SUMMARY
Active surveillance for laboratory-confirmed Salmonella serotype Enteritidis (SE) infection revealed a decline in incidence in the 1990s, followed by an increase starting in 2000. We sought to determine if the fluctuation in SE incidence could be explained by changes in foodborne sources of infection. We conducted a population-based case-control study of sporadic SE infection in five of the Foodborne Diseases Active Surveillance Network (FoodNet) sites during a 12-month period in 2002–2003. A total of 218 cases and 742 controls were enrolled. Sixty-seven (31%) of the 218 case-patients and six (1%) of the 742 controls reported travel outside the United States during the 5 days before the case's illness onset (OR 53, 95% CI 23–125). Eighty-one percent of cases with SE phage type 4 travelled internationally. Among persons who did not travel internationally, eating chicken prepared outside the home and undercooked eggs inside the home were associated with SE infections. Contact with birds and reptiles was also associated with SE infections. This study supports the findings of previous case-control studies and identifies risk factors associated with specific phage types and molecular subtypes.
INTRODUCTION
Salmonella causes an estimated 1·4 million infections, 15 000 hospitalizations and 400 deaths each year in the United States [1]. Salmonella serotype Enteritidis (SE) accounts for approximately one-fifth of all human Salmonella isolates reported to the Centers for Disease Control and Prevention (CDC) each year [2, 3]. Active surveillance for laboratory-confirmed SE infections is conducted by the Foodborne Diseases Active Surveillance Network (FoodNet). The incidence of laboratory-confirmed SE infections declined by 39% in FoodNet sites from 1996 to 1999 [4] but increased by 26% from 2000 to 2002 [5]. SE is also a common cause of foodborne outbreaks [6]. Most foodborne outbreaks of SE infection for which a vehicle is identified are associated with eating raw or undercooked eggs or foods containing undercooked eggs. Of 371 SE outbreaks reported to CDC between 1985 and 1999, in which a vehicle of transmission was identified, 80% were egg-associated [7].
Efforts to mitigate the burden of SE illness rely on understanding the source of SE infections. In 1996–1997, FoodNet conducted a population-based case-control study of SE infections to determine sources of sporadic illness [8]. This study found that travel outside the United States and eating chicken outside the home in the 5 days before illness onset were associated with infections; eating eggs in the 5 days before illness onset was not associated with sporadic infection. Although eggs have been a frequent source of SE outbreaks in Denmark, a case-control study of sporadic SE infections in 1997–1999 also failed to associate eating eggs with illness when exposure in the 5 days before illness onset was examined [9]. However, when exposure in the 1 day before illness onset was examined, eating eggs was strongly associated with illness.
To evaluate exposures associated with SE infection during this period of fluctuation in the SE incidence, and explore the impact of using a narrow exposure period, we conducted a second FoodNet population-based case-control study of sporadic SE infection in 2002–2003, that included both 1-day and 5-day exposure windows.
METHODS
Initiated in 1996 as part of the CDC's Emerging Infections Program, FoodNet is a collaboration between CDC, the United States Department of Agriculture's (USDA) Food Safety and Inspection Service, the United States Food and Drug Administration (FDA), and state health departments that conduct active surveillance and epidemiological studies of foodborne infections [10]. A 12-month case-control study of sporadic SE infection was conducted in 2002–2003 in five FoodNet sites (Connecticut, Minnesota, Tennessee and selected counties in Colorado and New York) representing a population of 17 million persons (6% of the US population).
Each month, FoodNet epidemiologists contact clinical laboratories in the participating sites to ascertain laboratory-confirmed Salmonella cases. Case-patients were eligible for enrolment if they were aged ⩾1 year, resided in the FoodNet catchment area and had SE isolated from stool or a normally sterile site (including blood, urine, or cerebral spinal fluid). Case-patients aged <1 year were enrolled in another FoodNet case-control study. Case-patients were excluded from enrolment if they could not be reached by FoodNet epidemiologists after 15 telephone attempts or within 45 days of specimen collection date, if they did not have a telephone number available, if they did not speak English or Spanish, if they did not report diarrhoea, if another household member reported diarrhoea before the case-patient's onset, or if they were part of an outbreak that was investigated by a health department and a vehicle of transmission was identified.
Controls were persons aged ⩾1 year living in the participating FoodNet sites identified using a two-stage random-digit dialling methodology similar to that used in CDC's Behavioral Risk Factor Surveillance Surveys (BRFSS) [11]. Control interviews were conducted by a professional telephone survey company [12]. At least 10 controls per month were interviewed in each state for the 12-month study period. Persons were excluded from being controls if they did not speak English or Spanish, or if they had diarrhoea within the previous 30 days.
The case-patient and control questionnaires asked about food and environmental exposures, demographics, and medical history using a standardized questionnaire. Case-patients were also asked questions about their illness. For food exposures, we asked case-patients about the 5 days and the 1 day prior to the case's illness onset date; controls were asked about the 5 days and 1 day prior to interview date. Verbal informed consent was obtained prior to beginning the interview from cases and controls aged ⩾18 years and from parents of children aged <18 years. The study was approved by the Human Subjects Research Committees at CDC and the participating sites.
Laboratory testing
Clinical laboratories, which perform primary isolation for Salmonella, forwarded Salmonella isolates to the appropriate state public health laboratory for confirmation and serotyping. Serotyping was conducted using the Kauffman–White scheme [13]. SE isolates were forwarded to CDC for phage typing. Phage typing was conducted using 16 phages obtained from the United Kingdom's Health Protection Agency (HPA). Phage typing followed HPA scheme with a modification of the recommended test dilution to achieve expected lysis results on control strains (PT1b, 3, 6a, 11b, 14 and 24). Three provisional phage types (PT911, 912, 913) assigned by the Health Protection Agency of Canada were used in addition to types designated by the HPA scheme [14, 15]. Molecular subtyping of isolates was performed by pulsed-field gel electrophoresis (PFGE) using the restriction enzyme XbaI [16] according to protocols of the National Molecular Subtyping Network for Foodborne Disease Surveillance (PulseNet) for Salmonella [17].
Statistical analysis
The association between study variables and SE infection was first explored using bivariate analysis. Multivariable logistic regression models were then used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for variables significant in bivariate analysis and variables of a priori interest based on previous studies of SE infection. All multivariable models included variables for FoodNet site and categorical age group (1–4, 5–17, 18–64, and ⩾65 years).
Egg exposures were defined by the type of egg preparation (i.e. scrambled, fried, boiled, or poached) and where the eggs were eaten (i.e. in the home or outside the home). ‘Runny eggs outside the home’ included the four types of egg preparation. ‘Undercooked eggs consumed inside the home’ included any runny eggs and any homemade products that usually contain raw eggs (e.g. uncooked cookie dough, homemade Caesar salad dressing, homemade mayonnaise, homemade ice-cream or custard containing raw eggs). The final multivariable models included nested variable coding to contrast exposures among subsets of the study population. For example, the exposure ‘ate runny eggs outside the home’ was a subset of the population who ‘ate eggs outside the home’. The estimated OR for eating runny eggs outside the home only applies to persons who consumed eggs outside the home.
To estimate the risk of disease by SE phage type, related phage types (PT1 and PT1B, PT4 and PT4B, PT6 and PT6A, PT8 and PT8A, PT13 and PT13A) were combined into phage-type complexes and multivariable polytomous regression models were created to assess risk factors for phage-type complexes, adjusting for region. Because of sparse data for some phage types, FoodNet sites were combined into mid-western (Colorado and Minnesota) and eastern (Connecticut, New York, and Tennessee) regions of the United States. We used the final multivariable model and restricted the population to the two most common phage types and to cases and controls that did not travel outside of the United States (domestic cases). We further restricted the population to the most common PFGE patterns within these two phage types.
The strength of the associations between exposures and SE illness for both a 5-day and 1-day exposure period was estimated. We calculated the population attributable fraction (PAF) for each exposure in the study population based on the OR in the final multivariable model, using the method of Bruzzi [18]. For the nested variables we calculated an overall PAF. The overall PAF applies to all cases in the study, regardless of their exposure to the parent variable. Data were analysed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
RESULTS
Enrolment
During the study period, 2347 cases of non-typhoidal Salmonella infection were ascertained by the five FoodNet sites. Of these, 340 (14%) were caused by SE; 327 (96%) of which were aged ⩾1 year old and were eligible for the case-control study. Of the 327 eligible case-patients, 218 (67%) were enrolled. Sixty-seven percent of the enrolled cases were interviewed within 21 days of specimen collection. Of the 109 non-enrolled case-patients: 41 (38%) were unreachable, 21 (19%) refused, 20 (18%) were outbreak-associated, 10 (9%) were secondary cases in a household, seven (6%) did not speak English or Spanish, seven (6%) had no diarrhoea, and three (3%) were unable to answer questions. The 20 outbreak-associated cases represented two outbreaks. Non-enrolled cases did not differ significantly from enrolled cases by gender, age, month in which the specimen was isolated, specimen source, or outcome (hospitalization or death).
Study personnel attempted 7350 telephone calls, successfully enrolling 742 controls. The response rate, calculated according to standards of the Council of American Survey Research Organizations, was 25% with an upper bound of 30% and a lower bound of 10% [19].
Clinical characteristics
Of the 218 enrolled case-patients, 182 (83%) reported abdominal pain, 178 (82%) had fever, 91 (42%) had bloody diarrhoea, 83 (38%) were given intravenous fluids, and 39 (18%) were hospitalized. Of the 218 enrolled case-patients, 151 (69%) were treated with an antibiotic after symptom onset; of the 136 case-patients who recalled the name of the antibiotic, 95 (70%) received a fluoroquinolone.
Demographic characteristics
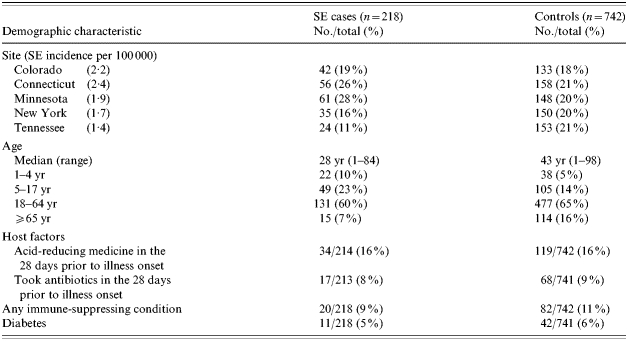
Enrolment varied by FoodNet site with over half of cases enrolled from Minnesota and Connecticut (Table 1). This variation reflects the incidence of laboratory-confirmed SE infections in these sites during the study period (incidence ranged from 1·4/100 000 population in Tennessee to 2·4/100 000 in Connecticut). The median age of case-patients (28 years) was less than the median age of controls (43 years) (P<0·001). Fifty-one percent of case-patients were female compared with 59% of controls (P=0·02). No differences in race or ethnicity were observed. No host factors were associated with illness (Table 1).
Table 1.
Demographic characteristics of Salmonella Enteritidis (SE) cases and controls, FoodNet sites 2002–2003 (including travellers)
Risk factor analysis
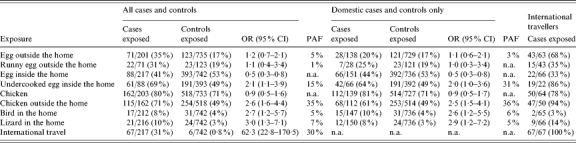
Of 218 enrolled case-patients, 67 (31%) reported travel outside the United States in the 5 days before illness onset compared with six (1%) controls (OR 53·4, 95% CI 22·7–125·3). In multivariable analysis, adjusting for site and age group, travel outside the United States was associated with the greatest risk (OR 62·3, 95% CI 22·8–170·5).
Eating undercooked eggs inside the home in the 5 days before illness onset, among those who ate eggs inside the home (OR 2·1, 95% CI 1·1–3·9), and eating chicken outside the home in the 5 days before illness onset, among those who ate chicken (OR 2·6, 95% CI 1·6–4·4) were independently associated with illness in the final multivariable model. Additionally, having a pet bird (OR 2·7, 95% CI 1·2–5·7) or lizard (OR 3·0, 95% CI 1·3–7·1) in the home in the 5 days before illness onset was associated with illness (Table 2). Multivariable analysis restricted to the 151 cases who did not report travel outside the United States (domestic cases) produced results similar to the analysis of all cases (Table 2).
Table 2.
Multivariable analysis of exposures, adjusted for age and state, in the 5 days before diarrhoea onset due to Salmonella Enteritidis infection, FoodNet sites 2002–2003
OR, Odds ratio; CI, confidence interval; PAF, population attributable fraction; n.a., not applicable.
For the domestic cases (i.e. those who did not travel outside the United States), using a 1-day exposure period instead of a 5-day period, the CIs for the exposures in the multivariable models were less precise and some variables were no longer significant, including eating undercooked eggs inside the home, among those who ate eggs (OR 0·8, 95% CI 0·3–2·1). However, eating chicken outside the home, among those consuming chicken (OR 3·6, 95% CI 1·5–8·5), and having a lizard in the home (OR 3·5, 95% CI 1·5–8·4) remained associated with illness.
Isolate subtype analysis
SE isolates were available for phage typing from 211 (97%) of the 218 cases. The most common phage type complexes were PT8 (n=61, 29%; 60 PT8 and 1 PT8A), PT13 (57, 27%; 42 PT13 and 15 PT13A), and PT4 (31, 15%; 29 PT4 and 2 PT4B). Travel outside the United States in the 5 days before illness onset was reported by 81% of 31 persons infected with PT4 complex (83% of PT4 and 50% of PT4B), 7% of 61 persons infected with PT8 complex (7% of PT8 and 0% of PT8A), and 2% of 57 persons infected with PT13 complex (2% of PT13 and 0% of PT13A).
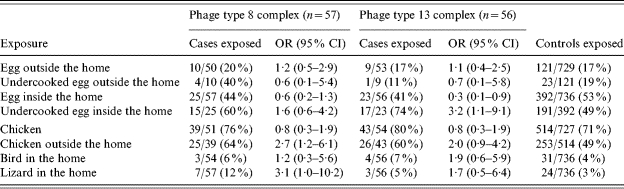
Among domestic cases, eating chicken outside the home in the 5 days before illness onset, among those who ate chicken (OR 2·7, 95% CI 1·2–6·1) and having a lizard in the home (OR 3·1, 95% CI 1·0–10·2) were associated with PT8 complex SE infection. Additionally, among domestic cases, eating undercooked eggs inside the home in the 5 days before illness onset, among those who ate eggs inside the home (OR 3·2, 95% CI 1·1–9·1) was associated with PT13 complex SE infection (Table 3).
Table 3.
Polytomous multivariable logistic analysis adjusted for age and region for domestic Salmonella Enteritidis cases by phage types, FoodNet sites 2002–2003
OR, Odds ratio; CI, confidence interval.
Among the 57 domestic PT8 cases, 54 (95%; 53 PT8 and 1 PT8A) were PFGE Pattern A (CDC JEGX01.0004). When restricting the analysis to PT8 cases with PFGE Pattern A, eating chicken outside the home in the 5 days before illness onset, among those who ate chicken (OR 2·5, 95% CI 1·1, 5·6) remained associated with illness and having a lizard in the home did not. Among the 56 domestic PT13 cases, 33 (59%) were PFGE Pattern B (CDC JEGX01.0005). When restricting the analysis to PT13 cases with PFGE Pattern B, eating undercooked eggs inside the home in the 5 days before illness onset, among those who ate eggs inside the home (OR 7·0, 95% CI 1·5, 32·6) and eating chicken outside the home in the 5 days before illness onset, among those who ate chicken (OR 3·6, 95% CI 1·2, 11·5) were more strongly associated with illness.
DISCUSSION
SE remains an important cause of human illness in the United States. We found however, that an important risk factor for SE infection was international travel; 31% of cases in our study were attributed to international travel. Previous case-control studies have identified international travel as an important risk factor for sporadic SE infections. SE is the most common Salmonella serotype identified throughout the world [20, 21]. Contaminated eggs and poultry have been identified as the source of human SE infections in several countries [9, 22–29]. Travellers are well advised to avoid dishes with raw or undercooked eggs.
Among cases who did not travel and ate eggs, eating undercooked eggs inside the home was a risk for SE infection. National efforts to reduce the risk of human SE infection due to eating SE-contaminated eggs have included diversion of contaminated eggs to pasteurization [30], labelling and refrigeration of shell eggs [31], on-farm prevention measures [32], egg quality assurance programmes [33], and educational programmes for consumers and food-service personnel. In 2004, an FDA-proposed rule to improve shell egg safety included interventions aimed at reducing SE prevalence in poultry egg-laying houses [32]. Egg quality assurance programmes containing preventive measures similar to those in the proposed rule, appear to have resulted in a lower incidence of human SE infection in areas where they have been implemented [33].
We also found that SE infection was associated with eating chicken outside the home in the 5 days before illness onset. These findings are not unexpected; previous case-control studies have identified eating chicken as an important risk factor for SE infection [8]. Furthermore, 12·8% of chickens sampled in slaughter plants in the Food Safety and Inspection Service (FSIS) Pathogen Reduction-Hazard Analysis Critical Control Point (PR/HACCP) Verification Testing Program were contaminated with Salmonella in 2003 [29]. Of the Salmonella-positive samples in 2003, 3·5% were SE (FSIS, unpublished data). In 2002, the CDC's National Antimicrobial Resistance Monitoring System (NARMS) Retail Food Study identified 60 Salmonella isolates from chicken breasts; of these, eight (13%) were SE [34]. These data support our finding that chicken is a source of SE infection in the United States. Regulations for HACCP systems and pathogen reduction have been implemented by FSIS to reduce the contamination of poultry products with Salmonella [35]. Efforts need to be enhanced to decrease the presence of Salmonella on chickens sold at retail and to improve poultry handling by restaurant food workers and consumers.
Among domestic cases in our study the most common phage types were PT8 and PT13. PT8 and PT13 have commonly been identified among SE isolates obtained from broilers and egg product samples in the United States (FSIS, unpublished data). In our study, SE infection with PT8 and PT13 was associated with eating chicken or eggs, respectively, in the 5 days before illness onset. Further subtyping of SE isolates by PFGE strengthened the association between SE infection and eggs and chicken, with a loss of precision due to small sample size. This finding suggests a specific relationship between some subtypes of SE and certain exposures, and demonstrates the added value of combining phage-type and PFGE data.
SE infection was also associated with having a bird or reptile in the home. Pet birds are known to harbour Salmonella spp., and close contact with fowl can transmit infection [36, 37]. Reptiles, including lizards and iguanas, have been associated with outbreaks of SE infections [38] and sporadic cases [39, 40]. Continued prevention efforts to educate the public about the risks from contact with these animals are needed [39, 41].
In this study we collected information on both a 5-day and 1-day exposure window. In a case-control study in Denmark association with eggs strengthened substantially if persons with SE were asked about exposures in the 1 day before illness onset, rather than in the 5 days before onset [9]. We also found that using a shorter exposure window increased the magnitude of association between SE infection and eggs, although the general conclusions did not change. Such analysis may be useful when studying exposures that are common.
In this case-control study, controls were selected using a random digit-dialling scheme commonly used by the CDC BRFSS. A limitation to this method is that cases and controls were interviewed by different groups of interviewers; however, to minimize interviewer bias, we used a standardized questionnaire and protocol for both case and control interviews and interviewers were unaware of the study hypothesis. Although the cases were not matched to the controls by demographic factors other than catchment area, they were similar in most characteristics except age group which was controlled for in the analysis. Case-patients were included in the study if interviewed within 45 days of the specimen collection date. While recall bias is an important consideration, two-thirds of case-patients were interviewed within 21 days and are likely to have good recall on exposures in the days preceding their illness. In addition, there was no association between the length of time between specimen collection and interview date and the number of unknown or missing responses.
A purpose of this study was to reassess the exposures leading to SE infection in the United States. The results are similar to the previous FoodNet case-control study [8]. Both identified international travel and eating chicken outside the home as risk factors. Eggs continue to be an important source as well. Although not assessed in our study, the previous study found that cases ate fewer meals prepared at home and they had lower dietary diversity (computed as the number of different food items consumed during the exposure period).
We excluded cases of SE associated with outbreaks. During the study period there were two outbreaks; one involving five cases identified fried rice with undercooked egg served at a private gathering outside the home. The other involving 15 cases suspected salsa served in a restaurant as the vehicle, although this was not confirmed epidemiologically or microbiologically. The outbreak suspected to be due to salsa was believed to be caused by contaminated cilantro included in the salsa. Recent outbreaks of Salmonella have also been linked to contaminated produce [42], including cilantro [43]. The FDA has developed a plan to decrease foodborne illness associated with fresh produce [44].
In this multisite case-control study, we provide further evidence that travel outside the United States, eating undercooked eggs, and eating chicken are the primary risk factors for SE infection. Among domestically acquired cases, consuming chicken outside the home, consuming undercooked eggs in the home, and contact with a bird or reptile in the home were all significant risk factors for infection. Efforts to reduce SE infection have focused on implementation of farm-to-table measures directed at egg producers, consumers, and food-service personnel. Prevention efforts need to be enhanced which focus on the live bird reservoir, poultry processing plants, food service workers, and consumers. Additional efforts are needed to prevent non-foodborne routes of infection especially by exposures to pet birds and reptiles [45].
ACKNOWLEDGEMENTS
The authors thank Rafiq Ahmed, National Laboratory for Enteric Pathogens, Health Canada; Nicole Ishill, Foodborne and Diarrheal Diseases Branch, CDC; George Maldonado, University of Minnesota; Susan Van Duyne, Foodborne and Diarrheal Diseases Branch, CDC.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Voetsch AC et al. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clinical Infectious Diseases. 2004;38:S127–S134. doi: 10.1086/381578. (Suppl. 3): [DOI] [PubMed] [Google Scholar]
- 2.CDC. Salmonella surveillance summary, 2002. US Department of Health and Human Services, CDC; 2003. [Google Scholar]
- 3.Olsen SJ et al. The changing epidemiology of Salmonella: trends in serotypes isolated from humans in the United States, 1987–1997. Journal of Infectious Diseases. 2001;183:753–761. doi: 10.1086/318832. [DOI] [PubMed] [Google Scholar]
- 4.Marcus R et al. Dramatic decrease in the incidence of Salmonella serotype Enteritidis infections in 5 FoodNet sites: 1996–1999. Clinical Infectious Diseases. 2004;38:S135–S141. doi: 10.1086/381579. (Suppl. 3): [DOI] [PubMed] [Google Scholar]
- 5.CDC. Preliminary FoodNet data on the incidence of foodborne illnesses – selected sites, United States, 2002. Morbidity and Mortality Weekly Report. 2003;52:340–343. [PubMed] [Google Scholar]
- 6.Olsen SJ et al. Surveillance for foodborne-disease outbreaks – United States, 1993–1997. Morbidity and Mortality Weekly Report. CDC Surveillance Summaries. 2000;49:1–62. [PubMed] [Google Scholar]
- 7.Patrick ME et al. Salmonella enteritidis infections, United States, 1985–1999. Emerging Infectious Diseases. 2004;10:1–7. doi: 10.3201/eid1001.020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura AC et al. Chicken consumption is a newly identified risk factor for sporadic Salmonella enterica serotype Enteritidis infections in the United States: a case-control study in FoodNet sites. Clinical Infectious Diseases. 2004;38:S244–S252. doi: 10.1086/381576. (Suppl. 3): [DOI] [PubMed] [Google Scholar]
- 9.Molbak K, Neimann J. Risk factors for sporadic infection with Salmonella enteritidis, Denmark, 1997–1999. American Journal of Epidemiology. 2002;156:654–661. doi: 10.1093/aje/kwf096. [DOI] [PubMed] [Google Scholar]
- 10.Allos BM et al. Surveillance for sporadic foodborne disease in the 21st century: the FoodNet perspective. Clinical Infectious Diseases. 2004;38:S115–S120. doi: 10.1086/381577. (Suppl. 3): [DOI] [PubMed] [Google Scholar]
- 11.CDC. Behavioral risk factor surveillance system user's guide. US Department of Health and Human Services, CDC; 1998. [Google Scholar]
- 12.Imhoff B et al. Burden of self-reported acute diarrheal illness in FoodNet surveillance areas, 1998–1999. Clinical Infectious Diseases. 2004;38:S219–S226. doi: 10.1086/381590. (Suppl. 3): [DOI] [PubMed] [Google Scholar]
- 13.Popoff M. Antigenic Formulas of the Salmonella Serovars. 8th edn. WHO Collaborating Centre for Reference and Research on Salmonella. Pasteur Institute; Paris, France: 2001. [Google Scholar]
- 14.Hickman-Brenner FW, Stubbs AD, Farmer 3rd JJ. Phage typing of Salmonella enteritidis in the United States. Journal of Clinical Microbiology. 1991;29:2817–2823. doi: 10.1128/jcm.29.12.2817-2823.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward LR, de Sa JDH, Rowe B. A phage-typing scheme for Salmonella enteritidis. Epidemiology and Infection. 1987;99:291–294. doi: 10.1017/s0950268800067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. Standardized molecular subtyping of foodborne bacterial pathogens by pulsed-field gel electrophoresis. Centers for Disease Control and Prevention; Atlanta, GA: 2003. [Google Scholar]
- 17.Swaminathan B et al. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerging Infectious Diseases. 2001;7:382–389. doi: 10.3201/eid0703.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruzzi P et al. Estimating the population attributable risk for multiple risk factors using case-control data. American Journal of Epidemiology. 1985;122:904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 19.Council of American Survey Research Organizations. http://www.casro.org/resprates.cfm. http://www.casro.org/resprates.cfm ). Accessed 24 January 2006.
- Galanis E et al. Web-based surveillance and global Salmonella distribution, 2000–2002. Emerging Infectious Diseases. 2006;12:381–388. doi: 10.3201/eid1203.050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herikstad H, Motarjemi Y, Tauxe RV. Salmonella surveillance: a global survey of public health serotyping. Epidemiology and Infection. 2002;129:1–8. doi: 10.1017/s0950268802006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Indar-Harrinauth L et al. Emergence of Salmonella enteritidis phage type 4 in the Caribbean: case-control study in Trinidad and Tobago, West Indies. Clinical Infectious Diseases. 2001;32:890–896. doi: 10.1086/319344. [DOI] [PubMed] [Google Scholar]
- 22.Delarocque-Astagneau E et al. Risk factors for the occurrence of sporadic Salmonella enterica serotype enteritidis infections in children in France: a national case-control study. Epidemiology and Infection. 1998;121:561–567. doi: 10.1017/s0950268898001460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC. Outbreaks of Salmonella serotype enteritidis infection associated with consumption of raw shell eggs – United States, 1994–1995. Morbidity and Mortality Weekly Report. 1996;45:737–742. [PubMed] [Google Scholar]
- 24.CDC. Outbreaks of Salmonella serotype enteritidis infection associated with eating shell eggs – United States, 1999–2001. Morbidity and Mortality Weekly Report. 2003;51:1149–1152. [PubMed] [Google Scholar]
- 25.Hedberg CW et al. Role of egg consumption in sporadic Salmonella enteritidis and Salmonella typhimurium infections in Minnesota. Journal of Infectious Diseases. 1993;167:107–111. doi: 10.1093/infdis/167.1.107. [DOI] [PubMed] [Google Scholar]
- 26.Morse DL et al. Outbreak and sporadic egg-associated cases of Salmonella enteritidis: New York's experience. American Journal of Public Health. 1994;84:859–860. doi: 10.2105/ajph.84.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trepka MJ et al. An increase in sporadic and outbreak-associated Salmonella enteritidis infections in Wisconsin: the role of eggs. Journal of Infectious Diseases. 1999;180:1214–1219. doi: 10.1086/314984. [DOI] [PubMed] [Google Scholar]
- 28.USDA. FSIS progress report on Salmonella testing of raw meat and poultry products, 1998–2003. USDA, FSIS; Washington, DC: 2004. [Google Scholar]
- 29.Presidential Council on Food Safety . Egg safety from production to consumption: an action plan to eliminate Salmonella Enteritidis illnesses due to eggs. President's Council on Food Safety; Washington, DC, 10 December, 1999 [Google Scholar]
- 30.FDA. Food labeling, safe handling statements, labeling of shell eggs; refrigeration of shell eggs held for retail distribution. Food and Drug Administration, HHS. Final rule. Federal Register. 2000;65:76092–76114. [PubMed] [Google Scholar]
- 31.FDA. Proposed rule: prevention of Salmonella enteritidis in shell eggs during production. Federal Register. 2004;69:60108–60110. [PubMed] [Google Scholar]
- 32.Mumma GA et al. Egg quality assurance programs and egg-associated Salmonella enteritidis infections, United States. Emerging Infectious Diseases. 2004;10:1782–1789. doi: 10.3201/eid1010.040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FDA, Center for Veterinary Medicine 2002. . National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): NARMS Retail Meat Annual Report, . Rockville, MD: U.S. Department of Health and Human Services, FDA, 2004.
- 34.USDA. Pathogen reduction/hazard analysis and critical control point (HACCP) U.S. Department of Agriculture [Final Rule] Federal Register. 1996;61:38805–38989. [Google Scholar]
- 35.CDC. Salmonella hadar associated with pet ducklings – Connecticut, Maryland, and Pennsylvania, 1991. Morbidity and Mortality Weekly Report. 1992;41:185–187. [PubMed] [Google Scholar]
- 36.CDC. Salmonellosis associated with chicks and ducklings – Michigan and Missouri, Spring 1999. Morbidity and Mortality Weekly Report. 2000;49:297–299. [PubMed] [Google Scholar]
- 37.Friedman C et al. An outbreak of salmonellosis among children attending a reptile exhibit at a zoo. Journal of Pediatrics. 1998;132:802–807. doi: 10.1016/s0022-3476(98)70307-5. [DOI] [PubMed] [Google Scholar]
- 38.CDC. Reptile-associated salmonellosis – selected states, 1998–2002. Morbidity and Mortality Weekly Report. 2003;52:1206–1209. [PubMed] [Google Scholar]
- 39.Mermin J et al. Reptiles, amphibians, and human Salmonella infection: a population-based, case-control study. Clinical Infectious Diseases. 2004;38:S253–S261. doi: 10.1086/381594. (Suppl. 3): [DOI] [PubMed] [Google Scholar]
- 40.Bradley T, Angulo FJ, Mitchell M. Public health education on Salmonella spp. and reptiles. Journal of the American Veterinary Association. 2001;219:754–755. doi: 10.2460/javma.2001.219.754. [DOI] [PubMed] [Google Scholar]
- 41.CDC. Outbreaks of Salmonella infections associated with eating roma tomatoes – United States and Canada, 2004. Morbidity and Mortality Weekly Report. 2005;54:325–328. [PubMed] [Google Scholar]
- 42.Campbell JV et al. An outbreak of Salmonella serotype Thompson associated with fresh cilantro. Journal of Infectious Diseases. 2001;183:984–987. doi: 10.1086/319254. [DOI] [PubMed] [Google Scholar]
- 43.FDA http://www.cfsan.fda.gov/~dms/prodpla2.html. http://www.cfsan.fda.gov/~dms/prodpla2.html . Produce safety from production to consumption: 2004 action plan to minimize foodborne illness associated with fresh produce consumption ( ). Accessed 15 April 2005.
- 44.CDC. Compendium of measures to prevent disease associated with animals in public settings, 2005. National Association of State Public Health Veterinarians, Inc. (NASPHV) Morbidity and Mortality Weekly Report. 2005;54:1–20. [PubMed] [Google Scholar]


