SUMMARY
In 1981, R. Edgar Hope-Simpson proposed that a ‘seasonal stimulus’ intimately associated with solar radiation explained the remarkable seasonality of epidemic influenza. Solar radiation triggers robust seasonal vitamin D production in the skin; vitamin D deficiency is common in the winter, and activated vitamin D, 1,25(OH)2D, a steroid hormone, has profound effects on human immunity. 1,25(OH)2D acts as an immune system modulator, preventing excessive expression of inflammatory cytokines and increasing the ‘oxidative burst’ potential of macrophages. Perhaps most importantly, it dramatically stimulates the expression of potent anti-microbial peptides, which exist in neutrophils, monocytes, natural killer cells, and in epithelial cells lining the respiratory tract where they play a major role in protecting the lung from infection. Volunteers inoculated with live attenuated influenza virus are more likely to develop fever and serological evidence of an immune response in the winter. Vitamin D deficiency predisposes children to respiratory infections. Ultraviolet radiation (either from artificial sources or from sunlight) reduces the incidence of viral respiratory infections, as does cod liver oil (which contains vitamin D). An interventional study showed that vitamin D reduces the incidence of respiratory infections in children. We conclude that vitamin D, or lack of it, may be Hope-Simpson's ‘seasonal stimulus’.
INTRODUCTION
Whoever wishes to investigate medicine properly should proceed thus: in the first place to consider the seasons of the year …
Hippocrates
(circa 400 b.c.)
… the characteristic microbe of a disease might be a symptom instead of a cause.
George Bernard Shaw
(Preface on Doctors, The Doctor's Dilemma, 1911)
Perhaps the most mysterious feature of epidemic influenza is its remarkable and recurrent seasonality – wintertime surfeit and summertime scarcity – a feature first explored in detail by R. Edgar Hope-Simpson, the British general practitioner and self-educated epidemiologist. After his celebrated discoveries of the cause of shingles [1] and the latency of varicella [2], Hope-Simpson dedicated much of the rest of his working life to the epidemiology of influenza. He believed that discovering the cause of influenza's seasonality would ‘provide the key to understanding most of the influenzal problems confronting us’ [3].
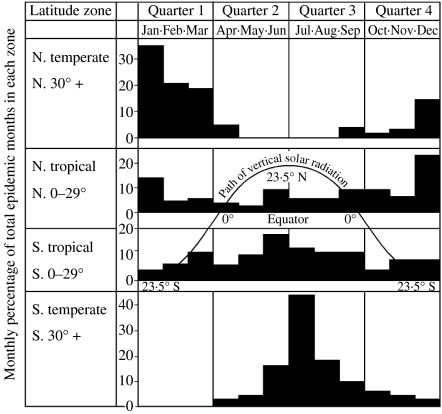
Hope-Simpson was the first to document that influenza A epidemics in temperate latitudes peak in the month following the winter solstice (Fig. 1). In both hemispheres, influenza rates rise significantly for about 2 months on either side of its peak.
Outbreaks are globally ubiquitous and epidemic loci move smoothly to and fro across the surface of the earth almost every year in a sinuous curve that runs parallel with the midsummer curve of vertical solar radiation, but lags about six months behind it … Latitude alone broadly determines the timing of the epidemics in the annual cycle, a relationship that suggests a rather direct effect of some component of solar radiation acting positively or negatively upon the virus, the human host, or their interaction … The nature of the seasonal stimulus remains undiscovered [4].
Fig. 1.
The seasonal and latitudinal distribution of outbreaks of type A influenza in the world, 1964–1975, summarized from the Weekly Epidemiological Record of the World Health Organization into major zones. The diagrams show for each calendar month the percentage of each zone's total outbreaks. In both north and south temperate zones the epidemics are distributed around the local midwinter, whereas the tropical zones show a transition, each approximating towards the distribution of its own temperate zone. The curve indicates the ‘midsummer’ path taken annually by vertical solar radiation. The ‘epidemic path’ seems to parallel it, but to lag 6 months behind it. (Reproduced with permission, Cambridge University Press, Hope-Simpson, 1981.)
Thus, he hypothesized that solar radiation produced a ‘seasonal stimulus’ that profoundly affected the pathogenesis of influenza A – but he had no idea of the mechanism. However, Hope-Simpson believed epidemiologists would eventually succeed with ‘the task of identifying the chain of intermediate mechanisms through which the prime cause (the variation in solar radiation) is operating its seasonal influence’ [3, p. 87].
Although serological and culture evidence of influenza infection has been documented in the summer, it seldom causes community outbreaks in summer [5–7]. About 2% of persons continuously surveyed seroconvert during periods when clinical influenza is not recognized [8]. In spite of being in the population year-round, epidemics in temperate latitudes usually peak in winter [9–13]. Furthermore, Hope-Simpson noted that ‘epidemics of influenza often occur contemporaneously at the same latitude even in localities widely separated by longitude’ [4, p. 43]. He noted influenza would abruptly attack 15% or more of the population around the winter solstice but virtually disappear in the sunny months despite a wealth of potential victims lacking virus-specific antibodies.
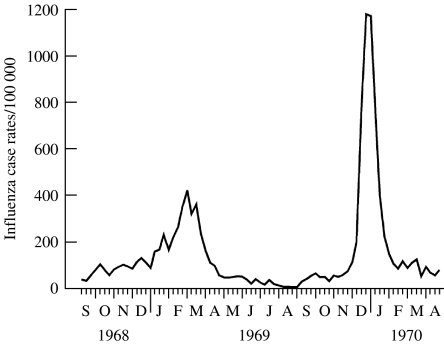
Hope-Simpson saw solar radiation as a stronger predictor of influenza epidemics than the presence of virus-specific antibodies. For example, Miller et al. [14] reported that the Hong Kong virus was first isolated in Britain in August 1968 but it did not cause significant summertime illness despite being a new antigenic variant in a non-immune population (Fig. 2). However, clinical case rates increased in intensity as the sun became progressively lower in the sky each day (autumn), waiting until the winter solstice of 1968 before the first community outbreaks appeared. Influenza case rates peaked for several months but waned as the sun rose higher in the sky each day (spring). Predictably, influenza virtually ceased following the summer solstice. Clinical case rates for Hong Kong influenza increased from September 1969, only to explode again in the days preceding the winter solstice, even though a much higher proportion of the British population had virus-specific antibodies at the beginning of the lethal second wave than they did at the beginning of its less lethal first wave.
Fig. 2.
Weekly consultation rates for illnesses diagnosed clinically as influenza or influenza-like, calculated from returns to the General Practice Research Unit of the Royal College of General Practitioners from about 40 general practices in various parts of England, Scotland and Wales, serving a population of about 150 000 persons, 1968–1970. (Reproduced/amended with permission, BMJ Publishing Group, Miller et al.)
Hope-Simpson also observed that influenza outbreaks in the tropics, where solar UV radiation is less seasonal, are also much less seasonal, but are generally more severe when solar radiation is impaired (the rainy season) – observations recently confirmed [15]. This intimate association with sunlight led the naturalist in Hope-Simpson to see influenza as a winter ‘crop, and, as with other crops, some years are good influenza years, and other years produce a poor crop of influenza cases’ [3, p. 92].
Influenza is but one of several respiratory viral pathogens that show a distinct predilection for infecting us in the wintertime. Noah found that in England and Wales respiratory syncytial virus and parainfluenza 1 and 2 display marked wintertime excess [16]. More than 200 viruses cause the common cold, which, as the name implies, also shows a distinct wintertime excess [17]. However, in clinical practice, and in much published research, specific identification of respiratory viral infections is frequently absent. With full appreciation of its inherent limitations, we will use the term viral respiratory infection in this review, unless the literature cited was more specific.
The seasonal stimulus?
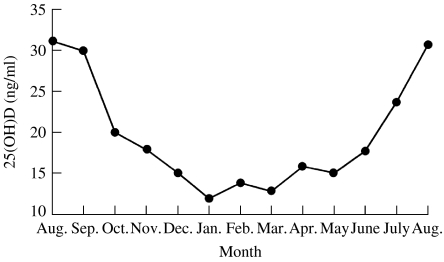
As Hope-Simpson pointed out, solar radiation may be affecting the ‘virus, the human host, or their interaction …’. That is, he theorized that humans might have a physiological system directly dependent on solar radiation that improves innate immunity around the summer solstice but impairs it in the winter. There is a seasonal steroid hormone system with profound effects on human immunity whose substrate levels reach their nadir during influenza season but peak when influenza is rare (Fig. 3) [18, 19].
Fig. 3.
Seasonal variation of 25(OH)D levels in a population-based sample of inhabitants of a small southern German town, aged 50–80 years. (Reproduced/amended with kind permission of Springer Science and Business Media, Scharla, S.H., 1998.)
Cholecalciferol (vitamin D) is a prehormone normally made in the skin during sunny months when UVB radiation triggers the conversion of 7-dehydrocholesterol in the skin into vitamin D [20]. The liver converts vitamin D into 25-hydroxyvitamin D [25(OH)D] and then cells all over the body convert 25(OH)D to 1,25-dihydroxyvitamin D [1,25(OH)2D] – a potent steroid hormone. Locally produced 1,25(OH)2D performs autocrine and paracrine functions in a wide variety of tissues, including the immune system. Local tissue levels of 1,25(OH)2D are dependent on available serum substrate [25(OH)D]. Dangers of vitamin D deficiency may include more than just low 25(OH)D levels. Vieth has proposed that progressively falling serum levels of 25(OH)D (as occurs in the autumn), may trigger intracellular deficiencies of 1,25(OH)2D, despite apparently adequate serum levels of 25(OH)D and 1,25(OH)2D [21].
Many distinctive features of the biology, physiology, and epidemiology of vitamin D point to it as a likely candidate for Hope-Simpson's ‘seasonal stimulus’.
Vitamin D has profound and multiple effects on human immunity [22, 23].
Inadequate vitamin D nutrition is endemic among the elderly in the winter [24–26].
Serum levels of 25(OH)D are low in many people of all ages who live at temperate latitudes, especially in the winter [20].
Humans acquire most of their vitamin D from casual sun exposure, and to a degree that is a function of skin surface area exposed [27, 28].
The elderly only make about 25% of the vitamin D as 20-year-olds do after exposure to the same amount of sunlight [29].
Seasonal variations – and vitamin D deficiency – occur in both subtropical and tropical latitudes [30, 31].
Routine daily supplementation with 400 IU of vitamin D does not prevent wintertime insufficiency [32].
Mechanism of action of vitamin D
The pathology of influenza involves a complex interaction between the virus, acquired immunity, and innate immunity. Macrophages rapidly release cytokines into infected respiratory tissue while virucidal antimicrobial peptides attempt to prevent viral replication [33]. The release of proinflammatory cytokines, as much as the virulence of the virus, may determine the clinical phenotype of influenza infection. Recent research confirms that the clinical phenotype of influenza correlates well with amount of cytokines released [34, 35]. Furthermore, the severity of the illness induced by genetically reproduced 1918 influenza virus also correlates with the ability of the virus to induce macrophage production of cytokines [36]. In avian influenza, the innate cytokine immune response can be overwhelming; levels of such cytokines are significantly higher in those with a fatal outcome [37, 38].
Recently, vitamin D has been found to modulate macrophages' response, preventing them from releasing too many inflammatory cytokines and chemokines [39, 40]. Vitamin D deficiency also impairs the ability of macrophages to mature, to produce macrophage-specific surface antigens, to produce the lysosomal enzyme acid phosphatase, and to secrete H2O2, a function integral to their antimicrobial function [41, 42]. The same authors found that the addition of 1,25(OH)2D increased expression of macrophage-specific surface antigens and the lysosomal enzyme acid phosphatase while stimulating their ‘oxidative burst’ function.
Perhaps most importantly, three independent research groups have recently shown that 1,25(OH)2D dramatically stimulates genetic expression of antimicrobial peptides (AMP) in human monocytes, neutrophils, and other human cell lines [43–45]. These endogenous antibiotics, such as defensins and cathelicidins, directly destroy invading microorganisms [46]. AMP display broad-spectrum antimicrobial activity, including antiviral activity, and have been shown to inactivate the influenza virus [47–49]. Not only do neutrophils, macrophages, and natural killer cells secrete AMP, but epithelial cells lining the upper and lower respiratory tract secrete them as well, where they play a major role in pulmonary defence [50, 51].
Influenza and solar radiation
In spite of people congregating on cruise ships, airplanes, nursing homes, factories, offices, subways, hospitals, etc., summertime outbreaks and the spread of influenza A are rare [52, 53]. Curwen found a strong inverse correlation between the incidence of influenza and temperature in England and Wales [54], temperature being strongly associated with solar radiation [55]. He found average monthly temperature dropped below around 7°C during the influenza season. It is of interest that no vitamin D is made in the skin at latitude 52° N (the latitude of London) from about October to March because atmospheric ozone easily filters out UVB radiation unless the sun is high enough in the sky [56].
Annual all-cause mortality peaks in the months following the winter solstice and most excess wintertime mortality is in the elderly, due to both influenza and cardiac disease; some believe influenza explains all the significant wintertime increase in cardiac mortality [57]. The average excess winter mortality in Great Britain alone is 30 000 persons per year [58], and is inversely related to hours of sunlight with 2·9% lower odds for every additional hour of sunshine; mortality from respiratory disease showed the greatest sunshine benefit.
If vitamin D is Hope-Simpson's ‘seasonal stimulus’, then countries with low 25(OH)D levels and marked wintertime troughs should have higher excess wintertime mortality than do countries with high 25(OH)D levels and little seasonal variation. For example, Norway has the highest 25(OH)D levels in Europe (thought to be due to its high year-round consumption of fish and cod liver oil) [59]. Levels of 25(OH)D in Scandinavia display the least seasonal variation in Europe; indeed there is virtually no 25(OH)D seasonal variation among the elderly in Scandinavia [60]. On the other hand, the elderly in Great Britain have low 25(OH)D levels and such deficiencies are much more common during the influenza season [61]. Excess wintertime mortality is twice as high in Great Britain as in Norway [62].
Global weather changes are associated with El Niño/Southern Oscillation (ENSO) [63]. Viboud et al. found an average of 3·7 million influenza cases in France during the 10 cold phases of ENSO but only 1·8 million cases during the eight warm phases [64]. The same authors reported that cold ENSO phases are associated with colder temperatures in Europe. Colder temperatures should lower mean serum population 25(OH)D levels by lessening outdoor activity and necessitating more clothes when outdoors. Ebi et al. studied six Californian counties and found that hospitalizations for viral pneumonia peaked around the winter solstice in all six counties [65]. They also found hospitalizations increased 30–50% for every 5 °F (3 °C) decrease in minimum temperatures in four counties and increased 25–40% for every 5 °F (3 °C) decrease in maximum temperatures in the other two.
Hope-Simpson was the first to note an association between severe influenza epidemics and solar flare activity [66]. In 1990, Hoyle and Wickramasinghe confirmed the association but von Alvensleben disputed it [67, 68]. Horgan [69] promptly derided the observations, connecting them to viral invasions from outer space, a theory Hope-Simpson dismissed in his 1992 book [3]. Since the controversy, science has learned that solar flare activity increases high-altitude ozone, which, in turn, absorbs more UVB radiation thereby decreasing surface UVB [70]. Thus, paradoxically, heightened solar activity reduces surface UVB; presumably, average 25(OH)D levels would be lower as well. Rozema et al. estimated the variations in surface UVB radiation due to the solar flare activity over the last 300 years and estimated that, beginning in the eighteenth century, ‘the dose of surface UV-B should be (about) 4% to 13% lower at maxima of the 11-year solar cycle’ [71]. Although modest, such reoccurring decreases in UVB radiation should trigger reductions in average 25(OH)D levels, which, in turn, could trigger nonlinear factors related to influenza infectivity.
Melanin retards the ability of sunlight to trigger vitamin D production in African-Americans' skin so they have much lower 25(OH)D levels than do whites [72]. If vitamin D is Hope-Simpson's ‘seasonal stimulus’ then African-Americans should have a higher incidence of influenza and higher age-adjusted influenza mortality than do whites. Although we could find no racial incidence data, the age-adjusted mortality from combined pneumonia and influenza deaths in the United States was higher for African-Americans than for whites in 2000 (10% excess), 2001 (11% excess), and 2002 (6% excess) [73–75]. The same statistics show African-Americans have a much higher age-adjusted mortality from heart disease, and a significant percentage of those who die from influenza are reported to have suffered a cardiac death [76, 77]. Furthermore, black children continue to have twice the pneumonia mortality of white children [78]. While some of these racial disparities may be due to socioeconomic factors, racial differences in 25(OH)D levels may also be important.
Attenuated influenza virus, the effect of season
If vitamin D is Hope-Simpson's ‘seasonal stimulus’, then humans inoculated with attenuated viruses during the summer [when 25(OH)D levels peak] should show less evidence of infection than those inoculated in winter. Shadrin et al. inoculated 834 non-immune males (age 16–18 years) with live attenuated influenza virus (B/Dushabbe/66 and B/Leningrad/2/67) in St Petersburg (62° N) and Krasnodar, Russia (45° N), during different seasons of the year, comparing them to 414 vehicle placebo controls [79]. In St Petersburg, they found that the attenuated virus was about eight times more likely to cause physical evidence of infection (fever) in the winter than the summer (6·7% vs. 0·8%). In Krasnodar, 8% of inoculated subjects developed a fever from the virus in January, but only 0·1% did so in May.
Zykov and Sosunov found that fever after inoculation with attenuated H3N2 (221 subjects) was twice as likely in February (10·7%) as in June (5%), compared to vehicle placebo controls [80]. They also confirmed that seroconversion varied by season, with the lowest rate of antibody formation in summer. When they attempted to recover the virus 48–72 h after inoculation, they found subjects were more likely to shed the virus in December (40%) than in September (16%), and the quantity of virus shed was significantly lower in summer than winter.
Vitamin D deficiency and viral respiratory infections
If vitamin D is Hope-Simpson's ‘seasonal stimulus’, then vitamin D deficiency should predispose patients to respiratory infections. Rickets is the classic vitamin D-deficient disease of childhood and a long-standing association exists between rickets and respiratory infection [81–88]. Mechanical impairment of pulmonary function due to clinical rickets is widely thought to explain the association. However, Wayse et al. recently compared 80 non-rachitic children with lower respiratory infections to healthy controls and found children with 25(OH)D levels <10 ng/ml were 11 times more likely to be infected [89]. This discovery makes it likely that it is vitamin D deficiency per se, and not mechanical impairment of pulmonary function, that explains the long-standing association of rickets with pulmonary infection.
UV radiation and viral respiratory infections
It is generally accepted that erythemal doses of UV radiation (UVR), which contains both UVB and UVA radiation, suppress human immune function [90, 91]. However, Termorshuizen et al. recently reviewed the literature on immune function and UVR, concluding it is dangerous to assume that such suppression will result in an increased incidence of infectious disease [92]. Furthermore, sub-erythemal doses of UVR, unlike erythemal doses, actually improve phagocytic activity in human volunteers. For example, Krause et al. reported that a 6- to 8-week course of sub-erythemal doses of UVR doubled the phagocytic activity in 21 children with recurrent respiratory tract infections [93]. Likewise, Csato et al. found five sub-erythemal doses of UVR increased polymorphonuclear chemotaxis in normal volunteers [94].
In 1990, Gigineishvili et al. administered sub-erythemal courses of UVR twice a year for 3 years to 410 teenage Russian athletes and compared them to 446 non-irradiated athletes [95]. The non-UVR controls had 50% more respiratory viral infections, 300% more days of absences and 30% longer duration of illness than did the UVR subjects. The irradiated subjects also had significant increases in salivary IgA, IgG and IgM compared to controls. In 2004, Termorshuizen et al. found that parents of Dutch children with the least sun exposure were twice as likely to report that their child developed a cough, and were three times as likely to report their child had a runny nose, compared to children with the most sun-exposure [96].
Cod liver oil and viral respiratory infections
Recently, Semba reviewed early literature on fish liver oils given as an ‘anti-infective’ [97]. These oils contain large amounts of vitamin D. All five cod liver oil studies listed by Semba showed it reduced the incidence of respiratory infections. Two controlled studies in the 1930s found similar results: the first found cod liver oil given to 185 adults for 4 months reduced colds by 50%; in the second study it reduced industrial absenteeism due to respiratory infections in 1561 adults by 30% [98, 99]. In 2004, Linday et al. reported that 600–700 IU of vitamin D, given as cod liver oil and a multivitamin, significantly reduced the mean number of upper respiratory tract visits over time when given to 47 young (mean age 2 years) New York City children from late autumn to early May, whereas in a medical record control site group, no decrease occurred over time [100]. Assuming the average 2-year-old weighs 13 kg, an equivalent dose in a 70 kg adult would be about 3500 IU/day.
Intervention with vitamin D
We are aware of only one modern paper that directly examined the relationship between vitamin D and respiratory infections. Rehman, in a letter, reported giving 60 000 IU of vitamin D a week and 650 mg of calcium daily for 6 weeks to 27 non-rachitic children (aged 3–12 years) with elevated alkaline phosphatases who were also suffering from frequent childhood infections, mostly respiratory infections [101]. He compared them to 20 age- and-sex matched control children who had not had more than one infectious episode per child during the previous 6 months. During the 6 months of observation after treatment, no difference was observed in the frequency of infection between the test and control groups of children. In fact, Rehman reported, ‘no recurrences were reported for a period of six months’, in the treated children.
DISCUSSION
The most common explanation for the seasonality of viral respiratory infections is that humans congregate indoors in the winter, thus increasing the chance for contagion. However, as Sir Christopher Andrewes pointed out, people also congregate indoors during the summer [102].
Many people regard (crowding) as the likeliest ‘winter factor’ to explain the facts (wintertime excess of respiratory infections). I have always had doubts about this. Indoor workers in towns spend their working hours in much the same way winter and summer; they are cheek-by-jowl in their offices or at the factory bench or canteen all through the year … If close contact were all, one would think the London Transport would ensure an all-the-year epidemic.
Summertime deaths due to influenza are rare except during pandemics – even during pandemics, most deaths occur during the colder months. Nor does the indoor theory of contagion explain why the administration of live attenuated influenza virus produces such seasonal results in non-immune volunteers, while the vitamin D theory would predict exactly such observations due to the stimulation of antimicrobial proteins and a suppression of cytokine response during the summer when UVB radiation induces the production of vitamin D. An indoor theory of contagion has difficulty explaining the strong association between vitamin D-deficient rickets (and simple vitamin D deficiency in non-rachitic children) and childhood infections, while the vitamin D theory explains both. Furthermore, the indoor contagion theory cannot explain why age-adjusted influenza deaths are more common among African-Americans than whites, whereas the striking racial differences in 25(OH)D levels readily explain it. Nor can an indoor theory explain the observations regarding sunlight, artificial UVB, cod liver oil, and supplemental vitamin D.
Eccles proposed that cooling of the nasal airways, which reduces mucociliary clearance and phagocytic activity, may explain the seasonality of viral respiratory infections [103]. The same group produced evidence in a controlled trial that chilling of the feet causes about 10% more subjects than controls to report the delayed onset of cold symptoms [104]. Furthermore, the common folklore that respiratory infections often follow exposure to cold air or to chilling of the body by wet hair, feet, or clothes is pervasive and unlikely to be entirely superstitious. Although this theory has evidence to support it and may explain some of the seasonality of respiratory infections, it, like the indoor contagion theory, fails to explain the observations detailed above while the vitamin D theory has plausible, albeit inadequately tested, explanations for them all.
Dosage of vitamin D
The seasonality hypothesis proposed here relates to sun-derived vitamin D. However, if vitamin D might be effective in preventing seasonal respiratory infections, then the daily oral dosage required for an effect remains to be addressed. Both the hypothesis and the dosage must be addressed through properly conducted clinical trials. A likely dose requirement can be estimated from existing knowledge of vitamin D nutrition.
The critical question of ‘What is an ideal 25(OH)D level?’ must be answered, ‘In regard to what?’ Levels needed to prevent rickets and osteomalacia (10 ng/ml) are lower than those that dramatically suppress parathormone levels (20 ng/ml) [105]. In turn, those levels are lower than those needed to increase intestinal calcium absorption maximally (34 ng/ml) [106]. In turn, neuromuscular performance in 4100 elderly patients steadily improved as 25(OH)D levels increased and maximum performance was associated with levels of 50 ng/ml [107]. If levels of 50 ng/ml are associated with further benefits, such as preventing viral respiratory infections, we are only now learning about it. Until more is known, it may be prudent to maintain wintertime 25(OH)D at concentrations achieved in nature by summertime sun exposure (50 ng/ml).
There are a number of factors to consider regarding the most appropriate dose of vitamin D. One minimal erythemal exposure of the full-body to artificial UVB radiation triggers the release of about 20 000 IU of vitamin D into the circulation of light-skinned persons within 48 h [108]. There was no evidence of toxicity in young men taking 50 000 IU of vitamin D a day for 6 weeks (although such a dose would be toxic if taken over a longer period) [109]. In 32 vitamin D-deficient elderly patients, 50 000 IU/day of vitamin D for 10 days showed no evidence of toxicity and only raised 25(OH)D levels by an average of 5 ng/ml 3 months after administration and in no patient did levels exceed 13 ng/ml at 3 months [110]. Single injections of 600 000 IU (15 mg) raised 25(OH)D levels from 2 ng/ml to 22 ng/ml at 2 weeks and to 26 ng/ml at 6 weeks in ten elderly subjects with no evidence of toxicity [111]. Indeed, a single injection of 600 000 IU of vitamin D is safe; such doses were recently recommended for the elderly to prevent vitamin D deficiency [112]. These studies indicate short-term administration of pharmacological doses of vitamin D is safe.
A vitamin D intake of 2000 IU/day for 1 year failed to achieve a 32 ng/ml target 25(OH)D concentration in 40% of the post-menopausal African-American women studied [113]. Administration of 4000 IU/day of vitamin D for more than 6 months to middle-age Canadian endocrinology outpatients, resulted in average 25(OH)D levels of 44 ng/ml and produced no side-effects other than an improved mood [114]. Heaney has estimated that about 3000 IU/day of vitamin D is required to assure that 97% of Americans obtain levels >35 ng/ml [115]. Dosage will depend upon age, latitude, season, skin type, body weight, sun exposure, and pre-existing 25(OH)D levels. Some groups – African-Americans, the obese, and the elderly – may require supplementation with 5000 IU/day during winter but less, or none, during the summer to obtain 25(OH)D levels of 50 ng/ml. These studies indicate that ideal daily doses of vitamin D exceed current recommendations by an order of magnitude.
If the ability of vitamin D to stimulate the production of virucidal antimicrobial peptides and to suppress cytokine and chemokine production is clinically significant, then pharmacological doses (1000–2000 IU/kg per day for several days) may be useful in the treatment of those viral respiratory infections that peak in wintertime. Physicians have successfully used pharmacological doses of vitamin D to prevent vitamin D deficiency, to prevent metabolic bone disease, and to treat severe hypoparathyroidism. Perhaps such doses have other effects, such as ameliorating symptoms of viral respiratory infections. As pointed out in a 1999 paper that heralded the current interest in the nutrient, the pharmacological potential of vitamin D remains unexplored [116].
CONCLUSION
There is much evidence to suggest that vitamin D may be Hope-Simpson's seasonal stimulus. Nevertheless, it is premature to recommend vitamin D for either the prevention or treatment of viral respiratory infections. It is not, however, too early to recommend that health-care providers aggressively diagnose and adequately treat vitamin D deficiency. Vitamin D deficiency is endemic and has been associated with many of the diseases of civilization [117, 118]. Vitamin D supplementation should stabilize 25(OH)D concentrations consistent with levels obtained by natural summertime sun exposure (50 ng/ml) while avoiding toxic levels. Those with large amounts of melanin in their skin, the obese, those who avoid the sun, and the aged may need up to 5000 IU/day to obtain such levels, especially in the winter.
The theory that vitamin D affects the course of viral respiratory infections should be tested. Are patients with low 25(OH)D levels more likely to contract viral respiratory infections? Does the clinical course correlate with 25(OH)D levels? Do patients with influenza have lower 25(OH)D levels than uninfected controls? Does sun exposure correlate with infection? Are patients who take physiological doses of vitamin D less likely to become infected? Should the concept of human herd immunity (the immune pressure on the virus due to the percentage of the population with acquired immunity) be expanded to include innate herd immunity [the immune pressure on the virus due to the percentage of the population with adequate 25(OH)D levels]? Is influenza infection a sign of vitamin D deficiency as much as Pneumocystis carinii pneumonia is a sign of AIDS? Does the administration of pharmacological doses of vitamin D, early in the course of a viral respiratory infection, ameliorate symptoms? As the annual mortality from influenza approaches one million worldwide, further studies testing this theory are warranted [119].
Today, in a rush from multiplex reverse transcriptase–polymerase chain reactions that rapidly subtype influenza viruses to complex mathematical formulas that explain its infectivity, many of us have forgotten Hope-Simpson's simple ‘seasonal stimulus’ theory for the lethal crop of influenza that sprouts around the winter solstice. The faith and humility that characterized his life and his writings insulated him from despairing that his ‘seasonal stimulus’ would not be sought. Among his last published words was the suggestion that ‘it might be rewarding if persons, who are in a position to do so, will look more closely at the operative mechanisms that are causing such seasonal behavior’ [3, p. 241].
ACKNOWLEDGEMENTS
We thank Professor Norman Noah of the London School of Hygiene and Tropical Medicine, Professor Robert Scragg of the University of Auckland and Professor Robert Heaney of Creighton University for reviewing the manuscript and making many useful suggestions.
DECLARATION OF INTEREST
Dr Cannell heads the non-profit educational group, ‘The Vitamin D Council’.
REFERENCES
- 1.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proceedings of the Royal Society of Medicine. 1965;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray DP. Robert Edgar Hope-Simpson. British Medical Journal. 2003;327:1111. [Google Scholar]
- 3.Hope-Simpson RE. The Transmission of Epidemic Influenza. New York: Plenum Press; 1992. p. 77. , pp. [Google Scholar]
- 4.Hope-Simpson RE. The role of season in the epidemiology of influenza. Journal of Hygiene. 1981;86:35–47. doi: 10.1017/s0022172400068728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monto AS, Kioumehr F. The Tecumseh Study of Respiratory Illness. IX. Occurence of influenza in the community, 1966–1971. American Journal of Epidemiology. 1975;102:553–563. doi: 10.1093/oxfordjournals.aje.a112193. [DOI] [PubMed] [Google Scholar]
- 6.Fox JP et al. Influenzavirus infections in Seattle families, 1975–1979. I. Study design, methods and the occurrence of infections by time and age. American Journal of Epidemiology. 1982;116:212–227. doi: 10.1093/oxfordjournals.aje.a113407. [DOI] [PubMed] [Google Scholar]
- 7.Thacker SB. The persistence of influenza A in human populations. Epidemiology Reviews. 1986;8:129–142. doi: 10.1093/oxfordjournals.epirev.a036291. [DOI] [PubMed] [Google Scholar]
- 8.Jordan WS, Jr. et al. A study of illness in a group of Cleveland families. XVII. The occurrence of Asian influenza. American Journal of Hygiene. 1958;68:190–212. doi: 10.1093/oxfordjournals.aje.a119962. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman D, Lieberman D, Friger MD. Seasonal variation in hospital admissions for community-acquired pneumonia: a 5-year study. Journal of Infection. 1999;39:134–140. doi: 10.1016/s0163-4453(99)90005-1. [DOI] [PubMed] [Google Scholar]
- 10.Marrie TJ, Huang JQ. Epidemiology of community-acquired pneumonia in Edmonton, Alberta: an emergency department-based study. Canadian Respiratory Journal: Journal of the Canadian Thoracic Society. 2005;12:139–142. doi: 10.1155/2005/672501. [DOI] [PubMed] [Google Scholar]
- 11.Saynajakangas P, Keistinen T, Tuuponen T. Seasonal fluctuations in hospitalisation for pneumonia in Finland. International Journal of Circumpolar Health. 2001;60:34–40. [PubMed] [Google Scholar]
- 12.Crighton EJ et al. Influenza and pneumonia hospitalizations in Ontario: a time-series analysis. Epidemiology and Infection. 2004;132:1167–1174. doi: 10.1017/s0950268804002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson WW et al. Influenza-associated hospitalizations in the United States. Journal of the American Medical Association. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 14.Miller DL, Pereira MS, Clarke M. Epidemiology of the Hong Kong-68 variant of influenza A2 in Britain. British Medical Journal. 1971;1:475–479. doi: 10.1136/bmj.1.5747.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shek LP, Lee BW. Epidemiology and seasonality of respiratory tract virus infections in the tropics. Paediatric Respiratory Review. 2003;4:105–111. doi: 10.1016/s1526-0542(03)00024-1. [DOI] [PubMed] [Google Scholar]
- 16.Noah ND. Cyclical patterns and predictability in infection. Epidemiology and Infection. 1989;102:175–190. doi: 10.1017/s0950268800029848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institutes of Health http://www.niaid.nih.gov/factsheets/cold.htm. http://www.niaid.nih.gov/factsheets/cold.htm . The common cold ( ). Accessed 17 May 2006.
- 18.Maxwell JD. Seasonal variation in vitamin D. Proceedings of the Nutrition Society. 1994;53:533–543. doi: 10.1079/pns19940063. [DOI] [PubMed] [Google Scholar]
- 19.Scharla SH. Prevalence of subclinical vitamin D deficiency in different European countries. Osteoporosis International. 1998;8:S7–S12. doi: 10.1007/pl00022726. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clinic Proceedings. 2006;81:297–299. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 21.Vieth R. Enzyme kinetics hypothesis to explain the U-shaped risk curve for prostate cancer vs. 25-hydroxyvitamin D in nordic countries. International Journal of Cancer. 2004;111:468. doi: 10.1002/ijc.20218. [DOI] [PubMed] [Google Scholar]
- 22.Amento EP. Vitamin D and the immune system. Steroids. 1987;49:55–72. doi: 10.1016/0039-128x(87)90079-1. [DOI] [PubMed] [Google Scholar]
- 23.Hayes CE et al. The immunological functions of the vitamin D endocrine system. Cellular and Molecular Biology (Noisy-le-Grand, France) 2003;49:277–300. [PubMed] [Google Scholar]
- 24.Hanley DA, Davison KS. Vitamin D insufficiency in North America. Journal of Nutrition. 2005;135:332–337. doi: 10.1093/jn/135.2.332. [DOI] [PubMed] [Google Scholar]
- 25.Thomas MK et al. Hypovitaminosis D in medical inpatients. New England Journal of Medicine. 1998;338:7777–7783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 26.Mosekilde L. Vitamin D and the elderly. Clinical Endocrinology. 2005;62:265–281. doi: 10.1111/j.1365-2265.2005.02226.x. [DOI] [PubMed] [Google Scholar]
- 27.Poskitt EM, Cole TJ, Lawson DE. Diet, sunlight, and 25-hydroxy vitamin D in healthy children and adults. British Medical Journal. 1979;1:221–223. doi: 10.1136/bmj.1.6158.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holick MF. Photosynthesis of vitamin D in the skin: effect of environmental and life-style variables. Federation Proceedings. 1987;46:1876–1882. [PubMed] [Google Scholar]
- 29.Holick MF. McCollum Award Lecture, 1994: vitamin D – new horizons for the 21st century. American Journal of Clinical Nutrition. 1994;60:619–630. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- 30.Levis S et al. Vitamin D deficiency and seasonal variation in an adult South Florida population. Journal of Clinical Endocrinology and Metabolism. 2005;90:1557–1562. doi: 10.1210/jc.2004-0746. [DOI] [PubMed] [Google Scholar]
- 31.Leung SS, Lui S, Swaminathan R. Vitamin D status of Hong Kong Chinese infants. Acta Paediatrica Scandinavica. 1989;78:303–306. doi: 10.1111/j.1651-2227.1989.tb11074.x. [DOI] [PubMed] [Google Scholar]
- 32.Vieth R et al. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. European Journal of Clinical Nutrition. 2001;55:1091–1097. doi: 10.1038/sj.ejcn.1601275. [DOI] [PubMed] [Google Scholar]
- 33.Rogan MP et al. Antimicrobial proteins and polypeptides in pulmonary innate defence. Respiratory Research. 2006;7:29. doi: 10.1186/1465-9921-7-29. . Published online: 17 February 2006. doi:10.1186/1465-9921-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pitrez PM, Brennan S, Sly PD. Inflammatory profile in nasal secretions of infants hospitalized with acute lower airway tract infections. Respirology. 2005;10:365–370. doi: 10.1111/j.1440-1843.2005.00721.x. [DOI] [PubMed] [Google Scholar]
- 35.Cheung CY et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 36.Kobasa D et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–707. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 37.Chan MC et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. http://respiratory-research.com/content/6/1/135. Respiratory Research. 2005;6:135. doi: 10.1186/1465-9921-6-135. ). Accessed 19 May 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beigel JH et al. Avian influenza A (H5N1) infection in humans. New England Journal of Medicine. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 39.Hewison M et al. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Molecular and Cellular Endocrinology. 2004;215:31–38. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Helming L et al. 1α,25-dihydroxyvitamin D3 is a potent suppressor of interferon γ-mediated macrophage activation. Blood. 2005;106:4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Amer Y, Bar-Shavit Z. Impaired bone marrow-derived macrophage differentiation in vitamin D deficiency. Cellular Immunology. 1993;151:356–368. doi: 10.1006/cimm.1993.1245. [DOI] [PubMed] [Google Scholar]
- 42.Cohen MS et al. 1,25-Dihydroxyvitamin D3 activates secretion of hydrogen peroxide by human monocytes. Journal of Immunology. 1986;136:1049–1053. [PubMed] [Google Scholar]
- 43.Wang TT et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Journal of Immunology. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 44.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB Journal. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 45.Liu PT et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 46.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nature Reviews. Immunology. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 47.Reddy KV, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. International Journal of Antimicrobial Agents. 2004;24:536–547. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Hiemstra PS et al. Antimicrobial peptides: mediators of innate immunity as templates for the development of novel anti-infective and immune thera-peutics. Current Pharmaceutical Design. 2004;10:2891–2905. doi: 10.2174/1381612043383566. [DOI] [PubMed] [Google Scholar]
- 49.Daher KA, Selsted ME, Lehrer RI. Direct inactivation of viruses by human granulocyte defensins. Journal of Virology. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schutte BC, McCray PB., Jr. β-defensins in lung host defense. Annual Review of Physiology. 2002;64:709–748. doi: 10.1146/annurev.physiol.64.081501.134340. [DOI] [PubMed] [Google Scholar]
- 51.Beisswenger C, Bals R. Antimicrobial peptides in lung inflammation. Chemical Immunology and Allergy. 2005;86:55–71. doi: 10.1159/000086651. [DOI] [PubMed] [Google Scholar]
- 52.Kohn MA et al. Three summertime outbreaks of influenza type A. Journal of Infectious Diseases. 1995;172:246–249. doi: 10.1093/infdis/172.1.246. [DOI] [PubMed] [Google Scholar]
- 53.Brammer L et al. Influenza surveillance – United States, 1992–93 and 1993–94. Morbidity and Mortality Weekly Report . CDC Surveillance Summaries. 1997;46:1–12. [PubMed] [Google Scholar]
- 54.Curwen M, Charlton J, Murphy M. The Health of Adult Britain 1841–1994. London: The Stationery Office; 1997. Excess winter mortality in England and Wales with special reference to the effects of temperature and influenza; pp. 205–216. : pp. [Google Scholar]
- 55.Scragg R. Seasonal variation of mortality in Queensland. Australian and New Zealand Journal of Public Health (Community Health Studies) 1982;6:120–129. [Google Scholar]
- 56.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. Journal of Clinical Endocrinology and Metabolism. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 57.Reichert TA et al. Influenza and the winter increase in mortality in the United States, 1959–1999. American Journal of Epidemiology. 2004;160:492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 58.Aylin P et al. Temperature, housing, deprivation and their relationship to excess winter mortality in Great Britain, 1986–1996. International Journal of Epidemiology. 2001;30:1100–1108. doi: 10.1093/ije/30.5.1100. [DOI] [PubMed] [Google Scholar]
- 59.van der Wielen RP et al. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 60.McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. American Medical Journal. 1992;93:69–77. doi: 10.1016/0002-9343(92)90682-2. [DOI] [PubMed] [Google Scholar]
- 61.Hirani V, Primatesta P. Vitamin D concentrations among people aged 65 years and over living in private households and institutions in England: population survey. Age and Ageing. 2005;34:485–491. doi: 10.1093/ageing/afi153. [DOI] [PubMed] [Google Scholar]
- 62.Laake K, Sverre JM. Winter excess mortality: a comparison between Norway and England plus Wales. Age and Ageing. 1996;25:343–348. doi: 10.1093/ageing/25.5.343. [DOI] [PubMed] [Google Scholar]
- 63.Glantz MH. Currents of Change: impacts of El Nino and La Nina on climate and society. 2nd edn. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 64.Viboud C et al. Association of influenza epidemics with global climate variability. European Journal of Epidemiology. 2004;19:1055–1059. doi: 10.1007/s10654-004-2450-9. [DOI] [PubMed] [Google Scholar]
- 65.Ebi KL et al. Association of normal weather periods and El Nino events with hospitalization for viral pneumonia in females: California, 1983–1998. American Journal of Public Health. 2001;91:1200–1208. doi: 10.2105/ajph.91.8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hope-Simpson RE. Sunspots and flu: a correlation. Nature. 1978;275:86. [Google Scholar]
- 67.Hoyle F, Wickramasinghe NC. Sunspots and influenza. Nature. 1990;343:304. doi: 10.1038/343304a0. [DOI] [PubMed] [Google Scholar]
- 68.Von Alvensleben A. Influenza according to Hoyle. Nature. 1990;344:374. [PubMed] [Google Scholar]
- 69.Horgan J. Space invaders. Extra! Extra! Flu linked to sunspots. Scientific American. 1990;262 : 26, 30. [PubMed] [Google Scholar]
- 70.Shindell D et al. Solar cycle variability, ozone, and climate. Science. 1999;284:305–308. doi: 10.1126/science.284.5412.305. [DOI] [PubMed] [Google Scholar]
- 71.Rozema J et al. Paleoclimate. Toward solving the UV puzzle. Science. 2002;296:1621–1622. doi: 10.1126/science.1070024. [DOI] [PubMed] [Google Scholar]
- 72.Zadshir A et al. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethnicity and Disease. 2005;15:97–101. (4 Suppl. 5): [PubMed] [Google Scholar]
- 73.Minino AM pp. 1–119. Deaths: final data for 2000. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 2002, vol. , pp. [PubMed]
- 74.Arias E pp. 1–115. Deaths: final data for 2001. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 2003, vol. , pp. [PubMed]
- 75.Kochanek KD pp. 1–115. Deaths: final data for 2002. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 2004, vol. , pp. [PubMed]
- 76.Sprenger MJ et al. Impact of influenza on mortality in relation to age and underlying disease, 1967–1989. International Journal of Epidemiology. 1993;22:334–340. doi: 10.1093/ije/22.2.334. [DOI] [PubMed] [Google Scholar]
- 77.Sprenger MJ et al. Influenza mortality and excess deaths in the elderly, 1967–82. Epidemiology and Infection. 1989;103:633–641. doi: 10.1017/s0950268800031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dowell SF et al. Mortality from pneumonia in children in the United States, 1939 through 1996. New England Journal of Medicine. 2000;342:1399–1407. doi: 10.1056/NEJM200005113421904. [DOI] [PubMed] [Google Scholar]
- 79.Shadrin AS, Marinich IG, Taros LY. Experimental and epidemiological estimation of seasonal and climato-geographical features of non-specific resistance of the organism to influenza. Journal of Hygiene, Epidemiology, Microbiology, and Immunology. 1977;21:155–161. [PubMed] [Google Scholar]
- 80.Zykov MP, Sosunov AV. Vaccination activity of live influenza vaccine in different seasons of the year. Journal of Hygiene, Epidemiology, Microbiology, and Immunology. 1987;31:453–459. [PubMed] [Google Scholar]
- 81.El-Radhi AS et al. High incidence of rickets in children with wheezy bronchitis in a developing country. Journal of the Royal Society of Medicine. 1982;75:884–887. doi: 10.1177/014107688207501112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beser E, Cakmakci T. Factors affecting the morbidity of vitamin D deficiency rickets and primary protection. East African Medical Journal. 1994;71:358–362. [PubMed] [Google Scholar]
- 83.Siddiqui TS, Rai MI. Presentation and predisposing factors of nutritional rickets in children of Hazara Division. Journal of Ayub Medical College, Abbottabad. 2005;17:29–32. [PubMed] [Google Scholar]
- 84.Mariam TW, Sterky G. Severe rickets in infancy and childhood in Ethiopia. Journal of Pediatrics. 1973;82:876–878. doi: 10.1016/s0022-3476(73)80087-3. [DOI] [PubMed] [Google Scholar]
- 85.Patwari A et al. Pulmonary changes in rickets in children. Indian Pediatrics. 1979;16:413–415. [PubMed] [Google Scholar]
- 86.Muhe L et al. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet. 1997;349:1801–1804. doi: 10.1016/S0140-6736(96)12098-5. [DOI] [PubMed] [Google Scholar]
- 87.Banajeh SM, al-Sunbali NN, al-Sanahani SH. Clinical characteristics and outcome of children aged under 5 years hospitalized with severe pneumonia in Yemen. Annals of Tropical Paediatrics. 1997;17:321–326. doi: 10.1080/02724936.1997.11747905. [DOI] [PubMed] [Google Scholar]
- 88.Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. Journal of Tropical Pediatrics. 2004;50:364–368. doi: 10.1093/tropej/50.6.364. [DOI] [PubMed] [Google Scholar]
- 89.Wayse V et al. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. European Journal of Clinical Nutrition. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 90.Leino L et al. Systemic suppression of human peripheral blood phagocytic leukocytes after whole-body UVB irradiation. Journal of Leukocyte Biology. 1999;65:573–582. doi: 10.1002/jlb.65.5.573. [DOI] [PubMed] [Google Scholar]
- 91.Hanneman KK, Cooper KD, Baron ED. Ultraviolet immunosuppression: mechanisms and consequences. Dermatology Clinics. 2006;24:19–25. doi: 10.1016/j.det.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 92.Termorshuizen F et al. A review of studies on the effects of ultraviolet irradiation on the resistance to infections: evidence from rodent infection models and verification by experimental and observational human studies. International Immunopharmacology. 2002;2:263–275. doi: 10.1016/s1567-5769(01)00178-3. [DOI] [PubMed] [Google Scholar]
- 93.Krause R, Holick MF, Jung EGet al. Suberythemal UV-irradiation increases immunological capacity in children with frequent cold Biological Effects of Light Proceedings of a Symposium, Basel, Switzerland, 1–3 November 1998Norwell, Massachusetts, USA: Kluwer Academic Publishers; 1998: pp. 49–51. [Google Scholar]
- 94.Csato M, Jablonski K, Tronnier H. Effect of ultraviolet irradiation on granulocyte chemotaxis and nitroblue tetrazolium reduction activity in healthy individuals. British Journal of Dermatology. 1984;111:567–570. doi: 10.1111/j.1365-2133.1984.tb06626.x. [DOI] [PubMed] [Google Scholar]
- 95.Gigineishvili GR et al. The use of UV irradiation to correct the immune system and decrease morbidity in athletes [in Russian] Voprosy Kurortologii, Fizioterapii, i Lechebnoǐ Fizicheskoǐ Kultury. 1990;3:30–33. [PubMed] [Google Scholar]
- 96.Termorshuizen F et al. Exposure to solar ultraviolet radiation and respiratory tract symptoms in 1-year-old children. Photodermatology, Photoimmunology and Photomedicine. 2004;20:270–271. doi: 10.1111/j.1600-0781.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 97.Semba RD. Vitamin A as ‘anti-infective’ therapy, 1920–1940. Journal of Nutrition. 1999;129:783–791. doi: 10.1093/jn/129.4.783. [DOI] [PubMed] [Google Scholar]
- 98.Holmes AD et al. Vitamins aid reduction of lost time in industry. Journal of Industrial and Engineering Chemistry. 1932;24:1058–1060. [Google Scholar]
- 99.Homes AD et al. Cod liver oil – a five-year study of its value for reducing industrial absenteeism caused by colds and respiratory diseases. Industrial Medicine. 1936;5:359–361. [Google Scholar]
- 100.Linday LA et al. Effect of daily cod liver oil and a multivitamin-mineral supplement with selenium on upper respiratory tract pediatric visits by young, inner-city, Latino children: randomized pediatric sites. Annals of Otology, Rhinology, and Laryngology. 2004;113:891–901. doi: 10.1177/000348940411301108. [DOI] [PubMed] [Google Scholar]
- 101.Rehman PK. Sub-clinical rickets and recurrent infection. Journal of Tropical Pediatrics. 1994;40:58. doi: 10.1093/tropej/40.1.58. [DOI] [PubMed] [Google Scholar]
- 102.Andrewes C. The Common Cold. New York: Norton; 1965. [Google Scholar]
- 103.Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Oto-laryngologica. 2002;122:183–191. doi: 10.1080/00016480252814207. [DOI] [PubMed] [Google Scholar]
- 104.Johnson C, Eccles R. Acute cooling of the feet and the onset of common cold symptoms. Family Practice. 2005;22:608–613. doi: 10.1093/fampra/cmi072. [DOI] [PubMed] [Google Scholar]
- 105.Lips P et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. Journal of Clinical Endocrinology and Metabolism. 2001;86:1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 106.Heaney RP et al. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. Journal of the American College of Nutrition. 2003;22:142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 107.Bischoff-Ferrari HA et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged >or=60 y. American Journal of Clinical Nutrition. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 108.Adams JS et al. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. New England Journal of Medicine. 1982;306:722–725. doi: 10.1056/NEJM198203253061206. [DOI] [PubMed] [Google Scholar]
- 109.Barger-Lux MJ et al. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporosis International. 1998;8:222–230. doi: 10.1007/s001980050058. [DOI] [PubMed] [Google Scholar]
- 110.Wu F et al. Efficacy of an oral, 10-day course of high-dose calciferol in correcting vitamin D deficiency. New Zealand Medical Journal. 2003;116:U536. [PubMed] [Google Scholar]
- 111.Burns J, Paterson CR. Single dose vitamin D treatment for osteomalacia in the elderly. British Medical Journal. 1985;290:281–282. doi: 10.1136/bmj.290.6464.281-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Diamond TH et al. Annual intramuscular injection of a megadose of cholecalciferol for treatment of vitamin D deficiency: efficacy and safety data. Medical Journal of Australia. 2005;183:10–12. doi: 10.5694/j.1326-5377.2005.tb06879.x. [DOI] [PubMed] [Google Scholar]
- 113.Aloia JF et al. A randomized controlled trial of vitamin D3 supplementation in African American women. Archives of Internal Medicine. 2005;165:1618–1623. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vieth R et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutrition Journal. 2004;3:8. doi: 10.1186/1475-2891-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heaney RP. The Vitamin D requirement in health and disease. Journal of Steroid Biochemistry and Molecular Biology. 2005;97:13–19. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 116.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. American Journal of Clinical Nutrition. 1999;69:842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 117.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? British Journal of Nutrition. 2003;89:552–572. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 118.Holick MF. The vitamin D epidemic and its health consequences. Journal of Nutrition. 2005;135:2739S–2748S. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 119.Pan American Health Organization (PAHO) Washington, D.C.: PAHO; 2004. . Final Report of the XVI Meeting on Vaccine Preventable-Diseases of the Pan American Health Organization. ). Accessed 30 April 2006. [Google Scholar]