SUMMARY
This study investigated 21 foodborne type-E botulism outbreaks, without antitoxin administration, from 1951 to 1965 in Hokkaido, Japan, to characterize the descriptive epidemiology and evaluate the relationship between case fatality and incubation period. The median (25–75% quartile) attack rate and case fatality, which were evaluated by outbreak, were 58·3% (38·0–73·2) and 25·7% (0·1–50·0) respectively. Individual records of 64 diagnoses, including 31 deaths, were also examined using logistic regression analysis, revealing that a shorter incubation period is likely to result in a significantly higher risk of death (P=0·01). The observed case fatality was more than 50% for those who developed symptoms within the first 18 h after exposure, possibly reflecting underlying dose-dependent mechanisms. In the event of intentional contamination of food with botulinum toxin, rapidly determining the incubation periods may be critical for guiding public health response efforts.
Botulinum toxin, a highly poisonous neurotoxin synthesized by Clostridium botulinum, causes foodborne botulism as a result of digestion. Moreover, it has also been intentionally used due to its toxicity and availability not only for military purposes but also in deliberate attacks against civilians [1]. Nevertheless, because of its rarity and the small size of each outbreak [2], our epidemiological knowledge remains insufficient. Shorter incubation periods have been associated with more severe disease manifestations in animal studies and in foodborne botulism outbreaks [3, 4]. However, additional epidemiological studies are useful to confirm and elucidate these relationships, particularly to help guide public health response efforts in the event of intentional contamination of food with botulinum toxin [1, 2, 5, 6].
Fermented preserved fish with rice, ‘izushi’, is a traditional food in Hokkaido, the northernmost prefecture of Japan. Because of environmental contamination by C. botulinum in this area [7, 8] and the need for anaerobic conditions in preparation of izushi, foodborne outbreaks have been repeatedly observed [9]. Of the known C. botulinum strains, which are distinguished by serological properties, Hokkaido suffers from those that produce type-E toxin. Izushi is eaten in northern Japan only, and therefore, the aetiology of related outbreaks differs from those in other areas caused by other types of neurotoxins [10, 11]. Although botulism is now extremely rare due to several factors such as public heath efforts, historical records of foodborne outbreaks are still useful for epidemiological analysis [12–22]. In particular, only analysis of historical outbreaks can help investigate the natural course of severe cases of botulism without antitoxin administration. In this paper, the records of 21 botulism outbreaks without antitoxin administration were analysed to characterize the descriptive epidemiology with particular emphasis on the attack rate (AR), case fatality (CF) and incubation period. Moreover, individual clinical histories of 64 izushi-related adult botulism diagnoses in the 1950s are investigated to evaluate the contribution of incubation period to the prognosis of this disease [12–19].
Twenty-one outbreaks involving 98 survivors and 40 non-survivors of type-E botulism in which antitoxin was not used were documented in Hokkaido from 1951 to 1965 [12–21]. Because of public health campaigns and other factors, foodborne outbreaks due to izushi have since decreased [22]. Before 1959, no therapeutic antitoxin was available and patients received supportive care only; however, treatment has become gradually available as a result of storage of the antitoxin in several hospitals [12–19]. After 1959, antitoxin was administered in some outbreaks to selected cases for treatment of symptoms, but not prophylactic or post-exposure purposes [20, 21].
Diagnosis was made based primarily on typical neurological manifestations (symmetric flaccid paralysis descending over time) with laboratory detection of botulinum toxin by means of neutralization or isolation of C. botulinum from implicated izushi as additional confirmatory evidence. Furthermore, traceback of izushi ingestion enabled diagnosis of mild botulism, even in those complaining of non-specific gastrointestinal symptoms only at an early stage of illness. Consequently, the subjects of this study included both adult botulism diagnoses with obvious neurological symptoms and those with only mild symptoms with diagnosis based on food traceback. Onset of disease in this study was defined as the time of initial recognition of symptoms [i.e. gastrointestinal symptoms (nausea, vomiting and diarrhoea) or ocular nerve palsy]. Accordingly, incubation period was defined as the time between eating implicated izushi and onset of disease. Although it was difficult to analyse each sign and symptom because thorough systematic descriptions of the clinical findings were not given, agreement of the time of onset (i.e. the time to develop gastrointestinal and neurological symptoms) was assessed among those manifesting both. The crude ARs of each outbreak were estimated based on traceback of implicated izushi, and CFs were determined based on the number of diagnoses.
First, distributions of ARs and CFs were obtained by outbreak as descriptive information. Second, survivors and non-survivors of botulism without antitoxin administration, for whom the incubation period was determined, were analysed to clarify the relationship between incubation period and fatality. Incubation period in relation to fatality was first examined using univariate logistic regression analysis. CF was also examined by sex and age as potential confounding variables. Multivariate logistic regression was then performed to determine the significance of sex, age and incubation period with respect to fatality. Furthermore, the incubation period-dependent CF was obtained using a Bayesian approach with independently fitted incubation periods stratified by fatality. A detailed description of the Bayesian methods is given in the Appendix. The level of statistical significance was set at α=0·05. All statistical data were analysed using JMP version 5.1 statistical software (SAS Institute Inc., Cary, NC, USA).
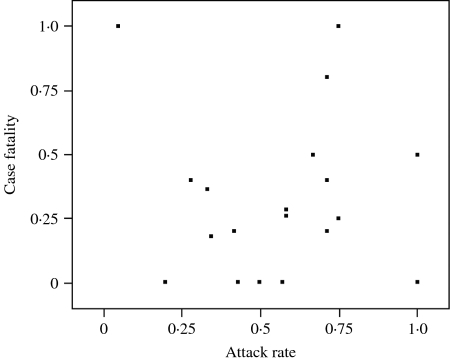
In 21 outbreaks, a total of 284 individuals were identified as having been exposed to implicated izushi, 138 were diagnosed with botulism and 40 died. The smallest and largest outbreaks involved one and 35 diagnosed individuals respectively, with a median size (25–75% quartile) of five individuals (2–8). Figure 1 shows the distribution of ARs and CFs in outbreaks in which identification of izushi-exposed individuals was completed. The median AR (25–75% quartile) was 58·3% (38·0–73·2) and estimates of CFs revealed a median of 25·7% (0·1–50·0); ARs and CFs ranged from 5·0 to 100% and from 0 to 100% respectively. Further descriptive information on the numbers exposed, diagnosed and deceased are provided for each outbreak, including those after introduction of antitoxin, in the supplementary online material (http://journals.cambridge.org/jid_HYG).
Fig. 1.
Distributions of attack rate (AR) and case fatality (CF) by outbreak. Plots indicate the observed AR and CF for each outbreak. Two plots (for those with AR=1·00 and CF=0·50 as well as AR=0·71 and CF=0·20) include two outbreaks for each. (See supplementary online material for raw data.)
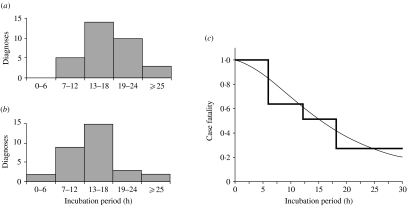
Incubation periods were obtained from individual records of 64 diagnoses, including 31 deaths, and were included in the following analysis. Although the exact proportion is not calculable, it is documented that most diagnosed individuals experienced gastrointestinal symptoms at onset of the disease [12–21]. The median (25–75% quartile) time lag from initial gastrointestinal symptoms to ocular nerve palsy among seven diagnosed individuals for whom time to manifestation of both symptoms was available was 4·0 h (0·0–7·0). The mean age (standard deviation, s.d.) of the individuals investigated was 36·7 years (17·5 years), and logistic regression analysis showed no significant influence of age on fatality (P=0·28). Approximately half were female (n=33, 51·6%), and similarly, sex was not associated with fatality (P=0·99). The distribution of incubation period ranged from 4·0 to 54·0 h, with a mean aNd median (25–75% quartile) of 17·5 and 16·0 h (12·3–20·0 h) respectively. Skewness and kurtosis were 2·0 and 7·7 respectively. Figure 2(a, b) shows the distributions of incubation period stratified by disease outcome (fatality). The mean incubation periods of those who survived and died were 20·7 (s.d.=11·0, median=16·0) and 14·1 h (s.d.=5·6, median=13·5) respectively. Univariate logistic regression revealed that those who died probably developed the disease within a significantly shorter incubation period than those who survived (P=0·01). Incubation period was neither associated with sex (P=0·27) nor correlated with age (P=0·92). Multiple logistic regression analysis also identified incubation period as the only variable significantly associated with botulism death (P=0·01). Figure 2 c shows the distribution of CF stratified by incubation period. The observed records revealed that more than half of those who developed the disease within 18 h died. Details of further analyses are given in the supplementary online material.
Fig. 2.
Botulism death and incubation period. Frequency distributions of incubation period for (a) those who survived and (b) those who died. (c) Case fatality (CF) stratified by incubation period.
 , Observed CF data; –––, prediction obtained using the Bayesian method (for details, see the Appendix and supplementary online material; overall CF=48·4%).
, Observed CF data; –––, prediction obtained using the Bayesian method (for details, see the Appendix and supplementary online material; overall CF=48·4%).
In this study, epidemiological investigations were performed based on historical records of type-E botulism outbreaks in Hokkaido from 1951 to 1965, when antitoxin was not available. Estimates of ARs based on food traceback and CFs varied by outbreak with medians of 58·3 and 25·7% respectively. The incubation period of type-E botulism in Hokkaido was shown to be associated with disease prognosis in the absence of specific treatment, in agreement with a previous study [3]. Moreover, CF was associated neither with age nor sex. A previous study suggested that disease severity is positively correlated with the quantity of toxin ingested [23], while another study demonstrated that a high dose of toxin results in a shorter incubation period [4]. Consequently, those showing a shorter incubation period are likely to be fatal [3], as also demonstrated in this study. Thus, incubation period probably reflects the underlying dose-dependent mechanisms of botulism. Despite the limitations of using historical data in accuracy of diagnosis as well as precision of incubation period, most diagnosed individuals initially developed gastrointestinal symptoms, and moreover, the time lag between the onset of gastrointestinal symptoms and ocular nerve palsy was not too dissociated among those for whom such information was available.
The observed individual records revealed that CF was >50% for those who developed the disease within the first 18 h after exposure, decreasing thereafter. A parametric model using the Bayesian method further yielded the incubation period-dependent CF (see supplementary online material). The CF by incubation period obtained here is likely to be helpful in predicting the natural course of this disease at an early stage of illness. Although in clinical practice, CF depends not only on the incubation period but also on other factors such as specific treatment [24], the obtained results suggest that determination of the time of exposure constitutes a critical component of history taking in addition to the crucial need for rapid diagnosis and supportive care [25, 26].
In conclusion, this study demonstrated a strong and consistent inverse association between incubation period and CF of type-E botulism, consistent with underlying dose–response mechanisms. These results suggest that in the event of a bioterrorist attack with intentional dissemination of a high dose of botulinum toxin, a large number of cases might develop the disease within a short incubation period. Rapidly determining the incubation period will, therefore, be critical to guiding public health response efforts.
ACKNOWLEDGEMENTS
The author thanks Reinhard Vonthein and Martin Eichner for technical help. This work was supported in part by Banyu Life Science Foundation International (Banyu Fellowship Program).
APPENDIX. Bayesian method
Frequency distributions of incubation period stratified by fatality were fitted to lognormal distributions using the maximum-likelihood method [here, g(t|θ1) and f(t|θ2) denote independent probability density functions of the incubation period, t, of those who died (g) and survived (f) respectively]. Based on the obtained parametric distributions, the posterior probability of death given incubation period t, P(Death|t), was then obtained using a Bayesian approach:
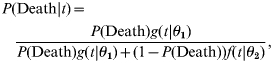 |
(1) |
where P(Death) denotes the prior probability of death, equivalent to the overall CF. The likelihood of death and survival with an incubation period of t are given by independently fitted probability density functions of incubation period, g(t|θ1) and f(t|θ2) respectively.
DECLARATION OF INTEREST
None.
NOTE
Supplementary information accompanies this paper on the Journal's website (http://journals.cambridge.org/jid_HYG).
REFERENCES
- 1.Arnon SS et al. Botulinum toxin as a biological weapon: medical and public health management. Journal of the American Medical Association. 2001;285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 2.Sobel J. Botulism. Clinical Infectious Diseases. 2005;41:1167–1173. doi: 10.1086/444507. [DOI] [PubMed] [Google Scholar]
- 3.Terranova W et al. Botulism type B: epidemiologic aspects of an extensive outbreak. American Journal of Epidemiology. 1978;108:150–156. doi: 10.1093/oxfordjournals.aje.a112599. [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki S, Sakaguchi G. Experimental botulism in chickens: the cecum as the site of production and absorption of botulinum toxin. Japanese Journal of Medical Science and Biology. 1978;31:1–15. doi: 10.7883/yoken1952.31.1. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro R et al. Botulism surveillance and emergency response: a public health strategy for a global challenge. Journal of the American Medical Association. 1997;278:433–435. [PubMed] [Google Scholar]
- 6.Shapiro RL, Hatheway C, Swerdlow DL. Botulism in the United States: a clinical and epidemiologic review. Annals of Internal Medicine. 1998;129:221–228. doi: 10.7326/0003-4819-129-3-199808010-00011. [DOI] [PubMed] [Google Scholar]
- 7.Kanzawa K. Ecological studies on Clostridium botulinum type E [in Japanese] Report of the Hokkaido Institute of Public Health. 1961;11:161–173. [Google Scholar]
- 8.Dolman CE, Chang H. The epidemiology and pathogenesis of type E and fish-borne botulism. Canadian Journal of Public Health. 1953;44:231–244. [PubMed] [Google Scholar]
- 9.Nakamura Y. Botulism in Japan. Japanese Journal of Medical Science and Biology. 1963;16:304–305. [PubMed] [Google Scholar]
- 10.Fukuda T et al. An outbreak of type B botulism occurring in Miyazaki Prefecture. Japanese Journal of Medical Science and Biology. 1970;23:243–248. doi: 10.7883/yoken1952.23.243. [DOI] [PubMed] [Google Scholar]
- 11.Otofuji T, Tokiwa H, Takahashi K. A food-poisoning incident caused by Clostridium botulinum toxin A in Japan. Epidemiology and Infection. 1987;99:167–172. doi: 10.1017/s0950268800066991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura Y, Iida H, Nakao R. Report on food-borne outbreak with suspicion of botulism [in Japanese] Report of the Hokkaido Institute of Public Health. 1952;2:29–34. [Google Scholar]
- 13.Nakamura Y et al. Food-borne outbreaks of botulism in several places in Hokkaido [in Japanese] Report of the Hokkaido Institute of Public Health. 1955;S3:1–39. [Google Scholar]
- 14.Nakamura Y et al. Observed outbreaks of botulism after that. Part 1 [in Japanese] Report of the Hokkaido Institute of Public Health. 1955;5:19–23. [Google Scholar]
- 15.Iida H et al. Two outbreaks of type E botulism encountered in Hokkaido in 1954 [in Japanese] Report of the Hokkaido Institute of Public Health. 1957;7:57–60. [Google Scholar]
- 16.Iida H, Karashimada T, Ohya T. Three outbreaks of type E ‘Izushi-borne’ botulism encountered in Hokkaido in 1955 [in Japanese] Report of the Hokkaido Institute of Public Health. 1957;S5:32–39. [Google Scholar]
- 17.Iida H, Karashimada T. Outbreak of type E botulism suspected due to ‘Izushi of Masu’ [in Japanese] Report of the Hokkaido Institute of Public Health. 1957;S5:40–41. [Google Scholar]
- 18.Iida H et al. Three outbreaks of type E botulism encountered in Hokkaido in 1956 [in Japanese] Report of the Hokkaido Institute of Public Health. 1959;9:31–38. [Google Scholar]
- 19.Iida H et al. Four outbreaks of ‘Izushi-borne’ type E botulism encountered in Hokkaido in 1957 [in Japanese] Report of the Hokkaido Institute of Public Health. 1960;10:19–30. [Google Scholar]
- 20.Iida H, Karashimada T, Saito T. Three outbreaks of ‘Izushi-borne’ type botulism encountered recently in Hokkaido [in Japanese] Report of the Hokkaido Institute of Public Health. 1962;12:16–20. [Google Scholar]
- 21.Iida H et al. Botulism outbreaks encountered in Hokkaido in 1962: with special reference to the therapeutic value of specific antitoxin [in Japanese] Report of the Hokkaido Institute of Public Health. 1964;14:6–18. [Google Scholar]
- 22.Anon. Botulism, Japan. http://idsc.nih.go.jp/iasr/21/241/tpc241.html Infectious Agents Surveillance Report. 2000;21:241. [Google Scholar]
- 23.Donadio JA, Gangarosa EJ, Faich GA. Diagnosis and treatment of botulism. Journal of Infectious Diseases. 1971;124:108–112. [Google Scholar]
- 24.Sandrock CE, Murin S. Clinical predictors of respiratory failure and long-term outcome in black tar heroin-associated wound botulism. Chest. 2001;120:562–566. doi: 10.1378/chest.120.2.562. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin JB et al. Botulism type E outbreak associated with eating a beached whale, Alaska. Emerging Infectious Diseases. 2004;10:1685–1687. doi: 10.3201/eid1009.040131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caya JG, Agni R, Miller JE. Clostridium botulinum and the clinical laboratorian: a detailed review of botulism, including biological warfare ramifications of botulism toxin. Archives of Pathology and Laboratory Medicine. 2004;128:653–662. doi: 10.5858/2004-128-653-CBATCL. [DOI] [PubMed] [Google Scholar]