SUMMARY
One strain of Salmonella Brandenburg began causing large numbers of human infections in New Zealand in 1998. We investigated the emergence of this strain using combined notification and laboratory data on human and animal disease and a case-control study. S. Brandenburg infection in humans was characterized by spring peaks and high rates in the southern half of the South Island. This epidemic pattern followed very closely that seen in sheep. The case-control study found that infection was significantly associated with occupational contact with sheep and having a household member who had occupational contact with sheep, during the 3 days prior to illness or interview. We conclude that S. Brandenburg has become established as a zoonotic disease in New Zealand. Preventing infection requires control of the epidemic in sheep through vaccination, changes in farm management practices, and promotion of hand washing and other precautions to protect farmers and their families.
INTRODUCTION
A new strain of Salmonella enterica Brandenburg, genetically distinct from any previously detected in New Zealand, was first isolated in this country in 1996. The first infection was from a sheep abortion and it subsequently caused outbreaks of abortion and deaths in ewes in the southern half of the South Island [1, 2]. In affected flocks, this infection resulted in ∼5% of ewes aborting. The strain also caused illness and deaths in cattle, foals, goats, and dogs [3]. There were 39 million sheep in New Zealand in 2003 compared with a human population of 4 million [Statistics New Zealand (http://www.stats.govt.nz/default.htm)]. Sheep are farmed throughout the country and are, therefore, a potentially important zoonotic reservoir for human infection, as well as a valuable economic resource.
S. Brandenburg began causing large numbers of human infections in 1998 and clinicians expressed concern that this new serotype was resulting in more invasive disease than other forms of salmonellosis [4]. The presence of this infection in sheep also raised concerns that this organism could become an important foodborne pathogen in sheep meat [5]. This investigation was undertaken to determine the sources of human infection with S. Brandenburg, particularly the potential contribution of foodborne transmission.
METHODS
Surveillance of human salmonellosis
Non-typhoidal salmonellosis has been notifiable in New Zealand since 1952. Notified cases require a clinical history of gastroenteritis and either laboratory confirmation of Salmonella species from a clinical specimen, contact with a confirmed case, or common exposure to the source of illness in a confirmed case(s). Notifications to the medical officer of health are stored on a computerized database, EpiSurv, which is installed in all public health services in New Zealand. These data are collated nationally by the Institute for Environmental Science and Research (ESR) for the Ministry of Health. Clinical laboratories throughout New Zealand refer all human salmonellae isolates to the Enteric Reference Laboratory (ERL) at ESR for typing which provides an additional source of surveillance data.
Electronic notification data were matched with laboratory data for all S. Brandenburg isolates identified over the 1995–2002 period. This set of data were then analysed to provide the epidemiological description of S. Brandenburg over this period. Crude incidence rates were calculated using population data from the 1996 and 2001 censuses as denominators, with linear interpolation used to estimate denominators for intercensal years.
Surveillance of salmonellosis in animals
Animal health laboratories refer animal isolates to the ERL for further characterization. Data on such isolates obtained for the years 1995–2002 were analysed according to animal source, month received, and the location of the referring laboratory.
Laboratory typing
ERL routinely serotypes salmonellae isolates using somatic (O) and flagellar (H) antigens according to the Kauffman–White scheme [6]. A selection of the human and animal S. Brandenburg isolates from the 2001–2003 period were tested with pulsed-field gel electrophoresis (PFGE) using the method described by Barrett et al. [7].
National case-control study
The study was conducted from February 2002 to April 2003. All cases of laboratory-confirmed S. Brandenburg infection occurring over that time period were eligible. Cases were excluded if any of the following occurred: refusal to participate; inability to speak English sufficiently well to answer questions; not contactable by telephone and non-responsive to mailed requests to return calls; no telephone at their primary residence; or no known onset of symptoms. Cases were recruited and interviewed by a study worker, but received prior information about the study at the time of their routine interview by the public health service or territorial authority.
One matched control was recruited for each case. The matching variables were: age group (<5, 5–15, 16–24, 25–64, ⩾65 years), geographic areas (16 geographic areas) and rurality (rural, small town or urban telephone number). Control participants were recruited and interviewed within 14 days of the case interview. The process used randomly selected telephone numbers matching the rurality and geographic area of the associated case. If the call was answered, the interviewer inquired in a standardized manner if a potential control in the matching age group lived at the residence. Telephone numbers were called a minimum of three times before moving onto the next telephone number. Controls were excluded from the study if any of the following occurred: refusal to participate; inability to speak English sufficiently well to answer the questions; no landline or listed telephone number at primary residence; or presence of diarrhoea in the 28 days prior to interview.
All interviews were carried out by telephone. If the subject was a child aged <13 years then the interview was carried out with the parent or guardian. If the subject was aged 13–15 years, the interviewer would ask the parent/guardian whether the potential participant could be interviewed directly. If consent was obtained, and no exclusion criteria applied, the interview was carried out. Interviews were conducted by staff employed by the market research company NFO New Zealand.
The questionnaire asked about exposures in the 3 days prior to illness for cases, or 3 days prior to interview for controls. These questions included: travel, recreational water contact, drinking water source, foods and drinks, animal contact, and contact with infected people. There were additional questions on usual food-handling practices in the household and demographic information. The questionnaire and study protocol were reviewed and approved by the Wellington Ethics Committee on behalf of all the health research ethics committees in New Zealand. The questionnaire was pre-tested and the entire process of recruitment and interviewing was piloted before the study started.
Interview data was entered directly into the database at the time of interview and subsequently exported into SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) for data cleaning and analysis. Initial examination of the data consisted of a descriptive analysis of the characteristics of cases and controls. A conditional logistic regression was performed on the matched data for both univariate and multivariate results. The variables in the multivariate model for S. Brandenburg were deliberately chosen to test the key hypotheses and other risk factors were allowed to be added to the model in a stepwise manner (however, no risk factors met the criteria to do so). For exposures that were associated with a significant increase in disease risk, the population-attributable risk was calculated using the method of Bruzzi et al. [8].
RESULTS
Human S. Brandenburg infection
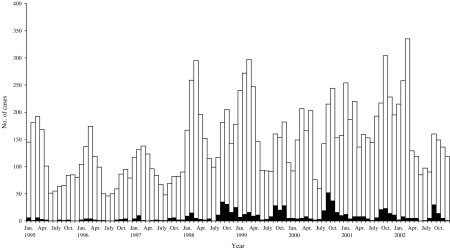
S. Brandenburg has historically been an uncommon cause of salmonellosis in New Zealand with an average of 27 cases a year from 1995 to 1997 (2·4% of all salmonellosis for that period). Its incidence rose markedly in 1998, causing an average of 134·2 cases per annum over the 1998–2002 period (6·9% of cases), which is a rate of 3·6/100 000 (Fig. 1). The distribution of this disease was also intensely seasonal with cases concentrated in spring, unlike other salmonellae infections which peak in late summer.
Fig. 1.
Salmonellae (□) and S. Brandenburg (■) cases by month from 1995 to 2002.
S. Brandenburg infections were concentrated in four health districts in the southern half of the South Island, notably Southland, Otago, South Canterbury, and Canterbury (Table 1). Other salmonellae infections were more evenly distributed across New Zealand.
Table 1.
Salmonellae and S. Brandenburg infections and rates by health district, 1998–2002

S. Brandenburg infection showed a similar age distribution to salmonellae generally, although the proportion in infants was relatively higher (10·4% for <1-year-olds compared with 6·2% for other salmonellae). There was also a relatively larger sex difference with 62·3% of S. Brandenburg cases being males, compared with 51·9% of salmonellae infections generally.
The outcomes of infection S. Brandenburg were similar to those seen for salmonellae infections generally. The hospitalization rate for S. Brandenburg over the 1995–2002 period was 11·6%, which was similar to the 12·5% for salmonellae generally. The case-fatality rates were also similar, being 0·3% for S. Brandenburg infection and 0·2% for salmonellae infection generally.
Animal S. Brandenburg infection
The ERL at ESR received 2035 S. Brandenburg isolates from animal sources over the 1995–2002 period. These were largely from sheep and lambs (1621, 79·7%) or cattle, including cows and calves (310, 15·2%). Other animal sources included dogs (24, 1·2%), poultry (26, 1·3%) and a range of other animals that all contributed <1% of isolates (pigs, horses, deer, goats, cats, and other birds).
The referral of such isolates increased markedly after 1997 (Fig. 2). The majority (88·1%) of sheep and lamb isolates and isolates from cattle (82·5%) came from animal health laboratories in the Otago region servicing the southern half of the South Island. As with human cases, isolates from animal sources were intensely seasonal with a pronounced spring peak each year from 1998 to 2002 (Fig. 2).
Fig. 2.
S. Brandenburg isolates from ovine sources (–■–), by month, 1995–2002, with human cases (□) for comparison.
Organism characteristics
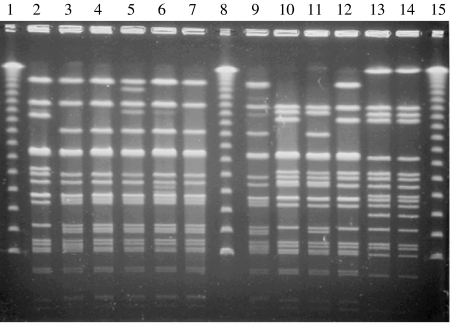
All 43 isolates from the case-control study were confirmed as S. Brandenburg serologically and by PFGE using the enzyme XbaI [7]. Thirty-eight of the 43 isolates were identified as the epidemic strain (36 from the South Island endemic area and two were from the North Island). The remaining five isolates had distinct PFGE patterns. Since 1995 an average of five bovine and 20 ovine isolates per annum have also been analysed by PFGE and all were the epidemic strain. Figure 3 demonstrates representative PFGE patterns seen in New Zealand isolates of S. Brandenburg, including the epidemic strain.
Fig. 3.
Gel image showing PFGE patterns seen in the New Zealand case-control study of S. Brandenburg. Lanes 1, 8 and 15, lambda ladder; lanes 13 and 14, the epidemic strain.
National case-control study
The case-control study recruited 43 cases of S. Brandenburg infection and 43 matched controls over the 15-month period from February 2002 to April 2003. A total of 85 cases of S. Brandenburg occurred during that period. Of these, 30 were excluded from the study for being unobtainable (18), being secondary cases (5), being unable to specify when their symptoms started (3), having no telephone (2), insufficient English language to understand the questions (1), and not being matched to a control (1). Of the remainder, 10 declined to participate when approached by the local public health service and two refused at interview. Telephone recruitment identified 88 potential controls who matched cases in terms of geographic region, rurality and age group. Of these 10 were excluded from the study for the following reasons: diarrhoea in the previous 28 days (8), and not interviewed within the time limit (2). Of the remaining eligible controls, 35 refused at interview.
The characteristics of cases and controls were the same for the matching variables of age group [20 (46·5%) were children aged <15 years], rurality [26 (60·5%) lived in rural areas] and geographic area [39 (90·7%) were in the three southern health districts]. A higher proportion of cases were male (24, 50·0%) than controls (16, 33·3%). The ethnicity, education level and incomes of cases and controls were similar. Illness reported by cases was typified by diarrhoea (93·0%), stomach pains or cramps (89·7%), fever (72·1%), and nausea (62·2%). Smaller proportions reported vomiting (32·6%) and blood in stool (20·9%). The median duration of symptoms was 7 days.
The univariate analysis identifies a number of exposures during the 3 days prior to illness or interview associated with an increased risk of S. Brandenburg infection (Table 2). The strongest effect size was seen for occupational contact with live or dead sheep, and occupational contact with animal carcases. Increased risk was also associated with household contact with a dog, household occupational contact with live animals or carcasses, and household contact with live and dead sheep (that is having another member of the household who had occupational contact with a dog, live animal, carcass, or sheep in the 3 days before illness or interview). The risk of disease was also increased if there were other people in the household with diarrhoea and where the usual method of cleaning the chopping board or other surface was reported as ‘Rinse with hot water only’ (this was in situations where the same chopping board or other surface used for cutting raw meat or poultry was also reported to be used for preparing other food such as salads and bread).
Table 2.
Univariate analysis of selected risk factors for S. Brandenburg infection, New Zealand 2002–2003

OR, Odds ratio; CI, confidence interval.
A large number of exposures were associated with a significantly decreased risk of S. Brandenburg infection in the univariate analysis. This protective effect appeared strongest for consumption of unpeeled fruit, uncooked vegetables, dairy products (pasteurized milk, ice cream, cheese, yoghurt), 2-minute noodles, processed meat products such as luncheon or baloney sausage, and eggs (particularly cooked eggs). There was also a protective effect associated with use of over-the-counter pharmaceuticals and anti-inflammatories (including aspirin, paracetamol and non-steroidal anti-inflammatories).
In the multivariate analysis (Table 3), two exposures were associated with a significant increase in S. Brandenburg disease risk: occupational contact with live or dead sheep or lambs during the 3 days prior to illness or interview [odds ratio (OR) 9·79, 95% confidence interval (CI) 1·69–190·38]; and having a household member who had occupational contact with sheep or lamb in the 3 days prior to illness or interview (OR 4·31, 95% CI 1·26–21·33). Collectively these two exposures were able to explain over half (52·6%) of the population-attributable risk for this infection. There was no association between eating sheep meat (lamb, mutton or hogget) in the 3 days before illness or interview and S. Brandenburg infection (OR 0·96, 95% CI 0·35–2·62) after adjusting for other risk factors.
Table 3.
Multivariate analysis of risk factors for S. Brandenburg infection, New Zealand 2002–2003

OR, Odds ratio; CI, confidence interval; PAR, population-attributable risk.
DISCUSSION
This study provides strong evidence that S. Brandenburg has emerged as a directly transmitted zoonotic infection in New Zealand. The spatial and temporal distribution of human cases coincides with a similar epidemic in sheep. The organisms have the same molecular type. Moreover, a national case-control study found that direct or indirect contact with sheep could explain over half of the human cases.
This study has a number of limitations. Surveillance of human salmonellosis typically only detects about a third of cases [9]. Surveillance of animal disease is likely to be even less complete. Once farmers establish that the disease is affecting livestock then they are unlikely to obtain testing on further sick and dead animals. The case-control study has a number of sources of bias and error. Selection bias is potentially important because of the difficulty in fully matching controls by rurality. Information bias is likely because of the lengthy process of case recruitment. Recall of some exposures, particularly foods, is likely to have been far from complete. The use of a 3-day exposure window also introduces a potential source of bias. First, although the incubation period for Salmonella infection is usually listed as 12–72 h, it is highly variable and dose-dependent [10]. A 3-day exposure window would only capture foods eaten frequently or foods that are heavily contaminated with Salmonella. This bias would tend to reduce findings towards the null, i.e. towards there being no measured association between specific exposures and disease risk. Consequently, this study cannot rule out foodborne transmission as making a contribution to S. Brandenburg transmission in New Zealand.
There are few published reports of zoonotic and occupational outbreaks of salmonellosis. Living on a livestock farm has been found to be a risk factor for some forms of salmonellae infection, for example S. Typhimurium definitive phage-type 104 (DT104) [11, 12]. Infection with a range of salmonellae serotypes has been associated with ownership of reptiles in the United States [13]. However, it is unusual to find an outbreak of human infection that so closely mirrors a corresponding outbreak in a single animal species. One reason for the lack of comparable examples is that salmonellosis surveillance in animals is usually far less complete than in humans. In this instance surveillance of infection in animals was only possible because this form of salmonellosis is highly pathogenic for animals and, therefore, was placed under surveillance by animal health authorities. New Zealand has recently witnessed another apparent zoonotic outbreak of salmonellosis caused by DT160 [14]. In that instance wild birds were implicated in its spread. Unlike the S. Brandenburg out-break described here, this outbreak rapidly spread throughout New Zealand rather than remaining confined to a single region.
The origins of this S. Brandenburg outbreak in sheep are unknown. In other countries this serotype has been reported in farmed pigs [15] and birds [16], but rarely in sheep. In humans, this serotype is relatively uncommon internationally [17], and few outbreaks have been previously reported [18, 19]. While abortion is a well documented manifestation of salmonellae infection in sheep, this has traditionally been associated with the serotype Abortusovis which is almost entirely host specific to sheep [20].
Measures to reduce the risk of human infection from S. Brandenburg include reducing infection in sheep, reducing contamination of the farm environment, and hygiene precautions to protect farm workers and their families [2]. Particular attention is needed to reduce the risk of salmonellosis for young children who are likely to have more contact with floors, carpets and other contaminated surfaces [21, 22]. The strong association between disease risk and household contact with a dog in the univariate analysis supports the use of hygiene measures after contact with either working or pet dogs, although this risk factor is also likely to be strongly correlated with household contact with sheep. In response to this outbreak, an existing sheep salmonellae vaccine was reformulated by addition of specific S. Brandenburg antigens. This vaccine was widely used in the southern half of the South Island from 2000 onwards, largely driven by the economic need to protect sheep flocks from death and abortion. Local public health authorities have encouraged farmers to take additional precautions when handling sick and dead sheep. There is evidence that hand washing after contact with animals and contaminated rural environments may help control transmission of salmonellae [23–25].
This study provides good evidence that S. Brandenburg has become an important directly transmitted zoonotic disease in New Zealand. It now represents a risk for farmers and others who have direct occupational contact with infected sheep and family members who have indirect contact with the farming environment. This report provides a further illustration of the importance of animal reservoirs in the emergence of new sources of infectious disease transmission to humans [26].
ACKNOWLEDGEMENTS
We thank the New Zealand Ministry of Agriculture and Forestry for funding this study, D. Duncan of the ESR Enteric Reference Laboratory for salmonellae typing, C. Kliem and M. Eglinton for assistance with data management, N. Russell, K. Ryde and other staff at NFO New Zealand for the interviewing component of this work, public health service staff for informing salmonellosis cases about the study, and Animal Health Laboratories for referring animal isolates to ESR.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Clark RG et al. Salmonella Brandenburg – emergence of a variant strain on a sheep farm in the South Island of New Zealand. New Zealand Veterinary Journal. 2003;51:146–147. doi: 10.1080/00480169.2003.36355. [DOI] [PubMed] [Google Scholar]
- 2.Clark RG et al. Salmonella Brandenburg – emergence of a new strain affecting stock and humans in the South Island of New Zealand. New Zealand Veterinary Journal. 2004;52:26–36. doi: 10.1080/00480169.2004.36387. [DOI] [PubMed] [Google Scholar]
- 3.Clark RG et al. Salmonella in animals in New Zealand: the past to the future. New Zealand Veterinary Journal. 2002;50:57–60. doi: 10.1080/00480169.2002.36269. [DOI] [PubMed] [Google Scholar]
- 4.Clarke R, Tomlinson P. Salmonella Brandenburg: changing patterns of disease in Southland Province, New Zealand. New Zealand Medical Journal. 2004;117:U1144. [PubMed] [Google Scholar]
- 5.Hathaway S, Davies P, Ashby K. Quantitative risk assessment model for Salmonella in sheep meat in New Zealand: Final report of Gore Technical Meeting. Wellington: Ministry of Agriculture and Forestry; 2000 [Google Scholar]
- 6.Popoff MY. Antigenic Formulas of the Salmonella Serovars. 8th. Paris: WHO Collaborating Centre for Reference and Research on Salmonella; 2001. [Google Scholar]
- 7.Barrett TJ et al. Laboratory investigation of a multistate food-borne outbreak of Escherichia coli O157:H7 by using pulsed-field gel electrophoresis and phage typing. Journal of Clinical Microbiology. 1994;32:3013–3017. doi: 10.1128/jcm.32.12.3013-3017.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruzzi P et al. Estimating the population attributable risk for multiple risk factors using case-control data. American Journal of Epidemiology. 1985;122:904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler JG et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. British Medical Journal. 1999;318:1046–1050. doi: 10.1136/bmj.318.7190.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe K et al. Prolonged incubation period of salmonellosis associated with low bacterial doses. Journal of Food Protein. 2004;67:2735–2740. doi: 10.4315/0362-028x-67.12.2735. [DOI] [PubMed] [Google Scholar]
- 11.Dore K et al. Risk factors for Salmonella Typhimurium DT104 and non-DT104 infection: a Canadian multi-provincial case-control study. Epidemiology and Infection. 2004;132:485–493. doi: 10.1017/s0950268803001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fone DL, Barker RM. Associations between human and farm animal infections with Salmonella typhimurium DT104 in Herefordshire. Communicable Disease Report. CDR Review. 1994;4:R136–140. [PubMed] [Google Scholar]
- 13.Mermin J et al. Reptiles, amphibians, and human Salmonella infection: a population-based, case-control study. Clinical Infectious Diseases. 2004;38:S253–261. doi: 10.1086/381594. (Suppl 3): [DOI] [PubMed] [Google Scholar]
- 14.Thornley CN et al. First incursion of Salmonella enterica serotype Typhimurium DT160 into New Zealand. Emerging Infectious Diseases. 2003;9:493–495. doi: 10.3201/eid0904.020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korsak N et al. Salmonella contamination of pigs and pork in an integrated pig production system. Journal of Food Protein. 2003;66:1126–1133. doi: 10.4315/0362-028x-66.7.1126. [DOI] [PubMed] [Google Scholar]
- 16.Reche MP et al. Incidence of salmonellae in captive and wild free-living raptorial birds in central Spain. Journal of Veterinary Medicine. B, Infectious Diseases and Veterinary Public Health. 2003;50:42–44. doi: 10.1046/j.1439-0450.2003.00623.x. [DOI] [PubMed] [Google Scholar]
- 17.Herikstad H, Motarjemi Y, Tauxe RV. Salmonella surveillance: a global survey of public health serotyping. Epidemiology and Infection. 2002;129:1–8. doi: 10.1017/s0950268802006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baquar N, Burnens A, Stanley J. Comparative evaluation of molecular typing of strains from a national epidemic due to Salmonella brandenburg by rRNA gene and IS200 probes and pulsed-field gel electrophoresis. Journal of Clinical Microbiology. 1994;32:1876–1880. doi: 10.1128/jcm.32.8.1876-1880.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada K, Tsuji H. Salmonella Brandenburg and S. Corvallis involved in a food poisoning outbreak in a hospital in Hyogo Prefecture. Japanese Journal of Infectious Diseases. 2001;54:195–196. [PubMed] [Google Scholar]
- 20.Uzzau S et al. Host adapted serotypes of Salmonella enterica. Epidemiology and Infection. 2000;125:229. doi: 10.1017/s0950268899004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schutze GE et al. The home environment and salmonellosis in children. Pediatrics. 1999;103:E1. doi: 10.1542/peds.103.1.e1. [DOI] [PubMed] [Google Scholar]
- 22.Rice DH et al. Household contamination with Salmonella enterica. Emerging Infectious Diseases. 2003;9:120–122. doi: 10.3201/eid0901.020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman CR et al. An outbreak of salmonellosis among children attending a reptile exhibit at a zoo. Journal of Pediatrics. 1998;132:802–807. doi: 10.1016/s0022-3476(98)70307-5. [DOI] [PubMed] [Google Scholar]
- Outbreaks of Escherichia coli O157:H7 associated with petting zoos – North Carolina, Florida, and Arizona, 2004 and 2005. Morbidity and Mortality Weekly Report. 2005;54:1277–1280. [PubMed] [Google Scholar]
- 25.Crump JA et al. Outbreaks of Escherichia coli O157 infections at multiple county agricultural fairs: a hazard of mixing cattle, concession stands and children. Epidemiology and Infection. 2003;131:1055–1062. doi: 10.1017/s0950268803001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marano N, Pappaioanou M. Historical, new, and reemerging links between human and animal health. Emerging Infectious Diseases. 2004;10:2065–2066. doi: 10.3201/eid1012.041037. [DOI] [PMC free article] [PubMed] [Google Scholar]