SUMMARY
To evaluate the epidemiology of hospital admissions for varicella in children, a 1-year prospective multicentre study was done in Northern France in the pre-varicella vaccine era. The 405 children aged <16 years seen at local hospitals for varicella or herpes zoster were included. Among them, 143 who had varicella and resided in the district were admitted. Admission incidence rates were 28/100 000 children aged <16 years (149/100 000 infants aged <1 year, 69/100 000 children aged 1–4 years, and 2/100 000 children aged 5–15 years). Most admissions (57%) were related to complications, usually skin infection (47%). Independent risk factors for admission were place of residence outside the district [adjusted odds ratio (aOR) 8·7], complication at admission (aOR 5·8), recurrent fever (aOR 4·5), recent varicella in a sibling (aOR 4·0), and previous physician visit for the same condition (aOR 2·0).
INTRODUCTION
Varicella is a viral infection considered until recently as a common and usually innocuous disease experienced by nearly every child in the world. Between 600 000 and 750 000 cases in children or adults are reported each year in France, with an annual incidence rate around 1–1·35/100 population [1, 2]. Among varicella cases, 95% occur in individuals <20 years of age [3]. Complications occur in 2–3% of varicella cases [2, 4], usually in otherwise healthy children [5]. The admission rate in children <15 years has been estimated at 23/100 000, and in all age groups combined the mean number of deaths is 19 per year from 1990 to 1997 [1]. Varicella affects not only individuals but also society, as the costs of medical care, work days missed by the patients or parents, and babysitting combine into a substantial financial burden [6, 7].
Immunization against varicella has been used for more than 20 years in Japan [8]. In the United States, it has been recommended since 1995 for children aged ⩾12 months, a strategy that has produced good results [9–11]. In 2004, the German health-care authorities issued a recommendation that children be routinely immunized against varicella [12]. In France, the varicella vaccine was licensed in December 2003 but is not recommended for routine administration to children [13]. Epidemiological data are needed to estimate the potential benefits of widespread varicella immunization.
The aim of this study was to prospectively determine the admission incidence rate in children with varicella infection, the reasons for admission with special attention to the role of skin infection, and the risk factors for admission.
PATIENTS AND METHODS
Study design and inclusion criteria
A prospective multicentre cohort study was carried out in Northern France, a well-defined geographic area with 572 347 children aged <16 years in 2003 [14]. The 11 hospitals in Northern France that have paediatric units participated in the study during the year preceding licensure of the varicella vaccine in France (from 6 January 2003 to 5 January 2004). Inclusion criteria were age <16 years and admission or emergency-department evaluation for varicella or herpes zoster (VZV) during the study period. Children were included at any stage of varicella or herpes zoster (from early vesicles to shedding of scabs) or if a VZV-related event occurred within 4 weeks after the onset of VZV symptoms. The diagnosis of VZV infection was based on typical physical findings. In children who were admitted more than once during the study period for VZV infection, only the first admission was counted; the outcomes of the subsequent admissions were added to the initial questionnaire form.
In the six hospitals that had a paediatric ward but no paediatric emergency department, only VZV-related admissions were recorded. In the five other hospitals, which had a paediatric ward and a paediatric emergency department, both VZV admissions and VZV cases seen at the emergency department on an outpatient basis were recorded. For each in-patient or outpatient who met the study inclusion criteria, a hospital paediatrician prospectively completed a standardized questionnaire. In each study centre, the local coordinating investigator was a paediatrician participating in the Hospital Network for Evaluating the Management of Common Childhood Diseases. The local coordinating investigator ensured that questionnaires were properly completed, validated each included patient, and sent the completed questionnaires to the main investigators.
Definitions
We used the textbook description of varicella [15] to diagnose the disease: generalized pruritic vesicular rash, usually with mild fever and systemic symptoms, followed after 5–6 days by crusting of the vesicles then by shedding of the scabs after about 2 weeks. Herpes zoster was defined as a similar rash in a dermatomal distribution [15]. We used textbook definitions for varicella complications and data from the literature for risk factors predisposing to severe or complicated varicella [16–21] (see below). Patients that could not be properly watched and treated at home were defined as having an underprivileged social background. We defined miscellaneous reasons for admission as admissions that were not ascribable to VZV complications, to risk factors for severe or complicated disease, or to an underprivileged social background. These miscellaneous admissions were validated independently by the local coordinating investigator and by one of the main investigators (F.D.).
Collected data
Among the questionnaire items, 81% required simple yes/no answers. The items collected information on demographics (age, sex, siblings, maternal educational background, hospital, and whether the patient lived in Northern France), potential risk factors for severe or complicated VZV infection (age <1 or >13 years, immunodeficiency due to a disease or treatment, asthma, atopic dermatitis, recent varicella cases in the family) [18, 19], clinical features (varicella or herpes zoster, fever, extent of the rash, involvement of the mucous membranes, suspected complications), whether the patient was admitted, and the reasons for admission.
Before study initiation, each local coordinating investigator was given a copy of the investigator's manual, which detailed the study objectives; recent data on VZV epidemiology; case definitions for varicella, herpes zoster, and their complications; and risk factors for VZV-related complications. The manual was designed to ensure that all centres and paediatricians involved in the study used the same diagnostic criteria. During the study, each local coordinating investigator was called on the phone every 2 or 3 weeks for a progress report. The data were keyboarded using EpiData software (EpiData Association, Odense, Denmark) with a check-in control. No information identifying the patients was entered into the database. At study completion, the data were compared to the discharge codes in each hospital during the study period in order to assess the efficiency of our case ascertainment procedure. Using a capture–recapture method, a correction coefficient was calculated to determine a corrected incidence rate of the varicella hospitalizations. Ethics Committee (CCPPRB Lille) declared that the study did not require specific agreement since the study protocol did not include any additional investigation or treatment other than routine care.
Statistics
Statistical analyses were done using Epi-Info 6.04 software (Centers for Disease Control and Prevention, Atlanta, GA, USA). First, a descriptive analysis of children admitted with varicella during the study period was presented. Second, the admission incidence rates and the corrected incidence rates of children <16 years of age having varicella and living in Northern France were determined using demographic estimates from the National Institute for Statistics and Economic Investigations [14]. Third, the reasons for admission were detailed separately for patients with and without a complication of varicella. Fourth, to identify risk factors for admission, we compared admitted and non-admitted patients in the five hospitals with paediatric emergency departments. For the univariate analysis, we used the χ2 test, Fisher's exact test, and comparisons of means. Variables associated with admission with P values of <0·20 in this univariate analysis, as well as risk factors for admission in published studies, were entered in a backward stepwise multivariate logistic regression model, using SPSS software (SPSS Inc., Chicago, IL, USA). Factors associated with admission at P levels <0·05 were taken as independent risk factors for admission.
RESULTS
Over the study period, 405 children with varicella (398) or herpes zoster (7) were included. Among them, 162 were admitted, including 52 in the six hospitals without and 110 in the five hospitals with paediatric emergency departments. In these five departments, 243 children were evaluated but not admitted.
The 162 admitted children had a median age of 21 months (range 2 months to 15 years) and a 1·4 male:female ratio. Before admission, 80% of the patients had seen an office-based physician for their symptoms; however, only 48% were referred to the hospital by a physician. Of all admissions, 67% were decided on in-house call duty. The parents were aware of the diagnosis of VZV infection in 85% of the cases. Of the 162 admitted patients, 159 had varicella and three had herpes zoster. At admission, 62% of patients had a fever (central temperature ⩾38°C; mean, 39·2°C), 34% a profuse vesicular rash (>100 lesions), 41% mucosal involvement, and 14% fewer than 10 skin lesions. A history of atopic dermatitis was noted in 15% of admitted patients, of asthma in 6%, and of immunodepression in 2%. Intravenous antibiotic therapy and acyclovir treatment were necessary respectively for 30% and 25% of admitted patients. The mean length of stay was 4·2 days [median 2 days, interquartile range (IQR) 2–5 days].
Of the 162 admitted patients, 146 lived in Northern France; 143 of these 146 had varicella and three had herpes zoster. The admission incidence rate for children (<16 years) with varicella living in Northern France in 2003 was 25/100 000. Our case ascertainment rate was 94% compared to discharge codes. Discharge codes missed 6% of VZV-related admissions recorded in our study. The overall correction coefficient was 1·13, and the corrected incidence rate was 28/100 000. Table 1 reports admission incidence rates in various age groups.
Table 1.
Admission incidence rates in children with varicella infection in Northern France, 2003
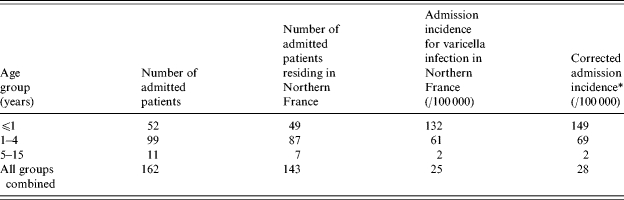
The case ascertainment rate was 94% compared to discharge codes. Discharge codes missed 6% of VZV-related admissions recorded in the study. The overall correction coefficient was 1·13.
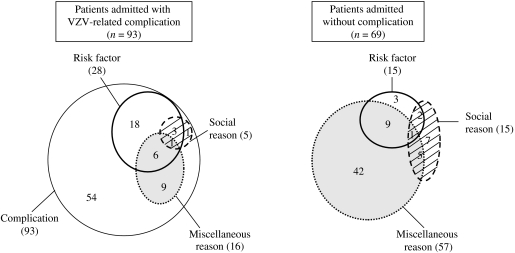
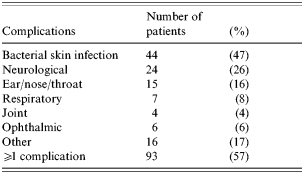
The distribution of reasons for admission is indicated in the Figure, considering separately patients with and without a VZV-related complication. A VZV-related complication was observed in 93 (57%) of the 162 admitted patients (Table 2). The main complication was a bacterial skin infection for 44 patients; some patients had more than one bacterial skin infection, and the diagnoses (n=49) were as follows: impetigo (n=21), cellulitis (n=15), ecthyma (n=5), scarlet fever (n=1), staphylococcal scalded skin syndrome (n=3), abscess (n=2), varicella gangrenosa (n=1), and felon (n=1). For patients with a complication, at least one risk factor of VZV complication was an associated reason for admission for 28 (30%): age <1 year (n=22), recent varicella infection in the family (n=9), non-steroidal anti-inflammatory drug therapy (n=6), atopic dermatitis (n=4). At least one complication occurred during the hospital stay in 11 patients (12%) and consisted of skin infection (n=6), neurological disorders (n=3), other infection (n=3), other complication (n=3). The mean length of stay was 4·7 days (median 3 days, IQR 2–5 days) for patients admitted with VZV-related complications. Three patients died of complications of varicella (n=2) or herpes zoster (n=1). A 4-month-old girl died of acute respiratory distress syndrome secondary to acute pneumonia. No bacterial agent was identified. The second patient, a 15-month-old boy, died after acute encephalitis, with autonomic system dysfunction. The third patient, a 15-year-old boy with a history of Gougerot–Sjögren syndrome presented with an ophthalmic herpes zoster complicated by a keratitis, high grade fever, and died of a septic shock. No bacterial agent was identified. The annual incidence rate of death associated with varicella infection in Northern France was 0·5/100 000 children <16 years of age.
Table 2.
VZV-related complications at the admission among the 162 admitted patients
Of the 69 patients admitted without any complication, at least one risk factor was a reason for admission for 15 (22%) [age<1 year (n=10), recent varicella infection in the family (n=2), non-steroidal anti-inflammatory drug therapy (n=3), atopic dermatitis (n=2), immunodepression (n=3)], and 57 had miscellaneous reasons for admission: 23 had gastrointestinal disorders (gastroenteritis in 16 and difficulty feeding in seven), 10 high grade fever, four other neurological disorders, five other respiratory reasons, three severe parental anxiety, 12 other reasons for admission. Five of them developed a complication during the hospital stay (four skin infections and one otitis media), but only one had risk factors of VZV complication. The length of stay was shorter (mean 3·5 days, median 2 days, IQR 1–4 days) for patients admitted without complication (P=0·005).
Risk factors for admission were studied in the 353 children evaluated at the five hospitals with paediatric emergency departments: we compared the 110 admitted patients to the 243 non-admitted patients. Table 3 lists the risk factors identified by univariate analysis. Independent predisposing factors for admission were identified in the multivariate analysis: residing elsewhere than in Northern France [adjusted odd ratio (aOR) 8·7], complication at admission (aOR 5·8), recurrent fever on or after day 3 of the disease (aOR 4·5), recent varicella infection in a sibling (aOR 4·0), and having previously seen a physician for the current VZV infection (aOR 2·0) (Table 3).
Table 3.
Univariate and multivariate analysis of risk factors for admission in 353 children with VZV infection evaluated in paediatric emergency departments
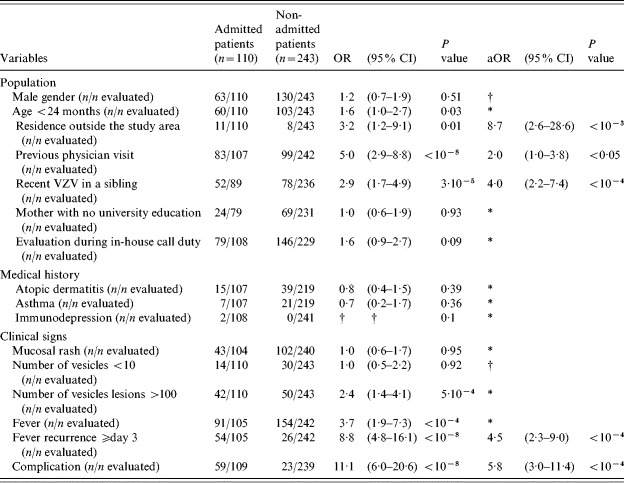
OR, Odds ratio; 95% CI, 95% confidence interval; aOR, adjusted odds ratio.
Excluded data during the multivariate analysis; † data not included in the multivariate analysis (see text).
DISCUSSION
This is the first prospective epidemiological study of the incidence, reasons, and risk factors for admission in children with VZV infection belonging to a large population (>2·5 million people). The admission incidence rate for varicella infection in children aged <16 years was high overall (corrected value 28/100 000) and very high in the youngest age groups (149/100 000 children aged <1 year old and 69/100 000 children aged 1–4 years old). VZV-related complications, and particularly skin infections, were the most common reasons for admission.
The overall admission incidence rate in our study is higher than in a previous retrospective nationwide survey conducted in France in 1990–1999 (23/100 000 children aged <16 years old) [1]. In previous studies from other countries in the pre-vaccine era (1990s), admission incidence rates were 1·4–12 times lower than in our study, in all age groups (Table 4) [21–24]. These discrepancies may be ascribable to differences in the quality of data collection. Previous studies were retrospective and, therefore, carried a risk of selection bias [23]. Our study was prospective and in each centre a coordinating investigator worked to maximize case ascertainment. Children seen at emergency departments of hospitals without paediatric units were referred to hospitals with paediatric units if admission was deemed necessary. For most questionnaire items, the missing data rate was <5%; exceptions were mother's educational background (12%), recent varicella in siblings (8%), and history of atopic dermatitis or asthma (7%). Thus, selection bias was probably minimal in our study.
Table 4.
Admission incidence rate by age group in our study (Northern France) and in several other geographic areas in the pre-vaccine era
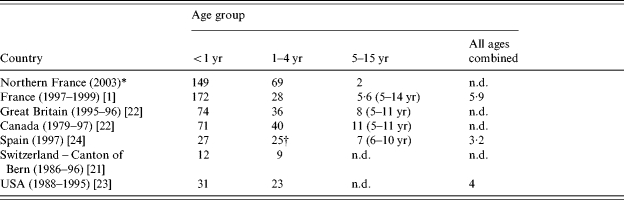
Prospective study; all other studies were retrospective.
Incidence for children aged 1–5 years.
n.d., No available data.
Our higher admission incidence rate may be ascribable to an increase in the incidence and severity of varicella in recent years. The incidence in France increased from 2001 (970/105) to 2004 (1420/100 000), according to the French Sentinelle network with an incidence rate at 1150/100 000 in the year under study [25]. Reports from the pre-vaccine era in the United States suggest a rise in the rate and severity of bacterial skin infections and other varicella complications [26–28]. Similar findings were reported in France in 2004 [29]. The incidence rate of varicella has increased markedly in infants and young children [4], who are known to be at higher risk for death and non-fatal complications [20]. These data are consistent with the higher admission incidence rate in young children and high complication rate in our study. However, we cannot exclude that prospective recruitment and reminding physicians of the complications and risk factors of varicella may have interfered with the admitting behaviour in increasing the admission incidence rate. A complication was the main reason for admission in 57% of our admitted patients, and the complication was a bacterial skin infection in 47% of these cases. Bacterial skin infections were the most common complications in earlier studies [30–32]. Risk factors for bacterial skin infection may include use of nonsteroidal anti-inflammatory drugs or topical agents [16, 33], which is still common although some reports recommend avoiding the use of these treatments [33, 34]. Neurological complications, which affected 26% of children in our study, were the leading reason for admission in other studies [21, 35].
Risk factors, underprivileged social background, or miscellaneous factors were associated reasons for admission in 66% of our admitted VZV patients (Fig.). In Northern France, low-income families often use hospital emergency departments instead of office-based physicians, which may have contributed to the high admission incidence rate in our study. The miscellaneous reasons category included factors clearly independent from the VZV infection (e.g. trauma or scheduled admission), factors clearly related to the VZV infection (e.g. high-grade fever or severe parental anxiety), and factors possibly related to the VZV infection (e.g. gastrointestinal symptoms). In a recent study, gastrointestinal symptoms (vomiting, diarrhoea, or difficulty feeding), with dehydration or gastric erosions in some patients, were classified among varicella complications [29]. Dehydration was considered a varicella complication in other studies [23, 32]; however, none of our patients with gastrointestinal symptoms had dehydration. Secondary gastrointestinal or respiratory tract symptoms consistent with a second viral infection seem common during or immediately after varicella. Whether these events are coincidences or reflect infections complicating VZV-induced immunosuppression is unclear. Lastly, this study showed the admitting behaviour of paediatricians as well as the epidemiology of complicated varicella.
Fig.
Distribution of the reasons for admission in 162 children with VZV infection.
Several independent risk factors for admission were identified in our study. These risk factors either reflected the occurrence of a complication or a predisposition to complications. Thus, fever recurrence on or after day 3 may indicate a complication. A recent case of VZV infection in a sibling may be associated with a larger viral inoculum and has been identified as a risk factor for invasive group A streptococcal infection complicating varicella [19]. A place of residence outside the study area and previous evaluation by a physician may indicate an unusual course of the disease consistent with the development of complications.
In conclusion, the admission incidence rate in children with VZV infection was high in Northern France overall and very high in children <5 years of age. Independent risk factors for admission included complications (the main reason for admission) and factors that may predispose to complications. Widespread VZV immunization may reduce the rate of varicella-related complications [36], admissions and deaths. Consistent with this possibility, a decrease in mortality has been reported in the United States after the introduction of varicella vaccine [11, 37]. In addition, co-administration of varicella vaccine and measles-mumps-rubella vaccine may reduce medical costs [38].
ACKNOWLEDGEMENTS
We are grateful to the physicians in Northern France participating in the Hospital Network for Evaluating the Management of Common Childhood Diseases who contributed to the present study: Dr Akitani, Seclin Hospital; Dr Bajja, Maubeuge Hospital; Dr Blondiaux, Cambrai Hospital; Dr Cixous, Roubaix Hospital; Dr Delepoulle, Dunkerque Hospital; Dr Dorkenoo, Lille Teaching Hospital; Dr Dumonceaux, Valenciennes Hospital; Dr El Kohen, Lille–Saint-Vincent-de-Paul Hospital; Dr Glowacki, Armentières Hospital; Dr Halna, Lille Teaching Hospital; Dr Racoussot, Douai Hospital; and Dr Segal, Tourcoing Hospital.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Boelle PY, Hanslik T. Varicella in non-immune persons: incidence, hospitalization and mortality rates. Epidemiology and Infection. 2002;129:599–606. doi: 10.1017/s0950268802007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deguen S, Chau NP, Flahault A. Epidemiology of chickenpox in France (1991–1995) Journal of Epidemiology and Community Health. 1998;52:46–49. (Suppl. 1): [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Evaluation of Varicella Reporting to the National Notificable Disease Surveillance System – United States, 1972–1997. Morbidity and Mortality Weekly Report. 1999;48:55–58. [PubMed] [Google Scholar]
- 4.Yawn BP, Yawn RA, Lydick E. Community impact of childhood varicella infections. Journal of Pediatrics. 1997;130:759–765. doi: 10.1016/s0022-3476(97)80019-4. [DOI] [PubMed] [Google Scholar]
- 5.Levrat V, Floret D. Clinical characteristics of varicella infections in French pediatric intensive care units, 1998–2001 [in French] Bulletin Epidémiologique Hebdomaire. 2003;9:51–52. [Google Scholar]
- 6.Saddier P et al. Cost of varicella in France: a study in day care centers. Journal of Infectious Diseases. 1998;178:58–63. doi: 10.1086/514275. (Suppl. 1): [DOI] [PubMed] [Google Scholar]
- 7.Law B et al. Cost of chickenpox in Canada: part II. Cost of complicated cases and total economic impact. The Immunization Monitoring Program – Active (IMPACT) Pediatrics. 1999;104:7–14. doi: 10.1542/peds.104.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi M. 25 years' experience with the Biken Oka strain varicella vaccine: a clinical overview. Paediatric Drugs. 2001;3:285–292. doi: 10.2165/00128072-200103040-00005. [DOI] [PubMed] [Google Scholar]
- 9.Anon. Decline in annual incidence of varicella – selected states, 1990–2001. Morbidity and Mortality Weekly Report. 2003;52:884–885. [PubMed] [Google Scholar]
- 10.Seward JF et al. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. Journal of the American Medical Association. 2002;287:606–611. doi: 10.1001/jama.287.5.606. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. New England Journal of Medicine. 2005;352:450–458. doi: 10.1056/NEJMoa042271. [DOI] [PubMed] [Google Scholar]
- 12.Rasch G, Hellenbrand W. Germany adds varicella vaccine to the national vaccination programme. www.eurosurveillance.org/ew/2004/040729.asp. Eurosurveillance Weekly. 8(31) ). 29 July 2004. ( ). Accessed August 2004. [Google Scholar]
- 13.Ministère de la Santé et de la Protection Sociale. www.sante.gouv.fr/htm/dossiers/cshpf/a_mt_190304_varicelle_def.pdf. www.sante.gouv.fr/htm/dossiers/cshpf/a_mt_190304_varicelle_def.pdf ) [in French]. Accessed April 2004.
- 14.INSEE www.insee.fr. www.insee.fr . National Institute for Statistics and Economic Studies ( ). Accessed April 2004.
- 15.Whitley RJ, Mandell GL, Bennett JE, Dolin R. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 6th edn. 2004. Varicella; pp. 1780–1786. , pp. [Google Scholar]
- 16.Lesko SM et al. Invasive group A streptococcal infection and nonsteroidal anti-inflammatory drug use among children with primary varicella. Pediatrics. 2001;107:1108–1115. doi: 10.1542/peds.107.5.1108. [DOI] [PubMed] [Google Scholar]
- 17.Abzug MJ, Cotton MF. Severe chickenpox after intranasal use of corticosteroids. Journal of Pediatrics. 1993;123:577–579. doi: 10.1016/s0022-3476(05)80954-0. [DOI] [PubMed] [Google Scholar]
- 18.Locksley RM et al. Infection with varicella-zoster virus after marrow transplantation. Journal of Infectious Diseases. 1985;152:1172–1181. doi: 10.1093/infdis/152.6.1172. [DOI] [PubMed] [Google Scholar]
- 19.Peterson CL et al. Risk factors for invasive group A streptococcal infections in children with varicella: a case-control study. Pediatric Infectious Disease Journal. 1996;15:151–156. doi: 10.1097/00006454-199602000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Meyer PA et al. Varicella mortality: trends before vaccine licensure in the United States, 1970–1994. Journal of Infectious Diseases. 2000;182:383–390. doi: 10.1086/315714. [DOI] [PubMed] [Google Scholar]
- 21.Jaeggi A, Zurbruegg RP, Aebi C. Complications of varicella in a defined central European population. Archives of Disease in Childhood. 1998;79:472–477. doi: 10.1136/adc.79.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brisson M et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiology and Infection. 2001;127:305–314. doi: 10.1017/s0950268801005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galil K et al. Hospitalizations for varicella in the United States, 1988 to 1999. Pediatric Infectious Disease Journal. 2002;21:931–935. doi: 10.1097/00006454-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Gil A et al. Epidemiology of primary varicella hospitalizations in Spain. Vaccine. 2001;20:295–298. doi: 10.1016/s0264-410x(01)00370-x. [DOI] [PubMed] [Google Scholar]
- 25.INSERM http://rhone.b3e.jussieu.fr/senti/ http://rhone.b3e.jussieu.fr/senti/ . French Sentinelle Network. Epidemiological situation in metropolitan France [in French]. ( ). Accessed December 2005.
- 26.Doctor A, Harper MB, Fleisher GR. Group A beta-hemolytic streptococcal bacteremia: historical overview, changing incidence, and recent association with varicella. Pediatrics. 1995;96:428–433. [PubMed] [Google Scholar]
- 27.Brogan TV et al. Group A streptococcal necrotizing fasciitis complicating primary varicella: a series of fourteen patients. Pediatric Infectious Disease Journal. 1995;14:588–594. doi: 10.1097/00006454-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 28.American Academy of Pediatrics. Committee on Infectious Diseases. Severe invasive group A streptococcal infections: a subject review. Pediatrics. 1998;101:136–140. [PubMed] [Google Scholar]
- 29.Mallet E et al. Evaluation of varicella complications through a retrospective hospital survey in a paediatric center over 16 years in France. Archives de Pédiatrie. 2004;11:1145–1151. doi: 10.1016/j.arcped.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Lin F, Hadler JL. Epidemiology of primary varicella and herpes zoster hospitalizations: the pre-varicella vaccine era. Journal of Infectious Diseases. 2000;181:1897–1905. doi: 10.1086/315492. [DOI] [PubMed] [Google Scholar]
- 31.Maharshak N, Somekh E. Hospitalization for varicella in central Israel. Acta Paediatrica. 1999;88:1279–1283. doi: 10.1080/080352599750030437. [DOI] [PubMed] [Google Scholar]
- 32.Peterson CL et al. Children hospitalized for varicella: a prevaccine review. Journal of Pediatrics. 1996;129:529–536. doi: 10.1016/s0022-3476(96)70117-8. [DOI] [PubMed] [Google Scholar]
- 33.Zerr DM et al. A case-control study of necrotizing fasciitis during primary varicella. Pediatrics. 1999;103:783–790. doi: 10.1542/peds.103.4.783. [DOI] [PubMed] [Google Scholar]
- 34.Dubos F et al. Assessment of out-patient treatment of varicella in children. Presse Médicale. 2004;33:992–996. doi: 10.1016/s0755-4982(04)98821-5. [DOI] [PubMed] [Google Scholar]
- 35.Ziebold C et al. Severe complications of varicella in previously healthy children in Germany: a 1-year survey. Pediatrics. 2001;108:e79. [PubMed] [Google Scholar]
- 36.Patel RA, Binns HJ, Shulman ST. Reduction in pediatric hospitalizations for varicella related invasive group A streptococcal infections in the varicella vaccine era. Journal of Pediatrics. 2004;144:68–74. doi: 10.1016/j.jpeds.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 37.McCoy L, Sorvillo F, Simon P. Varicella-related mortality in California, 1988–2000. Pediatric Infectious Disease Journal. 2004;23:498–503. doi: 10.1097/01.inf.0000129684.27717.d6. [DOI] [PubMed] [Google Scholar]
- 38.Coudeville L et al. The value of varicella vaccination in healthy children: cost-benefit analysis of the situation in France. Vaccine. 1999;17:142–151. doi: 10.1016/s0264-410x(98)00161-3. [DOI] [PubMed] [Google Scholar]