SUMMARY
Notifications of campylobacteriosis by New Zealand medical practitioners have increased steadily in the last two decades. To determine if this increase is real, as opposed to a surveillance artefact, we examined both available notification (1980–2003) and hospitalization data (1995–2003). The similarity in the temporal pattern of increasing hospitalizations for campylobacteriosis, with that of notifications, is suggestive that this increase is indeed real. Although some risk factors for this disease have been identified (e.g. uncooked poultry consumption) it is unclear what the likely causes of the increasing rates are. The overall disease burden is also high compared with other developed countries (an annual notification rate of 396 cases per 100 000 population in 2003), with highest rates in children aged 1–4 years, males, Europeans, and those living in urban areas. Given the large disease burden, further research and intervention studies should be public health priorities in this country.
INTRODUCTION
The incidence of reported campylobacteriosis has risen steadily in New Zealand since this disease first became notifiable in 1980. The cause of this increase is unknown and it is possible that it might be artefactual. For example, there may have been changes in the extent to which diagnosed disease is reported to public health authorities and hence to the national notifiable disease surveillance system. One previous New Zealand study surveyed laboratories and concluded that changes in laboratory methodologies (at least in the early 1990s) did not appear to account for the national increase in notifications [1]. To explore this issue of increasing campylobacteriosis notifications further, we analysed both notification and hospitalization data at the national level.
METHODS
Data from the national notifiable disease surveillance system was analysed for the period 1980–2003. Campylobacter infection has been legally notifiable by diagnosing medical practitioners since 1980 in New Zealand. These data are collated by the Institute of Environmental Science and Research Ltd (ESR) under contract to the Ministry of Health. In addition, data on campylobacteriosis hospitalization (primary diagnosis code) for 1995–2003 were obtained from the New Zealand Health Information Service (part of the Ministry of Health). This condition has been separately coded as a cause of hospital admission since 1995 (ICD9CM code 008.43 from 1995 and ICD10AM code A04.5 since 1998–1999).
The 3-year period 2001–2003 was analysed in detail. Also, to examine the potential role of environmental sources, notified and hospitalized cases for the 2001–2003 period were designated as ‘urban’ (settlements of ⩾1000 people) or ‘rural’ based on the Statistics New Zealand classification of the area unit in which they resided. At the 2001 Census 85·7% of the population was classified as ‘urban’, and 14·3% as ‘rural’.
The analyses were carried out in SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Rates were calculated using population data from the 2001 census. Rates for ethnic groups were directly age standardized to the age distribution of the New Zealand population in 2001. Rate ratios (RRs) and 95% confidence intervals (CIs) were calculated. Trends over time were tested using the χ2 test for trend. Trends in notification and hospitalization rates over time and across geographic areas (district health board districts) were compared using linear regression analysis.
RESULTS
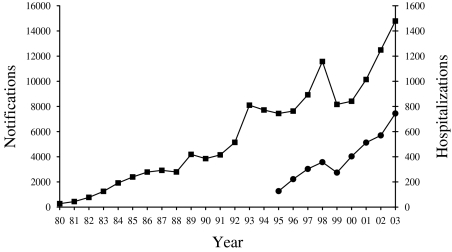
The incidence of notified campylobacteriosis has risen steadily in New Zealand since this disease first became notifiable in 1980 (Fig. 1). The annual incidence reached a new peak of 14 790 cases in 2003 (395·6/100 000).
Fig. 1.
Campylobacteriosis cases in New Zealand by year, based on notifications (–■–, 1980–2003) and hospitalizations (–●–, 1995–2003).
There were 744 hospitalizations attributable primarily to campylobacteriosis in 2003. This was 5·0% of the number of cases notified in that year. Hospitalized cases more than tripled from 1996 to 2003, which was even more than the relative increase in notifications over that period (nearly double). Furthermore, the incidence in notifications and hospitalizations both dipped in the same year (1999) and both increased in every other year over the 1995–2003 period (Fig. 1). During this period, notification and hospitalization rates were highly correlated (R 2=0·70) and showed a significant increase (χ2 test for trend P<0·0001). The analysis of data for the period 2001–2003 indicated seasonal variation in notification rates with 32·0% of notified cases occurring over the summer period (December–February in New Zealand) and only 21·2% occurring in winter (June–August). A similar distribution was seen for hospitalizations with 32·0% of cases over summer and 21·4% over winter (Table).
Table.
Campylobacteriosis notification and hospitalization numbers and rates (average annual rate per 100 000), by season, rural–urban domicile, age group, sex, and ethnicity, 2001–2003, New Zealand
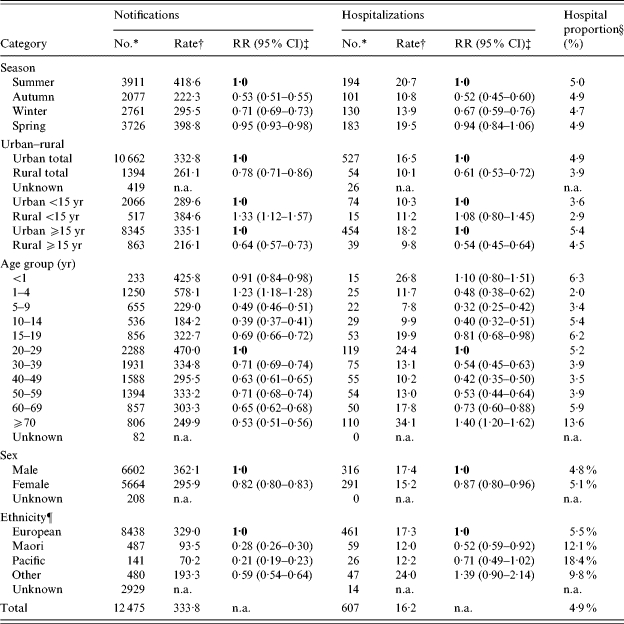
n.a., not applicable.
Number is the average annual number rounded to the nearest integer.
Rate is the average annual rate per 100 000 population.
RR, Rate ratio calculated in relation to reference value in bold; 95% CI, 95% confidence interval calculated based on 3-year period.
Proportion hospitalized is based on recorded hospitalizations expressed as a percentage of number notified.
Rates for ethnic groups were directly age standardized to the age distribution of the New Zealand population at the 2001 Census with confidence intervals calculated according to the methods used for age-standardized data [33].
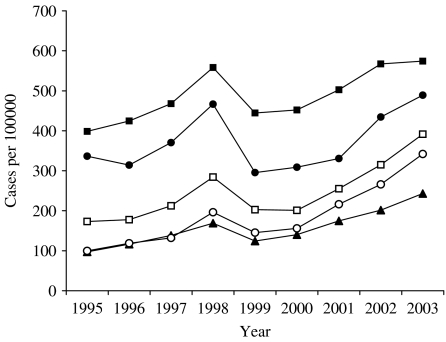
Also for the period 2001–2003, campylobacteriosis showed highest average annual notification rates in children aged 1–4 years (578·1/100 000), and adults aged 20–29 years (470·0/100 000). The average annual notification rate in males (362·1/100 000) was significantly higher than that in females (295·9/100 000). Hospitalization data showed a broadly similar age-sex distribution to notification data, albeit at much lower rates. Peak hospitalization rates were in infants (<1 year), in young adults (20–29 years), but unlike notifications, there was also a peak in the elderly population (⩾70 years). Notification and hospitalization rates in males were higher than females in almost all age groups (the one exception was for hospitalizations in the 15–19 years age group where the rate was slightly higher in females). Trends in age-specific notification rates (Fig. 2) for the period 1995–2003 show that the incidence of disease rose for all age groups, although this increase was relatively higher for those aged 5–14 years and ⩾30 years.
Fig. 2.
Age-specific rates of campylobacteriosis notifications in New Zealand, by year (1995–2003). –■–, <5; –▲–, 5–14; –●–, 15–29; –□–, 30–59; –○–, ⩾60.
When analysed by ethnicity, notification rates were higher among Europeans (329·0/100 000) than Maori (93·5/100 000), Pacific Peoples (70·2/100 000), and people of ‘Other’ ethnicity (193·3/100 000) (Table). These differences were less pronounced when considering hospitalized cases and actually disappeared in the case of people of ‘Other’ ethnicity.
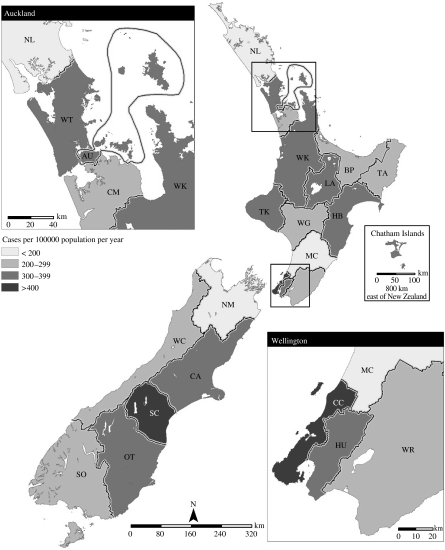
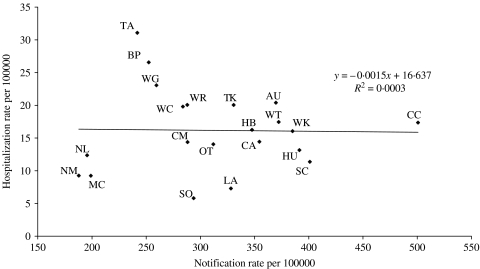
Rates of disease also showed marked regional variations over the period 2001–2003 (Fig. 3). Notification rates ranged from 187·8/100 000 (Nelson Marlborough district health board) to 500·8/100 000 (Capital and Coast district health board). Hospitalization rates ranged from 5·8/100 000 (Southland) to 31·1/100 000 (Tairawhiti). As shown in Figure 4, there was no correlation between notification and hospitalization rates at the district health board level (R 2=0·0003). Rates of campylobacteriosis notifications and hospitalizations were significantly lower for those living in rural areas compared to urban dwellers (Table). When the population was divided by age group, disease rates were higher for children (<15 years old) living in rural areas compared to those with urban home addresses. The opposite pattern was seen for adults (Table).
Fig. 3.

Fig. 4.
Rates of campylobacteriosis notification and hospitalization by New Zealand district health board (average annual rates for 2001–2003). For health-board abbreviations see Figure 3 legend.
DISCUSSION
Notification data suggest a steady rise in campylobacteriosis incidence for more than two decades in New Zealand. The similarities between the temporal patterns of notifications and hospitalizations strongly suggest that the increase in this disease is real. Changes in reporting behaviour by doctors are unlikely to have contributed to this observed trend as they have a fairly high level of notification of gastrointestinal diseases that are laboratory-confirmed [2]. For example, over the 1995–2001 period about 92% of laboratory-identified cases of salmonellosis were also notified [3]. Notifications for other enteric diseases, such as salmonellosis, have also followed similar temporal trends to hospitalizations, but none have risen in the same way as campylobacteriosis over this period [4].
Despite the conclusion that the increase in reported campylobacteriosis is probably real, a concurrent increase in known sequelae of this infection (particularly Guillain–Barré syndrome) is not apparent over this period [5]. One reason for this lack of increase may be that New Zealand possibly has a lower prevalence of the serological types of Campylobacter linked to immune-mediated illnesses.
There is no conclusively identified major cause for the increase in campylobacteriosis incidence in New Zealand. While a high proportion of the poultry on retail sale in New Zealand is contaminated with Campylobacter [6], the sparseness and quality of the data [7] are not adequate for discerning temporal patterns in contamination levels. Also, there is no clear evidence for changing levels of known behavioural risk factors over time (e.g. for consumption of undercooked poultry or for poultry away from home – both of which have been identified in case-control studies [8, 9]). Nevertheless, it is plausible that there are relevant temporal trends in: (i) the increased frequency of eating outside the home setting (likely to have been substantial); (ii) the barbequing of food; and (iii) the overall increase in poultry consumption (which nearly doubled during the 1990s in real terms) [10]. Unsafe domestic food handling has been identified in outbreaks involving Campylobacter [11] and there may also have been a decline in home cooking and food safety skills in recent decades (but confirmatory data on any such trends are lacking).
Increased average temperatures over the last two decades in New Zealand [12] may have also favoured the survival of Campylobacter in various settings, given the evidence that the timing of peak infection in various countries (including New Zealand) is weakly associated with high temperatures 3 months previously [13]. Climatic factors may also contribute to any role that flies play in transmission of this organism [14].
Pathogenic Campylobacter are also known to contaminate recreational water in several parts of New Zealand [15] and contaminated water supplies have been implicated in specific outbreaks [16, 17]. Yet water contamination is unlikely to be a major source for the increase since there has been substantial progress in improving the microbiological quality of reticulated water supplies in New Zealand since the early 1990s [18]. Although pastoral livestock are known to provide reservoirs for pathogenic Campylobacter in this country [19], there are no systematic surveillance data to assess temporal infection trends in this potential source.
The higher rates of disease among children living in rural areas identified in this analysis are suggestive that direct transmission from infected animals and contaminated environments contributes to the burden of disease for this population. An intensive investigation carried out in one rural area of New Zealand found that Campylobacter was widespread in farm animal faeces and river water providing many potential transmission routes to humans [20]. However, changes in such sources are unlikely to explain much of the increase and can only explain a relatively small proportion of the disease burden (and appear less important for adults than children).
This analysis has also described the characteristics of those most affected by campylobacteriosis. While such analyses are commonly carried out using notification and laboratory data, they have rarely used hospitalization data. These analyses show that rates are highest in children and young adults, males and those of European ethnicity. This age distribution is common to other developed countries [21]. Marked ethnic variations in campylobacteriosis rates have also been reported in England [22]. The findings for ethnicity are notable as Maori and Pacific people generally experience higher rates of infectious diseases in New Zealand, particularly serious infections such as meningococcal disease that are linked to socio-economic deprivation [23]. This ethnic difference was less marked for campylobacteriosis hospitalizations compared with notifications, suggesting that some of this observed difference is related to poorer access to primary care and diagnostic services resulting in lower rates of notified disease. The important role of surveillance factors in determining observed patterns of enteric disease notifications is also illustrated by the analysis of disease rates by district health board (Fig. 4). This analysis found no correlation in notification and hospitalization rates for these geographical areas.
These surveillance data also show that the rate of notified campylobacteriosis in New Zealand is relatively high compared to that reported for other developed countries. For example, the rate in 2003 (395·6/100 000) is higher than rates in the following countries: Australia (116·5 cases/100 000 population in 2003) [24], England and Wales (85·4/100 000 in 2003) (source http://www.hpa.org.uk/), Scotland (86·6/100 000 in 2003) [25], Iceland (116/100 000 in 2000) [26], The Netherlands (37·0/100 000 in 2001) [27], United States FoodNet sites (12·6/100 000 in 2003) [28], and Canada (40·1/100 000 in 2000) [29]. Although country comparisons need to be considered cautiously given different reporting systems, these findings are suggestive that New Zealand has relatively high levels of campylobacteriosis. Reasons for this higher rate are unknown. Furthermore, the true population rate is likely to be many times higher than notified cases suggest because only a small proportion of cases will seek medical attention and provide specimens for laboratory testing. Population-based studies carried out in England found that a multiplier of 7·6 applied when converting from nationally notified cases to the incidence occurring in the population [30]. Mainly because of its high incidence, campylobacteriosis is the largest contributor to the economic costs of foodborne diseases in New Zealand [31].
This analysis has the limitations associated with use of routinely collected surveillance data. Hospitalization data in particularly is affected by changes in coding practices, with this disease only being coded separately since 1995. In general, these disease estimates are highly conservative e.g. only a principal diagnosis of campylobacteriosis was included in the hospitalization data which will significantly underestimate numbers.
The evidence for a high and increasing burden of campylobacteriosis in New Zealand suggests that further research into the epidemiology of this disease should be a public health priority. Also, given the apparent success of reducing Campylobacter contamination of poultry in Iceland through various measures [26], and the evidence from a ‘natural experiment’ in Belgium [32], there is a strong case for conducting intervention studies in New Zealand. Such studies could investigate the health benefits of reducing Campylobacter contamination of New Zealand poultry intended for human consumption.
ACKNOWLEDGEMENTS
The Institute of Environmental Science and Research Ltd (ESR) supplied the notification data and the New Zealand Health Information Service supplied the hospitalization data. Kurt Janssen, Public Health Intelligence, New Zealand Ministry of Health, prepared the map.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.McNicholas AM et al. Is New Zealand's recent increase in campylobacteriosis due to changes in laboratory procedures? A survey of 69 medical laboratories. New Zealand Medical Journal. 1995;108:459–461. [PubMed] [Google Scholar]
- 2.Simmons G et al. Could laboratory-based notification improve the control of foodborne illness in New Zealand? New Zealand Medical Journal. 2002;115:237–240. [PubMed] [Google Scholar]
- 3.Thornley C, Baker M, Nichol C. The rising incidence of salmonella infection in New Zealand, 1995–2001. New Zealand Public Health Report. 2002;9:25–28. [Google Scholar]
- 4.Sneyd E, Baker M. Infectious Diseases in New Zealand: 2002 annual surveillance summary. Wellington: Institute of Environmental Science & Research Ltd (ESR); 2003. [Google Scholar]
- 5.Lake R et al. Lack of association between long-term illness and infectious intestinal disease in New Zealand. New Zealand Medical Journal. 2004;117:U893. [PubMed] [Google Scholar]
- 6.Hudson JA et al. Seasonal variation of Campylobacter types from human cases, veterinary cases, raw chicken, milk and water. Journal of Applied Microbiology. 1999;87:115–124. doi: 10.1046/j.1365-2672.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 7.Lake R Risk Profile: Campylobacter jejuni/coli in poultry (whole and pieces) Christchurch: Institute of Environmental Science & Research Ltd (ESR); 2003. [Google Scholar]
- 8.Ikram R et al. A case control study to determine risk factors for campylobacter infection in Christchurch in the summer of 1992–3. New Zealand Medical Journal. 1994;107:430–432. [PubMed] [Google Scholar]
- 9.Eberhart-Phillips J et al. Campylobacteriosis in New Zealand: results of a case-control study. Journal of Epidemiology and Community Health. 1997;51:686–691. doi: 10.1136/jech.51.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Statistics New Zealand. New Zealand Official Yearbook 2000. Wellington: Statistics New Zealand; 2000. [Google Scholar]
- 11.Lake R, Simmons G. How important is unsafe domestic food handling in the aetiology of foodborne illness in New Zealand. New Zealand Public Health Report. 2001;8:89–91. [Google Scholar]
- 12.National Institute of Water and Atmospheric Research (NIWA) Climate Variability and change: past climate variations over New Zealand (New Zealand annual temperature series updated to 2003) Wellington: NIWA; 2005. [Google Scholar]
- 13.Kovats RS et al. Climate variability and campylobacter infection: an international study. International Journal of Biometeorology. 2005;49:207–214. doi: 10.1007/s00484-004-0241-3. [DOI] [PubMed] [Google Scholar]
- 14.Nichols G. Fly transmission of Campylobacter. Emerging Infectious Diseases. 2005;11:361–363. doi: 10.3201/eid1103.040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyles R et al. Spatial and temporal patterns of Campylobacter contamination underlying public health risk in the Taieri River, New Zealand. Journal of Environmental Quality. 2003;32:1820–1828. doi: 10.2134/jeq2003.1820. [DOI] [PubMed] [Google Scholar]
- 16.Stehr-Green JK et al. Waterborne outbreak of Campylobacter jejuni in Christchurch: the importance of a combined epidemiologic and microbiologic investigation. New Zealand Medical Journal. 1991;104:356–358. [PubMed] [Google Scholar]
- 17.Bohmer P. Outbreak of campylobacteriosis at a school camp linked to water supply. New Zealand Public Health Report. 1997;4:58–59. [Google Scholar]
- 18.Ministry of Health. Annual Review of Drinking-water Quality in New Zealand 2003. Wellington: Ministry of Health; 2005. [Google Scholar]
- 19.Savill M et al. Elucidation of potential transmission routes of Campylobacter in New Zealand. Water Science and Technology. 2003;47:33–38. [PubMed] [Google Scholar]
- 20.Devane ML et al. The occurrence of Campylobacter subtypes in environmental reservoirs and potential transmission routes. Journal of Applied Microbiology. 2005;98:980–990. doi: 10.1111/j.1365-2672.2005.02541.x. [DOI] [PubMed] [Google Scholar]
- 21.Butzler JP. Campylobacter, from obscurity to celebrity. Clinical Microbiology and Infection. 2004;10:868–876. doi: 10.1111/j.1469-0691.2004.00983.x. [DOI] [PubMed] [Google Scholar]
- 22.The Campylobacter Sentinel Surveillance Scheme Collaborators. Ethnicity and Campylobacter infection: a population-based questionnaire survey. Journal of Infection. 2003;47:210–216. doi: 10.1016/s0163-4453(03)00072-0. [DOI] [PubMed] [Google Scholar]
- 23.Baker MG et al. A 10-year serogroup B meningococcal disease epidemic in New Zealand: descriptive epidemiology, 1991–2000. Journal of Paediatrics and Child Health. 2001;37:S13–S19. doi: 10.1046/j.1440-1754.2001.00722.x. [DOI] [PubMed] [Google Scholar]
- 24.Miller M et al. Australia's notifiable diseases status, 2003: Annual Report of the National Notifiable Diseases Surveillance System. Communicable Disease Intelligence. 2005;29:1–61. doi: 10.33321/cdi.2005.29.1. [DOI] [PubMed] [Google Scholar]
- 25.Scottish Centre for Infection and Environmental Health . Gastro-intestinal and foodborne infections: Campylobacter. SCIEH Weekly Report2004381–4.http://www.show.scot.nhs.uk/SCIEH/PDF/pdf2004/0401.pdf). Accessed 6 June 2005.
- 26.Stern NJ et al. Campylobacter spp. in Icelandic poultry operations and human disease. Epidemiology and Infection. 2003;130:23–32. doi: 10.1017/s0950268802007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Pelt W et al. Laboratory surveillance of bacterial gastroenteric pathogens in The Netherlands, 1991–2001. Epidemiology and Infection. 2003;130:431–441. [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food – selected sites, United States, 2003. Morbidity and Mortality Weekly Report. 2004;53:338–343. [PubMed] [Google Scholar]
- 29.Public Health Agency of Canada http://dsol-smed.hc-sc.gc.ca/dsol-smed/ndis/c_time_e.html. http://dsol-smed.hc-sc.gc.ca/dsol-smed/ndis/c_time_e.html . Notifiable disease incidence by year, 1988–2000 ( ). Accessed 6 June 2005.
- 30.Wheeler JG et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. British Medical Journal. 1999;318:1046–1050. doi: 10.1136/bmj.318.7190.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott WG et al. Economic cost to New Zealand of foodborne infectious disease. New Zealand Medical Journal. 2000;113:281–284. [PubMed] [Google Scholar]
- 32.Vellinga A, Van Loock F. The dioxin crisis as experiment to determine poultry-related campylobacter enteritis. Emerging Infectious Diseases. 2002;8:19–22. doi: 10.3201/eid0801.010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkin D. Cancer Incidence in Five Continents. Lyon, France: IARC Press; 2005. [Google Scholar]