SUMMARY
We examined the association between socioeconomic status and the level of serum antibodies to selected faeco-orally transmitted pathogens among Israeli adolescents. Random samples of eighty volunteers aged 12–15 years from high (HSL), medium (MSL) and low (LSL) standard of living towns were included in the study. Serum samples were examined by radioimmunoassay for HAV and by in-house-developed ELISA systems for IgA and IgG antibody levels against Shigella sonnei, S. flexneri, E. coli O157:H7 lipopolysacchride and Cryptosporidium parvum antigens. Seropositivity to HAV was highest (98·8%) in the LSL towns and lowest (25%) in the HSL towns, showing a statistically significant linear trend. Antibody levels to the other enteropathogens had gender variation, with higher titres in females. Significantly lower titres in the HSL towns were found for: IgA anti-S. sonnei in females (P<0·001); IgG anti-S. sonnei in females (P=0·024) and males (P=0·033); IgG anti-S. flexneri in females (P=0·016). Inverse linear association with socioeconomic status was found for IgA anti-C. parvum in females (P<0·001); IgA anti-E. coli O157:H7 in females (P<0·001) and males (P=0·024). A statistically significant association between HAV seropositivity and higher titres of IgA anti-S. sonnei and E. coli O157:H7 was shown. In conclusion, exposure to enteropathogens transmitted via the faecal–oral route in communities of lower socioeconomic status is reflected in a higher prevalence of lifelong lasting antibodies to HAV, and higher levels of antibodies to bacterial and protozoan enteropathogens. Among females, the levels of specific serum antibodies are higher and more strongly associated with low socioeconomic status.
INTRODUCTION
Most gastrointestinal pathogens are transmitted via the faecal–oral route. High transmission rates can be expected in populations of low socioeconomic status usually characterized by low income, high crowdedness, and increased contact with young children. The increased risk of exposure to such pathogens may be reflected in a high prevalence of antibodies to these organisms attained in the early years of life. The prevalence of antibodies to hepatitis A virus (HAV) is known to be associated with low socioeconomic status and varies in different parts of the world as a function of hygienic and sanitation factors [1, 2].
The association of other bacterial and protozoan enteric pathogens with low socioeconomic status is less established. A small study comparing Shigella antibodies from representative groups of Costa Rican and Swedish women suggested correlation with socioeconomic status [3]. In Israel, Shigella lipopolysaccharide (LPS) antibodies in young adults were associated with a low socioeconomic background [4]. Socioeconomic factors were important correlates of early exposure to Cryptosporidium parvum and high seroprevalence to the parasite in some studies [5, 6] but not in others [7]. The association of Escherichia coli O157:H7 with socioeconomic status is still not established.
Although it is assumed that recurrent bacterial and protozoan enteric infections will boost the immune system against the homologous antigens maintaining elicited levels of antibodies [8], it is not known how comparable this would be with the lifelong antibody response induced by HAV exposure. In this study, we reassessed the association between low socioeconomic status and seroprevalence of HAV antibodies in teenagers living in three communities in Israel and examined, in the same individuals, the antibody levels to other faeco-orally transmitted pathogens, bacterial and protozoan.
METHODS
Study population
The populations of three towns of different socioeconomic status served as sampling frame for the study: a high standard of living (HSL) town; a middle-low standard of living (MSL) town; and a low standard of living (LSL) Bedouin town. All three towns were located in the south part of Israel within 20 miles of each other, and had similar geographical characteristics (desert climate with hot dry summer from June to September and a short mild rainy winter from December to March). Although some interactions between the populations of the three towns occur (i.e. at work, market, hospital) they live separately, and attend separate schools.
Population and housing characteristics were thoroughly investigated in 1995 by the Israeli Central Bureau of Statistics. Socioeconomic status was measured by: number of children in the household (number of persons/room), percentage of people with only primary education, and income. The MSL town had a high percentage of residents of non-European origin compared to the HSL town. Potable water was supplied to the three towns by the same supplier (Mekorot Company) using different sources but following the same national policy of chlorination. The supply to the HSL town, pipe water from a local drill, was completely separate from the other communities (Table 1).
Table 1.
Socioeconomic characteristics and source of water supply of the three communities
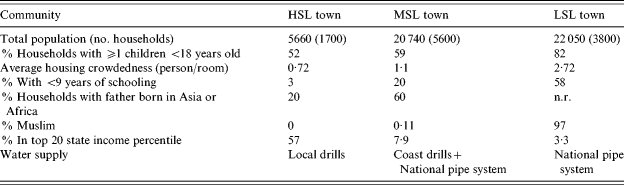
HSL, High standard of living; MSL, medium standard of living; LSL, low standard of living; n.r., Non-relevant data.
At the time of recruitment (1996), the children of the three towns were not vaccinated against hepatitis A. Large-scale enteric disease outbreaks had not been reported in any of the three town populations preceding the initiation of the study.
Data and specimen collection
During the winter of 1996, in each of the three communities, one investigator (O.A.) approached ∼200 children in one representative school. For each school, 1–2 classes (to cover ∼50 children) from each of the cohorts studied in 6th, 7th, 8th, and 9th grades (11–15 years old) were approached. Letters were sent to parents and in >90% both parental informed consent and children's assent were obtained. Thus, the initial sample size comprised 526 children. For each school (representing one community) we selected for the serological study, using a randomization list, 80 children (20 per cohort from 6th, 7th, 8th, and 9th grades) for analysis. Thus, a total of 240 samples was the final sample size. Parents were asked to complete questionnaires detailing demographic and socioeconomic status.
Blood samples were collected and transferred chilled to the Pediatric Infectious Disease Unit, Soroka University Medical Centre where sera were separated (3000 rpm, 15 min) and kept frozen (–70°C). The sera were analysed for serological markers of exposure to different enteric pathogens: HAV, Shigella sonnei, Shigella flexneri, C. parvum and E. coli O157:H7.
Laboratory procedures
HAV serology was performed at the virology laboratory of the Rambam Medical Center Hepatitis Laboratory. Other serological tests were performed at the Center for Vaccine Development and Evaluation, Israel Defense Force (IDF).
HAV serology
Serological evaluation was performed using a radioimmunoassay HAV kit (Abbott Laboratories, Abbott Park, IL, USA). Qualitative results were given for anti-HAV. Specimens with count rates less than the cut-off values (one half the combined negative control mean and positive control mean) were considered positive for anti-HAV antibodies.
S. sonnei, S. flexneri, E. coli O157 serology
ELISA was performed as previously described [9]. S. sonnei, S. flexneri 2a and E. coli O157 LPS extracted by the Westfal phenol–water method, were used as antigens to coat the microtitre plates. Goat anti-human IgG, IgA or IgM, conjugated to alkaline phosphatase (Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA) were employed as second antibodies. Control sera were included in each microtitre plate. Adjusted optical densities (aOD), derived from a linear regression analysis of eight doubling dilutions, were expressed as end-point titres (at aOD=0·3), and geometric mean titres (GMTs) were calculated.
When the identification of S. sonnei and S. flexneri infection by stool culture served as gold standard, the sensitivity and specificity of the ELISA protocol for detection of S. sonnei LPS antibodies were 90 and 100% respectively. The corresponding figures for S. flexneri were 85 and 85% respectively [9].
C. parvum serology
ELISA was performed in polystyrene microtitre plates (Costar, model 3590, Cambridge, MA, USA) according to the method of Ungar et al. [10] with some modifications [11]. Briefly, 100 μl of coating buffer (0·05 m carbonate buffer, pH 9·6) containing lysate of 104 calf oocysts after 20 freeze–thaw cycles was added to each of 96 wells, and the plates were incubated for 1 h at 37°C. After removal of the coating solution, the plates were incubated for 1 h at 37°C with 0·05 m phosphate-buffered saline (PBS) supplemented with casein and bovine serum albumin (both at 5 g/l). The plates were then washed twice in PBS–Tween-20 washing solution. Sera were diluted in blocking buffer (1:25 for IgG and IgM, and 1:5 for IgA) and added to the first line of wells in the microtitre plates. The sera were then double-diluted seven times in blocking buffer and incubated overnight at room temperature. After four further washings, goat anti-human IgG, or IgA, or IgM conjugated to alkaline phosphatase (Kirkegaard & Perry Laboratories), was added to the wells. The plates were incubated overnight at room temperature, washed and ELISA was completed by the addition of the enzyme-substrate solution containing p-nitrophenylphosphate (1 mg/ml) in diethanolamine buffer at pH 9·8. The reactions were stopped with 3 m NaOH and optical density was read at 405 nm with an automatic ELISA biokinetics EL340 reader (Bio-Tek Instruments, Winooski, VT, USA). The consecutive serum samples of each subject were tested within the same assay; positive and negative control sera were included in every microtitration plate in each of the assays. The adjusted optical densities derived from a linear regression analysis of eight doubling dilutions were expressed as end-point titres (at aOD=0·3), and GMTs were calculated. The sensitivity and specificity of this assay was 95% [12].
Statistical analysis
Gender distribution among the HSL, MSL and LSL towns was compared using the χ2 test. Differences in age, children per family, housing density (person/room), fathers and mothers years of education as well as the ln of antibody titres in these three groups were tested using ANOVA and DUNCAN tests for multiple comparisons. GMTs of antibodies against the bacterial and protozoan antigens, in volunteers who were positive and negative for hepatitis A, were compared using t test. Data were analysed with SPSS version 11 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Study population
The socio-demographic characteristics of the participating children and their families in each community (Table 2) showed no significant differences in age, as anticipated by study design. In the HSL town group, 65% were males, compared with 49% in the MSL town (49%) and 44% in the LSL town. Overall, the socio-demographic characteristics of the children participating in the study were consistent with data collected during the 1995 census on the entire populations of the three towns. In the LSL town (all Arab Bedouins, born in Israel), there were more children per family and more crowding, together with lower parent education. In the HSL town there were fewer children per family and less crowding, parents had high school or university education and few households (26%) had a father of Asian (mainly Middle East) or African (Mainly North Africa) origin. MSL town characteristics were intermediate, with a high (75%) percentage of the fathers born in Asia or Africa.
Table 2.
Socioeconomic characteristics of the study subjects
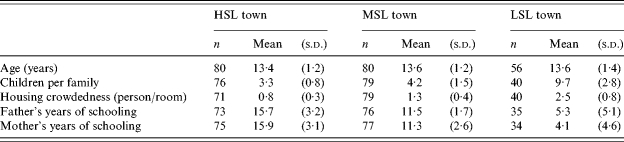
HSL, High standard of living; MSL, medium standard of living; LSL, low standard of living.
Serological results
Serological results were analysed separately for males and females.
Antibodies to HAV
The distribution of HAV seropositivity by gender and community (Table 3) shows almost 100% seropositivity rate in the LSL town, and a low rate (∼25%) in the HSL town (statistically significant linear trend). No significant differences were found between males and females of the same community.
Table 3.
HAV antibodies positivity rate by gender and community
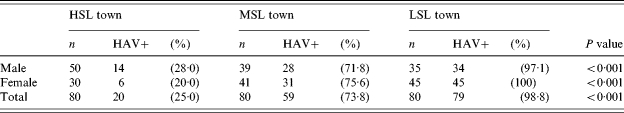
HSL, High standard of living; MSL, medium standard of living; LSL, low standard of living.
Antibodies to S. sonnei, S. flexneri, C. parvum, E. coli O157:H7
Anti-S. sonnei LPS IgA levels in females were significantly lower in the HSL town (P<0·001) than in the MSL and LSL towns. There was no significant difference with males. IgG levels were significantly lower in the HSL town compared to the LSL town in females and males (P=0·024 and P=0·033 respectively).
Anti-S. flexneri LPS IgA titres were similar in the three groups. IgG titres were significantly lower in the HSL town compared to the LSL town in females (P=0·016) but not in males.
Anti-C. parvum IgA titres in females showed a linear trend, with lowest values in the HSL town and highest values in the LSL town (P<0·001). IgA titres for males and IgG titres for both sexes showed no significant differences.
Anti-E. coli O157:H7 IgA titres showed a linear trend in females, with lowest values in the HSL town and highest values in the LSL town (P<0·001). In males a similar significant trend was found (P=0·024) but only IgA levels in the HSL town were significantly different from the LSL town. IgG titres for both sexes showed no significant differences among the three communities.
Females showed a tendency for higher GMTs for all antibodies tested than males (Table 4). This was statistically significant for anti-C. parvum IgG and anti-E. coli O157:H7 IgG in the HSL town; anti-S. sonnei IgA, IgG, anti-C. parvum IgA, IgG, and anti-E. coli O157:H7 IgA, IgG in the MSL town; and anti-S. sonnei IgG, anti-C. parvum IgA and anti-E. coli O157:H7 IgG in the LSL town (Table 4).
Table 4.
Geometric mean titres of IgA and IgG antibodies to Shigella sonnei, Shigella flexneri, Cryptosporidium parvum and Escherichia coli O157:H7 in the three communities of different socioeconomic status
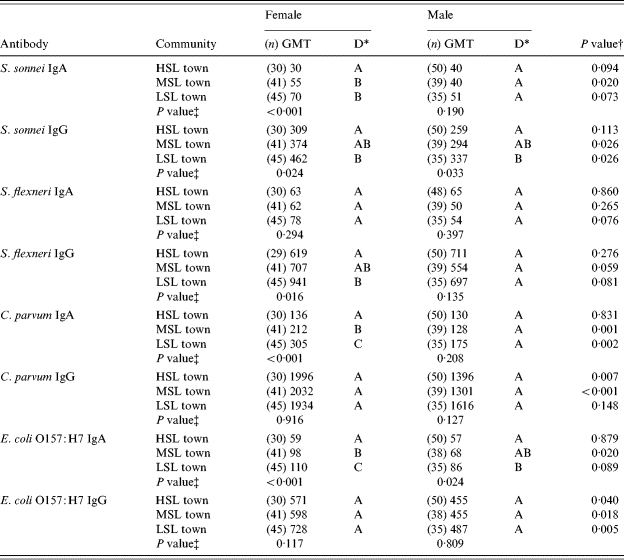
HSL, High standard of living; MSL, medium standard of living; LSL, low standard of living.
Duncan different letters (A, B and C) stand for statistical differences among the three communities.
P value, t test difference between genders.
P value, ANOVA F test difference among communities.
A strong multi-collinearity was identified among the variables composing the complex variable ‘town’ (children per household, crowdedness, father's years of schooling, mother's years of schooling) which prevented the performance of multiple regression analysis. Nevertheless, when we included in the regression analysis just two variables, ‘town’ and ‘number of children in a household’, in more than half of the comparisons the complex variable ‘town’ maintained the statistically significant correlation with the level of antibodies while in none of the comparisons ‘number of children in household’ emerged as a significant predictor of the level of antibodies.
Association of HAV seropositivity and antibody response to the other pathogens
The association between HAV seropositivity and the level of serum antibodies to S. sonnei, S. flexneri, C. parvum and E. coli O157:H7 was examined for the HSL and MSL towns. In the LSL town, analysis could not be carried out because all samples were positive for HAV. In the HSL town, no statistically significant differences were found in the GMT levels of S. sonnei, S. flexneri, C. parvum and E. coli O157:H7 anti-LPS IgG and IgA in HAV-positive compared to HAV-negative subjects. The S. sonnei Ig GMT was slightly higher in the HAV-positive than in the HAV-negative group without reaching statistical significance (GMTs 45 vs. 33, P=0·091). In children of the MSL town, the IgA GMTs against S. sonnei and E. coli O157:H7 LPS were significantly higher in the HAV-positive group: 52 and 91 respectively, compared to the HAV-negative one: 36 and 63 respectively (P=0·021 and P=0·043 respectively). Anti-S. flexneri LPS IgG levels were also slightly higher in the HAV-positive than in the HAV-negative group, without reaching statistical significance (P=0·099).
DISCUSSION
In this study we investigated in teenagers the association between the socioeconomic status of the community, the seroprevalence of antibodies to HAV and the level of serum antibodies to other faeco-orally transmitted pathogens, including S. sonnei, S. flexneri, C. parvum and E. coli O157:H7. The feature common to these organisms is the low inoculum (a few hundred cells) that may trigger disease. This biological characteristic confers the enteropathogens with multiple ways of transmission such as person-to-person, food-, water-, fly- and fomite-borne, which we assumed would be more available in communities of lower socioeconomic status.
The collection of data on the study population and of serum samples was done in 1996. Serology for hepatitis was performed a short time after the collection of sera while the other serological assays as well as the analysis of data concerning all the various antigens were conducted more recently. Since the study deals with an aetiological question (the association between socioeconomic status and the level of serum antibodies to selected faeco-orally transmitted pathogens) we do not feel that its findings and message could be affected by the relatively long time that elapsed from collection of data to publication.
To investigate a spectrum of socioeconomic levels and standards of life we chose three geographically adjacent communities representing high, middle and low socioeconomic levels (HSL, MSL and LSL towns). Thus, ‘town’ represented a complex variable characterized by number of children per household, crowdedness, father's years of schooling, mother's years of schooling, etc. The study groups had similar socioeconomic characteristics as the community to which they belonged. The ethnic origin of the three communities differed, with most (80%) of the households in the HSL town of European Jewish origin. In the MSL town most (60%) were Jews from African or Asian origin and in the LSL town almost all were of Arab ethnicity.
We could not control for ethnicity in the present study. While in Israel ethnic origin is associated with socioeconomic status, there is no evidence of an independent association between ethnicity and seroprevalence of antibodies to the various enteropathogens. The Bedouin community of the LSL town had also a higher risk of contact with domestic animals.
HAV seroprevalence had a significant and linear association with low socioeconomic status. Seropositivity rate ranged from 25% in the high-status town to 99% in the low-status community, with no difference between males and females. In Israel, HAV was endemic before the introduction of universal immunization of toddlers in 1999 with high seropositivity in the adult population [1]. It has been shown that low socioeconomic status was associated with a higher prevalence of anti-HAV antibodies [13]. In a study conducted in Jewish males aged 25–44 years sibship size emerged as the strongest correlate of anti-HAV antibodies [14].
Separate analysis for males and females revealed significant differences between the study groups regarding the levels of specific antibodies to the other enteropathogens. Consistently, the antibody GMTs in females were significantly higher than in males and showed stronger inverse association with socioeconomic status. In females, significant differences in the GMTs were detected for antibodies to all enteropathogens with the exception of IgA antibodies to S. flexneri, and IgG antibodies to C. parvum and E. coli O157:H7. A ‘dose–response’ association was found between the GMT of IgA antibodies to C. parvum and to E. coli O157, and the socioeconomic level of the communities. In males, only the anti-S. sonnei IgG titres and the anti-E. coli O157 IgA titre were significantly lower in the HSL community compared with the LSL community.
The high levels of specific IgG antibodies reflect old and new exposures and re-exposures to the various enteropathogens studied while the high levels of specific IgA antibodies reflect mostly recent infection with the homologous enteropathogen. It has been shown that following natural infection, anti-S. sonnei and S. flexneri LPS IgG antibodies remain elevated for months and even years while IgA serum antibodies return to pre-exposure levels much earlier, i.e. after 2–3 months [9]. The level of S. sonnei and S. flexneri LPS antibodies was found to be associated with immunity against new infections caused by homologous Shigella species [15].
Female gender was associated with higher GMTs of antibodies to Shigella, E. coli O157 and C. parvum antigens but not with the hepatitis A seropositivity. We assume that a more vigorous immune response to previous symptomatic and asymptomatic natural infections with the corresponding bacterial and protozoan organisms in females compared to males may explain the difference between the two genders in the levels of specific antibodies to the various enteropathogens. This may also explain the stronger inverse association of these antibodies with the socioeconomic status found in females. A more vigorous immune response in females is possibly the reason for these findings rather than gender differences in exposure to the various enteropathogens at the ages at which the subjects were examined. While we are not aware of other reports on sex differences in the immune response to bacterial antigens following natural exposure, significantly higher levels of IgG antibody directed to rubella virus structural proteins were observed in convalescent females compared with males [16]. It has been also shown that female young adults induced a more vigorous immune response to vaccination with the live-attenuated measles vaccine [17], although similar differences were not shown in infants' reactogenicity to the MMR vaccine [18].
The levels of serum antibodies detected in the subjects in this study reflect previous symptomatic and asymptomatic infections with the various enteropathogens that are more common in communities of lower socioeconomic status. Israel is highly endemic for shigellosis caused by S. sonnei and S. flexneri [19–24] and the involvement of C. parvum in diarrhoeal diseases in children in Israel has been reported [11, 25]. There are, however, few reports on E. coli O157 causing outbreaks of diarrhoea in Israel [26] and the incidence rate of haemolytic–uraemic syndrome is estimated to be lower in Israel compared with other developed countries. We assume that the major part of the antibodies to the LPS of E. coli O157 detected in higher levels among the study subjects of low socioeconomic status (with higher risk of exposure to faecal material) are cross-reactive antibodies elicited to LPS of other E. coli serotypes as previously reported [27]. A high extent of exposure to E. coli LPS cross-reacting with E. coli O157 LPS may confer natural cross-immunity and explain, at least in part, the low level of morbidity associated with E. coli O157 in countries highly endemic to enteric infections such as Israel.
The anti-S. sonnei and anti-E. coli O157 IgA levels were significantly associated with HAV seropositivity, indicating common routes of transmission for the three enteric pathogens.
In conclusion, the findings of our study demonstrate that the higher risk of exposure to enteropathogens transmitted via the faecal–oral route in communities of low socioeconomic status is reflected not only in a higher prevalence of lasting antibodies to HAV but also in higher antibody levels to bacterial and protozoan enteropathogens spread by the same route. In females, the levels of the specific serum antibodies were higher and more strongly associated with low socioeconomic status.
ACKNOWLEDGEMENTS
The authors thank Ruhama Ambar for her technical assistance in the performance of the serological tests.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Dienstag JL et al. Hepatitis A virus infection: new insights from seroepidemiologic studies. Journal of Infectious Diseases. 1978;137:328–340. doi: 10.1093/infdis/137.3.328. [DOI] [PubMed] [Google Scholar]
- 2.Termorshuizen F et al. The prevalence of antibodies to hepatitis A virus and its determinants in The Netherlands: a population-based survey. Epidemiology and Infection. 2000;124:459–466. doi: 10.1017/s0950268899003842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achi R, Mata L, Lindberg AA. Serum antibody titres to Shigella lipopolysaccharides and invasion plasmid antigens in healthy Costa Rican and Swedish women. Scandinavian Journal of Infectious Diseases. 1994;26:329–337. doi: 10.3109/00365549409011803. [DOI] [PubMed] [Google Scholar]
- 4.Cohen D, Slepon R, Green MS. Sociodemographic factors associated with serum anti-Shigella lipopolysaccharide antibodies and shigellosis. International Journal of Epidemiology. 1991;20:546–550. doi: 10.1093/ije/20.2.546. [DOI] [PubMed] [Google Scholar]
- 5.Chacin-Bonilla L et al. Cryptosporidium infections in a suburban community in Maracaibo, Venezuela. American Journal of Tropical Medicine and Hygiene. 1993;49:63–67. doi: 10.4269/ajtmh.1993.49.63. [DOI] [PubMed] [Google Scholar]
- 6.Kuhls TL et al. Seroprevalence of cryptosporidial antibodies during infancy, childhood, and adolescence. Clinical Infectious Diseases. 1994;18:731–735. doi: 10.1093/clinids/18.5.731. [DOI] [PubMed] [Google Scholar]
- 7.Pereira MD et al. Intra-familial and extra-familial risk factors associated with Cryptosporidium parvum infection among children hospitalized for diarrhea in Goiania, Goias, Brazil. American Journal of Tropical Medicine and Hygiene. 2002;66:787–793. doi: 10.4269/ajtmh.2002.66.787. [DOI] [PubMed] [Google Scholar]
- 8.Cohen D et al. Natural immunity to shigellosis in two groups with different previous risks of exposure to Shigella is only partly expressed by serum antibodies to lipopolysaccharide. Journal of Infectious Diseases. 1992;165:785–787. doi: 10.1093/infdis/165.4.785. [DOI] [PubMed] [Google Scholar]
- 9.Cohen D et al. Immunoglobulin M, A, and G antibody response to lipopolysaccharide O antigen in symptomatic and asymptomatic Shigella infections. Journal of Clinical Microbiology. 1989;27:162–167. doi: 10.1128/jcm.27.1.162-167.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungar BL, Mulligan M, Nutman TB. Serologic evidence of Cryptosporidium infection in US volunteers before and during Peace Corps service in Africa. Archives of Internal Medicine. 1989;149:894–897. [PubMed] [Google Scholar]
- 11.Robin G et al. Cryptosporidium infection in Bedouin infants assessed by prospective evaluation of anticryptosporidial antibodies and stool examination. American Journal of Epidemiology. 2001;153:194–201. doi: 10.1093/aje/153.2.194. [DOI] [PubMed] [Google Scholar]
- 12.Ungar BL et al. Enzyme immunoassay detection of immunoglobulin M and G antibodies to Cryptosporidium in immunocompetent and immunocompromised persons. Journal of Infectious Dieases. 1986;153:570–578. doi: 10.1093/infdis/153.3.570. [DOI] [PubMed] [Google Scholar]
- 13.Green MS et al. Sociodemographic correlates of neutralizing poliovirus and hepatitis A virus antibodies as markers of different modes of acquiring immunity. American Journal of Public Health. 1990;80:1270–1271. doi: 10.2105/ajph.80.10.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green MS, Zaaide Y. Sibship size as a risk factor for hepatitis A infection. American Journal of Epidemiology. 1989;129:800–805. doi: 10.1093/oxfordjournals.aje.a115194. [DOI] [PubMed] [Google Scholar]
- 15.Cohen D et al. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. Journal of Infectious Diseases. 1988;157:1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell LA, Zhang T, Tingle AJ. Differential antibody responses to rubella virus infection in males and females. Journal of Infectious Diseases. 1992;166:1258–1265. doi: 10.1093/infdis/166.6.1258. [DOI] [PubMed] [Google Scholar]
- 17.Green MS et al. Sex differences in the humoral antibody response to live measles vaccine in young adults. International Journal of Epidemiology. 1994;23:1078–1081. doi: 10.1093/ije/23.5.1078. [DOI] [PubMed] [Google Scholar]
- 18.Shohat T et al. Gender differences in the reactogenicity of measles-mumps-rubella vaccine. Israeli Medical Association Journal. 2000;2:192–195. [PubMed] [Google Scholar]
- 19.Green MS et al. Four decades of shigellosis in Israel: epidemiology of a growing public health problem. Review of Infectious Diseases. 1991;13:248–253. doi: 10.1093/clinids/13.2.248. [DOI] [PubMed] [Google Scholar]
- 20.Admoni O et al. Epidemiological, clinical and microbiological features of shigellosis among hospitalized children in northern Israel. Scandinavian Journal of Infectious Diseases. 1995;27:139–144. doi: 10.3109/00365549509018994. [DOI] [PubMed] [Google Scholar]
- 21.Cohen D et al. Prospective cohort studies of shigellosis during military field training. European Journal of Clinical Microbiology and Infectious Diseases. 2001;20:123–126. doi: 10.1007/s100960000428. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg D et al. Shigella bacteremia: a retrospective study. Clinical Pediatrics (Philadelphia) 2003;42:411–415. doi: 10.1177/000992280304200504. [DOI] [PubMed] [Google Scholar]
- 23.Finkelman Y et al. Epidemiology of Shigella infections in two ethnic groups in a geographic region in southern Israel. European Journal of Clinical Microbiology and Infectious Diseases. 1994;13:367–373. doi: 10.1007/BF01971992. [DOI] [PubMed] [Google Scholar]
- 24.Lerman Y, Slepon R, Cohen D. Epidemiology of acute diarrheal diseases in children in a high standard of living rural settlement in Israel. Pediatric Infectious Disease Journal. 1994;13:116–122. doi: 10.1097/00006454-199402000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Fraser D et al. Natural history of Giardia lamblia and Cryptosporidium infections in a cohort of Israeli Bedouin infants: a study of a population in transition. American Journal of Tropical Medicine and Hygiene. 1997;57:544–549. doi: 10.4269/ajtmh.1997.57.544. [DOI] [PubMed] [Google Scholar]
- 26.Lerman Y et al. A cluster of cases of Escherichia coli O157 infection in a day-care centre in a communal settlement (Kibbutz) in Israel. Journal of Clinical Microbiology. 1992;30:520–521. doi: 10.1128/jcm.30.2.520-521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig K et al. Immune response to non-O157 Vero toxin-producing Escherichia coli in patients with hemolytic uremic syndrome. Journal of Infectious Diseases. 1996;174:1028–1039. doi: 10.1093/infdis/174.5.1028. [DOI] [PubMed] [Google Scholar]


