity of 82%, subserotypes 1b and 1c could be separated. Fourteen of the 18 S. flexneri 1b isolates formed a cluster consisting of multiple AST profiles but had a uniform biochemical profile. A single S. flexneri serotype 1c isolate, expressing the same biochemical profile, was the only non-serotype 1b isolate in this cluster. Besides the previously mentioned S. flexneri 1b isolate grouping among the 2a cluster, the remaining 1b isolates grouped to a separate serotype 1b cluster.
Fourteen of the 17 S. flexneri serotype 1c isolates also formed a related cluster. This cluster also contained all 10 of the isolates from Abees and two additional isolates from AH recovered in 1999. The major 1c cluster was heterogeneous with respect to biochemical and AST composition. A S. flexneri 2a isolate was the only non-1c strain grouping in this cluster. One of the remaining S. flexneri 1c isolates (from AH) was present in the previously described 1b cluster; a second AH isolate shared similarity with S. flexneri 3a and 4a isolates and the remaining isolate (from BH) formed a unique group. At this level of similarity, it appears that there are four distinct lineages of S. flexneri 1c in Egypt.
set, but not sen, aids in discriminating between S. flexneri serogroups
PCR was used to determine the distribution of the Shigella enterotoxin genes set and sen among the Egyptian S. flexneri serotypes (data not shown). Thirty-six isolates carried the set toxin gene; all of these isolates were subserotype 2a. All isolates were positive for sen and ipaH.
DISCUSSION
In summary, we report the clinical presentation, epidemiology, phenotypical and genetical characterization of S. flexneri isolates acquired from children <5 years of age presenting for diarrhoea from three sites in lower Egypt. Paediatricians and general practitioners in Egypt typically treat acute watery diarrhoea using oral rehydration therapy and compounds that either reduce the water content in stools (e.g. a solution of kaolin-pectin) or reduce the irritation of the intestinal lining (e.g. bismuth subsalicylate). However, if blood is observed in the first presentation of the diarrhoeal episode or if it persists more than 2–3 days, the common practice is to empirically administer antibiotics in the form of SXT, amoxicillin or a third-generation cephalosporin. In Egypt, ciprofloxacin is not currently used to treat diarrhoea in individuals aged <18 years. SXT is still widely as a first-line antibiotic. SXT resistance is commonly carried as part of class I integrons in enteric Gram-negative bacteria and, therefore, selection of resistance to this antibiotic may inadvertently confer resistance to a wide range of other antimicrobials such as ampicillin [35, 36]. Antimicrobial resistance has emerged as a major issue for public health services worldwide [20, 37–39]. MDR S. flexneri were prevalent in our samples regardless of serotype, although the AST distributions differed between serotypes. This finding is similar to a recent report from Calcutta [38]. Overall, the frequency of resistance reported here is higher than that reported for isolates from the preceding 5 years, and the trend to MDR is more pronounced [18]. Higher prevalence of resistance was also noted for ampicillin, SXT, chloramphenicol and tetracycline. We observed little overt resistance to nalidixic acid in contrast to some other developing countries [38, 39], suggesting that nalidixic acid remains a viable therapeutic option for management of shigellosis in Egypt. Similar to a recent study from Turkey, no resistance to ciprofloxacin was recorded [12]. However, recent reports from Israel and Iran suggest that fluoroquinolone-resistant S. flexneri isolates are emerging in the region [10, 40]. If the percentage of intermediate isolates are combined with the resistance isolates, nalidixic acid non-susceptibility increases to nearly 10%. The WHO recommends that nalidixic acid is not administered for the treatment of shigellosis as the widespread usage of nalidixic acid may reduce the efficacy of ciprofloxacin [16]. Further surveillance must be conducted to monitor the development of S. flexneri quinolone/fluoroquinolone resistance in Egypt.
Within each of the serotypes, a single dominant clone was observed. However, within each of these clonal lineages, phenotypic differences were observed. Serotype 2a was the most prevalent serotype in children seeking medical care in Egypt, constituting 37% of the total S. flexneri population. This is consistent with previous studies examining the serotype distribution of S. flexneri isolates in children aged <5 years [1, 20, 37, 41, 42]. The Egyptian serotype 2a isolates were phenotypically diverse. Three of the five biochemical profiles observed in this study were expressed by S. flexneri 2a isolates. Additionally, isolates of serotype 2a expressed the most diverse assortment of antibiotic-resistance profiles, dominated by resistance to AP/TE/C/E/STX followed by AP/TE/STX. These observations are in line with previous studies but differences are noted in the details. An Israeli study [20] reported that 55% of serotype 2a isolates were resistant to AP/TE/C/STX and 23·2% were resistant to AP/C/TE; in contrast to 11 and 6% respectively, in this study. A Chilean study [37], while not determining S. flexneri subgroups, reported <1% S. flexneri resistance to AP/TE/STX, although this resistance pattern was the second most prevalent in our serotype 2a population. The majority of the serotype 2a isolates (32/36) formed a single, highly related cluster. The presence of the set and sen genes in serotype 2a and other S. flexneri serotypes has been shown to be variable [23, 25, 26, 33]. All of the Egyptian isolates contained both the sen toxin-encoding gene, however, only serotype 2a isolates contained the set gene.
S. flexneri serotype 1 (b and c) was the second most common serotype identified. We noted that isolates of S. flexneri 1b and 1c differed in their susceptibility to chloramphenicol, erythromycin and STX, resulting in very different AST profile distributions. A similar observation was made previously [9], in that a notable difference between serotype 1b and 1c isolates with respect to SXT was observed. Unfortunately, Talukder et al. did not examine C-sensitivity as part of their study, so it is difficult to determine whether C-sensitivity is a general property of serotype 1c. We did not expect to find serotypes 1b and 1c at almost identical frequencies in this study. Genetically, XbaI mrp–PFGE profiling could distinguish between the majority of serotype 1b and 1c isolates and separated the 1c group into four genetically diverse lines. Isolates of serotype 1c recovered from Abees, AH, and MH, spanning the period of time from 1994 to 2004, formed a single, large cluster. This is similar to results reported from a collection of serotype 1c isolates from Bangladesh [9]. From 1995 to 1998, no serotype 1c was observed in Egypt despite active community-based surveillance (data not shown) and the serotype reappeared in two separate children in AH in 1999. Since 1999, the frequency of isolation of serotype 1c has been increasing [in 2000, 1/17 (5·9%); 2001, 0/7; 2002, 2/6 (33·3%); 2003, 7/30 (23·3%); 2004 7/37 (18·9%)] and now this serotype is among the most frequently isolated S. flexneri serotypes in Egypt. A similar trend was reported in Bangladesh [9]. Further surveillance will be necessary to determine whether this is a stable or transient change in the S. flexneri dynamic in Egypt.
Serotype 6 was found in this study to be a common isolate with a bias towards young children (median age 12·5 months). In contrast, serotype 6 was more commonly associated with diarrhoea in children of kindergarten/early school age [7]. However, since our study only examined children aged ⩽5 years, it is difficult to determine the significance of this observation. Serotype 6 isolates were almost exclusively obtained from the warm season. A similar seasonal bias was also observed in a previous study [7]. In our study, S. flexneri serotype 6 isolates formed a closely related cluster that was distinct from the majority of other S. flexneri isolates and has been noted previously [43]. This genetic diversity may be reflected in the observation that current vaccine configurations targeting serotypes 2a and 3a are not cross-protective towards serotype 6 isolates [44–46].
The clinical features of shigellosis in these populations punctuate the importance of this pathogen as a disease among children in the developing world. Whereas others have found differences among severity of disease between serotypes [7], we did not find a similar clear association. The finding of relatively higher rates of rectal prolapse in serotypes 1b and 1c is interesting and could be an indicator that these circulating serotypes are more virulent. However, our study was not well powered to detect these differences, nor assess for potential confounding between other predictive factors. We found two different factors associated with differential probability of hospitalization. There was a higher probability of admission at BH Fever Hospital compared to the other two study sites. This finding could be explained by the different hierarchies of care represented at the three different sites, or by differences in the sociodemographic features of the paediatric populations in the respective catchments. Further studies designed to particularly evaluate the multiple host, agent and environmental factors which may be associated differential severity of presentation for shigellosis are clearly needed. We also noted that one particular resistance phenotype (AP/C/E/TE/SXT) was associated with higher probability of admission among children seeking care. A number of other studies have demonstrated an association between antimicrobial resistance and virulence determinants [47, 48] and this could explain our finding. Increased antimicrobial resistance in gastrointestinal bacterial pathogens is likely to increase the burden of disease [48]. Thus, in an environment where antibiotics are readily available and often abused, colonization by antimicrobial resistant bacterial pathogens followed by antibiotic therapy (perhaps for an unrelated illness) may lead to severe infections. Similarly, exposure to a resistant gastrointestinal pathogen after antibiotic therapy may also lead to serious consequences. However, this finding could also be confounded by the association between study site and resistance phenotype. Unfortunately this study was not powered to adjust for potential confounding and thus the true relationship between the probability of hospitalization, study site and resistance phenotype cannot be known. Despite these limitations, the higher probability of hospitalization and dehydration associated with Shigella compared to other enteric pathogens in these populations [43] serves as a reminder as to the burden of this pathogen and the further research that is needed to understand the epidemiology of this disease in Egypt.
ACKNOWLEDGEMENTS
The authors acknowledge the contributions of Dr Nils Carlin (subserotype specific antisera 1c) and Dr Atef El-Gendy (critical comments regarding serotyping) and Mr Abdel-Hakam Abdel-Fattah (data collection). Thanks are also due to Drs David Bean, Hind Shaheen and Stan Tai, for advice and critique of the manuscript. This work was funded in part by NBFAC 703242-4093-N921.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Kotloff KL et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bulletin of the World Health Organization. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Vaccine research and development. New strategies for accelerating Shigella vaccine development. Weekly Epidemiological Record. 1997;72:73–79. [PubMed] [Google Scholar]
- 3.Wehler T, Carlin NI. Structural and immunochemical studies of lipopolysaccharide from a new provisional serotype of Shigella flexneri. European Journal of Biochemistry. 1988;176:471–476. doi: 10.1111/j.1432-1033.1988.tb14304.x. [DOI] [PubMed] [Google Scholar]
- 4.Carlin NIA et al. Use of monoclonal antibodies to type Shigella flexneri in Bangladesh. Journal of Clinical Microbiology. 1989;27:1163–1166. doi: 10.1128/jcm.27.6.1163-1166.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coimbra RS, Grimont F, Grimont PAD. Identification of Shigella serotypes by restriction of amplified O-antigen gene cluster. Research Microbiology. 1999;150:543–553. doi: 10.1016/s0923-2508(99)00103-5. [DOI] [PubMed] [Google Scholar]
- 6.Talukder KA et al. Altering trends in the dominance of Shigella flexneri serotypes and emergence of serologically atypical S. flexneri strains in Dhaka, Bangladesh. Journal of Clinical Microbiology. 39:3757–3759. doi: 10.1128/JCM.39.10.3757-3759.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasilev V et al. Variability of Shigella flexneri serotypes in Israel during a period of two years: 2000 and 2001. Epidemiology and Infection. 2004;132:51–56. doi: 10.1017/s095026880300147x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Gendy A et al. Identification of Shigella flexneri subserotype 1c in rural Egypt. Journal of Clinical Microbiology. 1999;37:873–874. doi: 10.1128/jcm.37.3.873-874.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talukder KA et al. Phenotypic and genotypic characterization of provisional serotype Shigella flexneri 1c and clonal relationships with 1a and 1b strains isolated from Bangladesh. Journal of Clinical Microbiology. 2003;41:110–117. doi: 10.1128/JCM.41.1.110-117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MoezArdalan K et al. Prevalence and pattern of antimicrobial resistance of Shigella species among patients with acute diarrhoea in Jaraj, Tehran, Iran. Journal of Health, Population and Nutrition. 2003;21:96–102. [PubMed] [Google Scholar]
- 11.Nguyen TV et al. Antibiotic resistance in diarrheagenic Escherichia coli and Shigella strains isolated from children in Hanoi, Vietnam. Antimicrobial Agents and Chemotherapy. 2005;49:816–819. doi: 10.1128/AAC.49.2.816-819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Özmert E et al. Shigella antibiotic resistance in Central Turkey: comparison of the years 1987–1994 and 1995–2002. Journal of Pediatric Gastroenterology and Nutrition. 2005;40:359–362. doi: 10.1097/01.mpg.0000153006.38363.7e. [DOI] [PubMed] [Google Scholar]
- 13.Wasfy MO et al. Isolation and antibiotic susceptibility of Salmonella, Shigella, and Campylobacter from acute enteric infections in Egypt. Journal of Health, Population and Nutrition. 2000;18:33–38. [PubMed] [Google Scholar]
- 14.Green S, Tillotson G. Use of ciprofloxacin in developing countries. Pediatric Infectious Disease Journal. 1997;16:150–159. doi: 10.1097/00006454-199701000-00041. [DOI] [PubMed] [Google Scholar]
- 15.Jick S. Ciprofloxacin safety in a pediatric population. Pediatric Infectious Disease Journal. 1997;16:130–133. doi: 10.1097/00006454-199701000-00037. [DOI] [PubMed] [Google Scholar]
- 16.WHO . Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae 1 (http://whqlibdoc.who.int/publications/2005/9241592330.pdf). Accessed 1 March 2006.
- 17.Putnam SD et al. Antimicrobial susceptibility trends in Campylobacter jejuni and Campylobacter coli isolated from a rural Egyptian pediatric population with diarrhea. Diagnostic Microbiology and Infectious Disease. 2003;47:601–608. doi: 10.1016/s0732-8893(03)00154-8. [DOI] [PubMed] [Google Scholar]
- 18.Putnam SD et al. Antimicrobial susceptibility trends among Escherichia coli and Shigella spp isolated from rural Egyptian paediatric populations with diarrhoea between 1995 and 2000. Clinical Microbiology and Infection. 2004;10:804–810. doi: 10.1111/j.1469-0691.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 19.Wasfy MO et al. Trends of multiple-drug resistance among Salmonella serotype Typhi isolates during a 14-year period in Egypt. Clinical Infectious Diseases. 2002;35:1265–1268. doi: 10.1086/343052. [DOI] [PubMed] [Google Scholar]
- 20.Vasilev V et al. Antimicrobial resistance of Shigella flexneri serotypes in Israel during a period of three years: 2000–2002. Epidemiology and Infection. 2004;132:1049–1054. doi: 10.1017/s0950268804002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nataro JP et al. Identification and cloning of a novel plasmid-encoded enterotoxin and enteroinvasive Escherichia coli and Shigella strains. Infection and Immunity. 1995;63:4721–4728. doi: 10.1128/iai.63.12.4721-4728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talukder KA et al. Phenotypic and genotypic characterization of serologically atypical strains of Shigella flexneri Type 4 isolated in Dhaka, Bangladesh. Journal of Clinical Microbiology. 2002;40:2490–2497. doi: 10.1128/JCM.40.7.2490-2497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noriega FR et al. Prevalence of Shigella enterotoxin 1 among Shigella clinical isolates of diverse serotypes. Journal of Infectious Diseases. 1995;172:1408–1410. doi: 10.1093/infdis/172.5.1408. [DOI] [PubMed] [Google Scholar]
- 24.Venkatesan MM, Buysee JM, Kopecko DJ. Use of Shigella flexneri ipaC and ipaH gene sequences for the general identification of Shigella spp and enteroinvasive Escherichia coli. Journal of Clinical Microbiology. 1989;27:2687–2691. doi: 10.1128/jcm.27.12.2687-2691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niyogi SK, Vargas M, Vila J. Prevalence of the sat, set and sen genes among diverse serotypes of Shigella flexneri strains isolated from patients with acute diarrhoea. Clinical Microbiology and Infection. 2004;10:574–576. doi: 10.1111/j.1469-0691.2004.00897.x. [DOI] [PubMed] [Google Scholar]
- 26.Vargas M et al. Prevalence of Shigella enterotoxins 1 and 2 among Shigella strains isolated from patients with traveler's diarrhea. Journal of Clinical Microbiology. 1999;37:3608–3611. doi: 10.1128/jcm.37.11.3608-3611.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdel-Messih I et al. Diarrhea associated with Cryptosporidium parvum among young children on the Nile River Delta in Egypt. Journal of Tropical Pediatrics. 2005;51:154–159. doi: 10.1093/tropej/fmh105. [DOI] [PubMed] [Google Scholar]
- 28.Rao MR et al. Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. American Journal of Epidemiology. 2001;154:166–173. doi: 10.1093/aje/154.2.166. [DOI] [PubMed] [Google Scholar]
- 29.Wierzba TF et al. Clinic-based surveillance for bacterial- and rotavirus-associated diarrhea in Egyptian children. American Journal of Tropical Medicine and Hygiene. 2006;74:148–153. [PubMed] [Google Scholar]
- 30.WHO 1997. . Workbook on management and prevention of diarrhoea, vol. WHO/CDR/95.7,
- 31.CLSI 2005. . Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement M100-S15. Clinical and Laboratory Standards Institute,
- 32.Hunter SB et al. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. Journal of Clinical Microbiology. 2005;43:1045–1050. doi: 10.1128/JCM.43.3.1045-1050.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yavzori M, Cohen D, Orr N. Prevalence of the genes for shigella enterotoxins 1 and 2 among clinical isolates of shigella in Israel. Epidemiology and Infection. 2002;128:533–535. doi: 10.1017/s0950268802006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sethabutr O et al. Detection of PCR products of the ipaH gene from Shigella and enteroinvasive Escherichia coli by enzyme linked immunosorbent assay. Diagnostic Microbiology and Infectious Disease. 2000;37:11–16. doi: 10.1016/s0732-8893(00)00122-x. [DOI] [PubMed] [Google Scholar]
- 35.Jones ME et al. Widespread occurrence of integrons causing multiple antibiotic resistances in bacteria. Lancet. 1997;349:1742–1743. doi: 10.1016/S0140-6736(05)62954-6. [DOI] [PubMed] [Google Scholar]
- 36.Siu LK et al. β-lactamases in Shigella flexneri isolates from Hong Kong and Shanghai and a novel OXA-1-like β-lactamase, OXA-30. Antimicrobial Agents and Chemotherapy. 2000;44:2034–2038. doi: 10.1128/aac.44.8.2034-2038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fullá N et al. Surveillance for antimicrobial resistance profiles among Shigella species isolated from a semirural community in the northern administrative area of Santiago, Chile. American Journal of Tropical Medicine and Hygiene. 2005;72:851–854. [PubMed] [Google Scholar]
- 38.Pazhani GP et al. Species diversity and antimicrobial resistance of Shigella spp isolated between 2001 and 2004 from hospitalized children with diarrhoea in Kolkata (Calcutta), India. Epidemiology and Infection. 2005;133:1089–1095. doi: 10.1017/S0950268805004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zafar A, Sabir N, Bhutta ZA. Frequency of isolation of Shigella serogroups/serotypes and their antimicrobial susceptibility pattern in children from slum areas in Karachi. Journal of the Pakistan Medical Association. 2005;55:184–188. [PubMed] [Google Scholar]
- 40.Ashkenazi S et al. Growing antimicrobial resistance of Shigella isolates. Journal of Antimicrobial Chemotherapy. 2003;51:427–429. doi: 10.1093/jac/dkg080. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Elyazeed R et al. Epidemiology of Shigella-associated diarrhea in rural Egyptian children. American Journal of Tropical Medicine and Hygiene. 2004;71:367–372. [PubMed] [Google Scholar]
- 42.Dutta S et al. Shifting serotypes, plasmid profile analysis and antimicrobial resistance pattern of shigellae strains isolated from Kolkata, India during 1995–2000. Epidemiology and Infection. 2002;129:235–243. doi: 10.1017/s0950268802007240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pupo GM, Lan R, Reeves PR. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proceedings of the National Academy of Sciences USA. 2000;97:10567–10572. doi: 10.1073/pnas.180094797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noriega FR et al. Strategy for cross-protection among Shigella flexneri serotypes. Infection and Immunity. 1999;67:782–788. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simmon DA, Romanovska E. Structure and biology of Shigella flexneri O antigens. Journal of Medical Microbiology. 1987;23:289–302. doi: 10.1099/00222615-23-4-289. [DOI] [PubMed] [Google Scholar]
- 46.Van de Verg LL et al. Cross-reactivity of Shigella flexneri serotype 2a O antigen antibodies following immunization or infection. Vaccine. 1996;14:1062–1068. doi: 10.1016/0264-410x(96)00006-0. [DOI] [PubMed] [Google Scholar]
- 47.Bii CC et al. Detection of virulence-related genes by multiplex PCR in multidrug-resistant diarrhoeagenic Escherichia coli isolates from Kenya and Japan. Epidemiology and Infection. 2005;133:627–633. doi: 10.1017/s0950268805003870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mølbak K. Human health consequences of antimicrobial drug-resistant Salmonella and other foodborne pathogens. Clinical Infectious Diseases. 2005;41:1631–1640. doi: 10.1086/497599. [DOI] [PubMed] [Google Scholar]