SUMMARY
There is little UK data on hospital admission rates for childhood pneumonia, lobar pneumonia, severity or risk factors. From 13 hospitals serving the catchment population, demographic and clinical details were prospectively collected between 2001 and 2002 for children aged 0–15 years, seen by a paediatrician with community-acquired pneumonia (CAP) and consistent chest X-ray changes. From 750 children assessed in hospital, incidence of CAP was 14·4 (95% CI 13·4–15·4)/10 000 children per year and 33·8 (95% CI 31·1–36·7) for <5-year-olds; with an incidence for admission to hospital of 12·2 (95% CI 11·3–13·2) and 28·7 (95% CI 26·2–31·4) respectively. Where ascertainment was confirmed, incidence of CAP assessed in hospital was 16·1 (95% CI 14·9–17·3) and 41·0 (95% CI 37·7–44·5) in the 0–4 years age group, whilst incidence for hospital admission was 13·5 (95% CI 12·4–14·6) and 32 (95% CI 29·1–35·1) respectively. In the <5 years age group incidence of lobar pneumonia was 5·6 (95% CI 4·5–6·8)/10 000 per year and severe disease 19·4 (95% CI 17·4–21·7)/10 000 per year. Risk of severe CAP was significantly increased for those aged <5 years (OR 1·50, 95% CI 1·07–2·11) and with prematurity, OR 4·02 (95% CI 1·16–13·85). It also varied significantly by county of residence. This is a unique insight into the burden of hospital assessments and admissions caused by childhood pneumonia in the United Kingdom and will help inform future preventative strategies.
INTRODUCTION
Community-acquired pneumonia (CAP) in children remains an important reason for hospital admission. Severity ranges from a very mild illness to systemic disease requiring ventilation, and death. It is surprising how little is known of the epidemiology of CAP in UK children today. There is no reliable data on incidence from the United Kingdom, little from developed countries, with most data over 20 years old [1–5].
The availability of an effective conjugate pneumococcal vaccine [6] and its very recent introduction into the UK primary immunization schedule, makes it essential to establish the current incidence of childhood pneumonia, lobar pneumonia and severe pneumonia in Britain. Although in an individual child chest radiograph (CXR) changes do not reliably correlate with a viral or bacterial aetiology, lobar pneumonia is more frequently bacterial [7] and could be used as a diagnostic indicator for bacterial infection within populations for which vaccination may provide an effective preventative strategy. Such data will provide a baseline against which, in the absence of a controlled trial, vaccine effectiveness could be measured. This would also inform health outcome studies to determine the cost effectiveness of universal vaccination.
PATIENTS AND METHODS
We conducted a prospective survey of paediatric pneumonia in children aged 0–15 years seen by a paediatrician in 13 hospitals in the North East of England (excluding Cumbria), between 1 August 2001 and 31 July 2002.
Previously the area involved was part of a single health administrative region (the Northern Regional Health Authority) and hence this is a well-defined region with established health service links and a stable population (0–16 years: 522158) which is served only by these hospitals.
In the United Kingdom most children are initially seen by a General Practitioner or A&E staff and then, if further assessment required, referred to a hospital paediatrician.
Children were included if seen urgently by a paediatrican, but not necessarily admitted, with history, signs or symptoms indicative of lower respiratory tract infection and a CXR consistent with infection, as determined by the local paediatrician. Symptoms and signs included any of fever, tachypnoea, dyspnoea, cough and respiratory distress and were prospectively recorded on a standard form. Also recorded were date of birth, postcode, parental occupation, parental smoking, gestational age, clinical history, investigations performed. Treatment including oxygen and antibiotics as well as intensive care, length of stay, complications and organisms isolated were then recorded after discharge.
Radiological findings were confirmed by the local radiologist and children were excluded if this CXR was deemed normal. Where reports differed between the paediatrician and radiologist the films were reviewed by a paediatric radiologist (blinded to the clinical picture) and their report taken as final. Children were excluded if there was a clinical diagnosis of bronchiolitis, if they had been admitted to hospital for any reason in the preceding 3 weeks, or if their main place of residence was not in the study area.
Case ascertainment audit was performed in six of the 13 hospitals. Three were involved in a concurrent study for aetiology and a research nurse checked and registered all patients. Three further sites were visited by the research nurse, ward books were searched and coding data obtained, then cross checked with CXR results. All patients identified through this process were compared to those originally notified, duplicates were removed.
CXR changes were categorized into one of three broad groups – lobar, patchy consolidation, and perihilar infiltrates using the radiologists’ reports. These categories are very similar to the recently developed WHO criteria [8].
Severity criteria were derived from severity definitions within the management guidelines from the British Thoracic Society [9]. Any of the following led to a classification of ‘Severe’: tachypnoea (RR>70 for infants ⩽1 year old, RR>50 for children >1 year old); dyspnoea; oxygen saturation <93%; oxygen given; nasogastric feeds; intravenous fluid infusion; septicaemia; empyema; high dependency or intensive care. ‘Mild’ included immediate home discharge or hospital stay of <3 days and no oxygen, no intravenous or nasogastric feeds, or ‘Moderate’ (neither category).
Social class was derived from fathers’ occupation. Admission decisions and care were entirely by local paediatricians according to local practice or protocol. Data was entered onto a central database and anonymized. North Tyneside Ethical Committee and North and Yorkshire MREC chairpersons reviewed the protocol; no formal committee approval was required.
Statistical methods
Incidence rates of CAP were determined by gender, age and county of residence. Population estimates were obtained from the 1991 census [10]. Risk factors for severe CAP (compared to mild/moderate CAP) were investigated using logistic regression. The statistical package stata 8.0 (StataCorp., College Station, TX, USA) was used for the analysis.
RESULTS
A total of 792 children were identified, 81 were removed at review (six duplicates, 72 with normal CXRs and three outside the age range), leaving 711 eligible cases. Of these, 636 were admitted, 65 sent home with antibiotics and 10 sent home without antibiotics. There were no deaths.
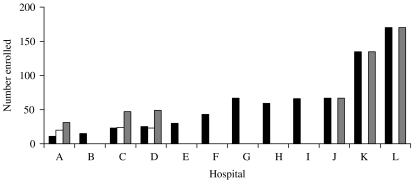
The number notified by each hospital is illustrated in the Figure. An audit in six hospitals found three hospitals had complete notification, but three had missed around half of the children admitted. The five other hospitals notified the number expected for their catchment area and their numbers were accepted. The 39 further cases thus identified were added to the dataset making 750 in total. Only age, gender and postcode were available for these cases, these were thus not included in the analysis of disease severity.
Fig.
Enrolment and validation numbers per hospital. ■, Number enrolled; □, number missed;  , validated total.
, validated total.
To further check completeness of ascertainment, we obtained Department of Health (England) Hospital Episode Statistics (HES) for 2001/2002, covering all-cause hospital admissions (1 655 961 in total) for children aged 0–15 years. This information was used in conjunction with the number of admissions for pneumonia-related diagnoses (12 412) to estimate the proportion of pneumonia-related admissions (0·75%). The total number of admissions for the study area (94 546) was estimated from admission numbers for each of 16 Primary Care Trusts (administrative and funding bodies for health) in the study area, and using the proportion of pneumonia-related admissions previously calculated we estimated that based on HES data, there would be 709 admissions during the study time period for CAP in children aged 0–15 years.
The overall observed incidence rate of children acutely seen in hospital with CAP aged <16 years in the Northern Region (excluding Cumbria) was 14·4 (95% CI 13·4–15·4)/10 000 children per year and for <5-year-olds, 33·8 (95% CI 31·1–36·7). Incidence for hospital admission was 12·2 (95% CI 11·3–13·2) and 28·7 (95% CI 26·2–31·4) respectively. The incidence rate was highest for males and those aged 0–4 years (Table 1).
Table 1.
Incidence rates per 10 000 per year of hospital assessments for CAP
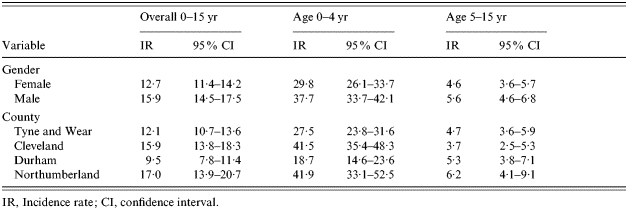
IR, Incidence rate; CI, confidence interval.
Using only those centres where ascertainment was confirmed, the estimated overall incidence was 16·1 (95% CI 14·9–17·3)/10 000 children per year and 41·0 (95% CI 37·7–44·5)/10 000 in the 0–4 years age group and incidence for admission to hospital was 13·5 (95% CI 12·4–14·6) and 32·0 (95% CI 29·1–35·1) respectively.
Incidence rates by age and gender for mild, moderate and severe forms of CAP are shown in Table 2. Males have higher rates of CAP than females in both age groups and for severe and moderate pneumonia. Rates of CAP for both genders were six times higher in the 0–4 years age group than the 5–15 years age group.
Table 2.
Incidence rates per 10 000 per year of CAP (mild, moderate and severe) by age and gender
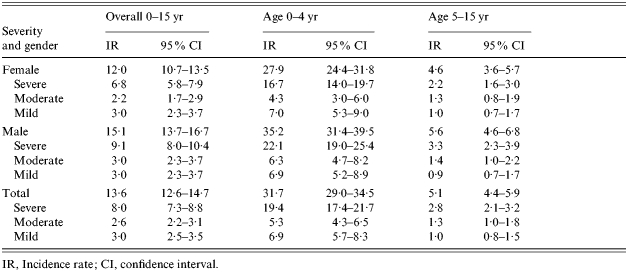
IR, Incidence rate; CI, confidence interval.
The incidence rates of CAP by CXR characteristic, age and gender, are shown in Table 3. Patchy changes were the most common CXR finding in both age groups comprising approximately half of all CXR patterns. Lobar pneumonia comprised one-sixth of all pneumonia seen in the 0–4 years age group. Perihilar changes were most uncommon in those older than 4 years.
Table 3.
Incidence rates per 10 000 per year of CAP (patchy, lobar and perihilar) by age and gender
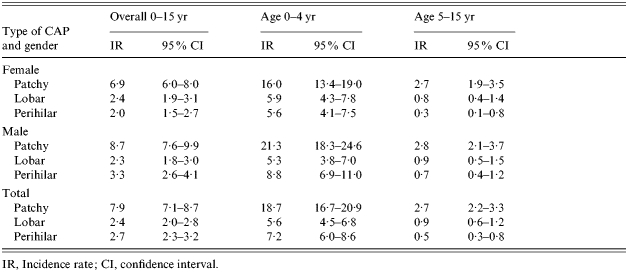
IR, Incidence rate; CI, confidence interval.
Logistic regression was used to identify the characteristics of children with severe disease, compared to those with less severe disease; the results are shown in Table 4.
Table 4.
Univariate risk factors of severe versus mild/moderate CAP
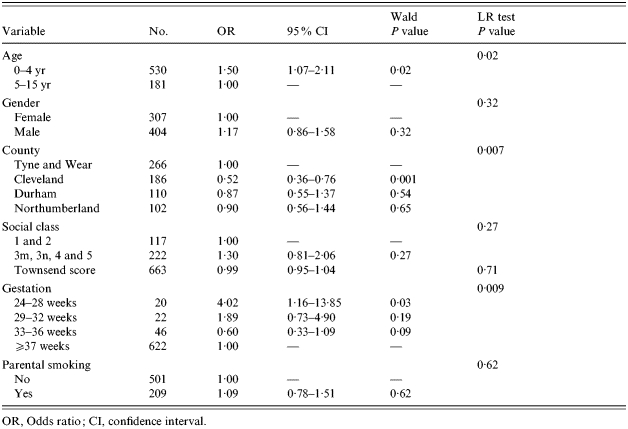
OR, Odds ratio; CI, confidence interval.
Children aged <5 years were significantly more likely to have severe disease than older children. Boys were more likely to have severe disease than girls, but not significantly so. Those with pneumonia in Cleveland were significantly less likely to have severe pneumonia than children in Tyne and Wear. Those born at 24–28 weeks gestation were significantly more likely to have severe disease than those with pneumonia born at ⩾37 weeks. Children presenting with pneumonia had a trend to more severe disease if they were from less advantaged socio-economic groups.
Multivariate analysis suggests that the associations between severity of CAP and gestational age and county were not accounted for by socio-economic factors.
No increase in numbers of pathogens associated with epidemics or outbreaks were noted (Table 5).
Table 5.
Numbers of pathogens identified
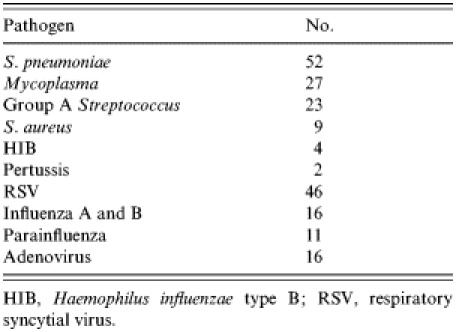
HIB, Haemophilus influenzae type B; RSV, respiratory syncytial virus.
DISCUSSION
This is the largest prospective study of incidence of children seen in hospital with pneumonia and the first from the United Kingdom.
Our annual incidence rates for hospital admission at 13·5/10 000 for all ages and 32/10 000 for those aged <5 years are 1·4 times higher than reported from previous HES data in the South of England [11]. HES data may be less reliable due to incomplete or confounded coding data and radiology findings and severity cannot be evaluated. However incidence is generally lower than other previous studies evaluating hospital admissions due to CAP from Germany [12], Finland [3], Australia [13], the United States [14] and of European children in New Zealand [15] (Table 6). Previous community rates, capturing all cases of CAP, are obviously higher (Table 6), although these, with the exception of the Finnish study [4] and US vaccine study [6] are rarely radiologically confirmed. Interestingly, more recent community estimates are lower at 150/10 000 per year in the <6 years age group [6, 16].
Table 6.
Incidence of CAP from previous studies
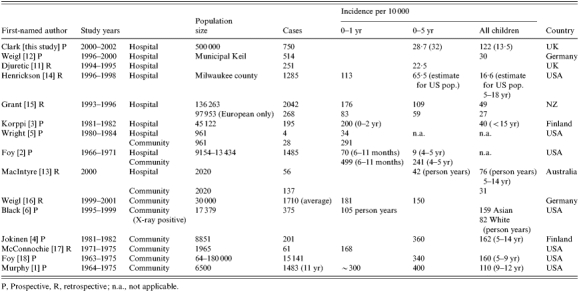
P, Prospective, R, retrospective; n.a., not applicable.
Differences in these rates may be explained by several factors. Hospital admission patterns may have changed over the last 20 years, with fewer children being admitted. However, community rates also appear to have declined suggesting that changes in admission policies are not the only reason for the current lower rates. Hospital data depends on local referral and admitting patterns which will vary even within countries [4] as well as between them. There is likely to be differences in the underlying rate between countries and ethnic groups as described in New Zealand, where the average annual incidence was 140/10 000 for Pacific Island children, 67/10 000 for Maori children but only 27/10 000 for children of European descent [15]. Annual incidence can also be affected by epidemics and local outbreaks, but no evidence to support any unexpected increase in particular pathogens such as Mycoplasma or influenza was noted over the study period.
We found substantial variation in incidence across counties. While some variation may be due to incomplete ascertainment, other factors such as differences in ethnic groups, primary care, hospital-seeking behaviour and hospital admissions procedures can produce regional variation in hospital presentation. Hospital data depends on local referral and admitting patterns which will vary even within countries [4] and between ethnic groups [15]. The differences we observed remained after socio-economic factors were considered.
Perihilar CXR changes are seen in the <5 years age group more frequently than in older children. Lobar pneumonia is only seen in 5·6/10 000 per year of <5-year-olds, less than the 10/10 000 per year identified by HES data [11] and in 0·9/10 000 per year in the 5–15 years age group.
Risk factors previously associated with any CAP include being a boy and a history of attending child care, passive smoking, overcrowding [19, 20], wheezing, previous acute respiratory tract infections and acute otitis media [21], diminished airway function [22], asthma, bronchitis and chronic illness [13], deprivation [23, 24] and malnutrition [25]. Household smoking has a recognized impact on children’s respiratory infections [26].
Risk factors for severe pneumonia have not been explored, although deprivation has been associated with severe bronchiolitis [27]. We found that a history of significant prematurity (possibly reflecting chronic lung disease) indicated a greater risk of severe disease, as did being of a young age (<5 years). There was also a trend for severe disease to be associated with being male and from a lower socio-economic class. Parental smoking did not have a definable impact in our figures, although recording may have been unreliable.
Younger children have higher rates of pneumonia in all surveys including ours and are also more likely to be hospitalized [4]. This may be because they are more likely to have severe disease, as we have shown, but there may also be different admission criteria for young children compared with older children.
There were significantly proportionately fewer severe cases admitted in Cleveland than elsewhere. There is no obvious explanation for this as this was an area where ascertainment was high and it is not an especially affluent county. It cannot be explained by the inclusion of nasogastric and intravenous fluids within the severity criteria as no one was classified as severe only on the basis of either of these. There was always at least one other factor which would have denoted them as severe. It may reflect a local population seeking hospital-based rather than primary-care emergency care (hence earlier hospital presentation), primary-care referral practices or hospital admission policies.
This dataset gives a unique insight into the burden of paediatric assessments and hospital admissions caused by childhood pneumonia in the United Kingdom. Rates are lower than described 20 years ago in Finland but consistent with US rates in the 1990s [14] before the introduction of the conjugate pneumococcal vaccine, which decreased radiologically proven pneumonia by 32% in the first year of life [6]. There is a definite association of disease severity with young age and being born prematurely and variation exists in rates of severe CAP with gender and socio-economic factors. We have suggested that admission rates and severity may depend on local factors. These are important health economic factors and should be taken into account when considering future preventative strategies for childhood pneumonia.
ACKNOWLEDGEMENTS
Thanks are due to paediatric staff in the following hospitals for their help and participation: North Tyneside, South Tyneside, Sunderland Royal, North Durham, Hartlepool, North Tees, James Cook University Hospital, Darlington Memorial, Queen Elizabeth Gateshead, Bishop Auckland, Newcastle General, Royal Victoria Infirmary. Jayne Kelly and Carol Barwick, Research Nurses, collected and entered data. This work was supported by a grant from Wyeth Vaccines UK. The sponsor played no role in the design, collection or analysis of data.
DECLARATION OF INTEREST
J.E.C. has had research support and received sponsorship for meetings and lecture fees from Wyeth Vaccines UK. D.S. has had research support from Wyeth Vaccines UK.
REFERENCES
- Murphy TF et al. Pneumonia: an eleven-year study in a pediatric practice. American Journal of Epidemiology. 1981;113:12–21. doi: 10.1093/oxfordjournals.aje.a113061. [DOI] [PubMed] [Google Scholar]
- Foy HM et al. Incidence and etiology of pneumonia, croup and bronchiolitis in preschool children belonging to a prepaid medical care group over a four-year period. American Journal of Epidemiology. 1973;97:80–92. doi: 10.1093/oxfordjournals.aje.a121492. [DOI] [PubMed] [Google Scholar]
- Korppi M et al. Aetiology of community-acquired pneumonia in children treated in hospital. European Journal of Pediatrics. 1993;152:24–30. doi: 10.1007/BF02072512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen C et al. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. American Journal of Epidemiology. 1993;137:977–988. doi: 10.1093/oxfordjournals.aje.a116770. [DOI] [PubMed] [Google Scholar]
- Wright A et al. The Tuscan Children’s Respiratory Study. II. Lower respiratory tract illness in the first year of life. American Journal of Epidemiology. 1989;129:1232–1246. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- Black SBM et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatric Infectious Disease Journal. 2002;21:810–815. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Virkki R et al. Differentiation of bacterial and viral pneumonia in children. Thorax. 2002;57:438–441. doi: 10.1136/thorax.57.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Pneumonia Vaccine Trial Investigators Group http://www.who.int/vaccine_research/documents/en/pneumonia_children.pdf. http://www.who.int/vaccine_research/documents/en/pneumonia_children.pdf . Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. In: WHO vaccine development team; 2001. ( ). Accessed 28 December 2005.
- British Thoracic Society of Standards of Care Committee. BTS Guidelines for the management of community acquired pneumonia in childhood. Thorax. 2002;57:1i–24. doi: 10.1136/thorax.57.90001.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONS Manchester Information and Associated Services (MIMAS), University of Manchester; 1991. . Small Area Statistics, 1991 Census, Crown Copyright. ESRC Purchase. [Google Scholar]
- Djuretic T et al. Hospital admissions in children due to pneumococcal pneumonia in England. Journal of Infection. 1998;37:54–58. doi: 10.1016/s0163-4453(98)90604-1. [DOI] [PubMed] [Google Scholar]
- Weigl JA et al. Population-based incidence of severe pneumonia in children in Kiel, Germany. Klinische Padiatrie. 2005;217:211–219. doi: 10.1055/s-2004-822699. [DOI] [PubMed] [Google Scholar]
- MacIntyre C, McIntyre P, Caney M. Community-based estimates of incidence and risk factors for childhood pneumonia in Western Sydney. Epidemiology and Infection. 2003;131:1091–1096. doi: 10.1017/s0950268803001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrickson KJ et al. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatric Infectious Disease Journal. 2004;23:S11–S18. doi: 10.1097/01.inf.0000108188.37237.48. (1 Suppl.): [DOI] [PubMed] [Google Scholar]
- Grant CC et al. Hospitalization for pneumonia in children in Auckland, New Zealand. Journal of Paediatrics & Child Health. 1998;34:355–359. doi: 10.1046/j.1440-1754.1998.00237.x. [DOI] [PubMed] [Google Scholar]
- Weigl JA et al. Population-based burden of pneumonia before school entry in Schleswig-Holstein, Germany. European Journal of Pediatrics. 2003;162:309–316. doi: 10.1007/s00431-002-1140-4. [DOI] [PubMed] [Google Scholar]
- McConnochie K, Hall C, Barker W. Lower respiratory tract infection in the first two years of life: epidemiologic patterns and costs in a suburban pediatric practice. American Journal of Public Health. 1988;78:34–39. doi: 10.2105/ajph.78.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy H et al. Rates of pneumonia during Influenza epidemics in Seattle, 1964 to 1975. Journal of the American Medical Association. 1979;241:253–258. [PubMed] [Google Scholar]
- Koch A et al. Risk factors for acute respiratory tract infections in young Greenlandic children. American Journal of Epidemiology. 2003;158:374–384. doi: 10.1093/aje/kwg143. [DOI] [PubMed] [Google Scholar]
- Louhiala P et al. Form of day care and respiratory infections among Finnish children. American Journal of Public Health. 1995;85:1109–1112. doi: 10.2105/ajph.85.8_pt_1.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiskanen-Kosma T et al. Risk factors for community-acquired pneumonia in children: a population-based case-control study. Scandinavian Journal of Infectious Disease. 1997;29:281–285. doi: 10.3109/00365549709019043. [DOI] [PubMed] [Google Scholar]
- Castro-Rodriguez JA et al. Association of radiologically ascertained pneumonia before age 3 year with asthma like symptoms and pulmonary function during childhood. American Journal of Respiratory Care Medicine. 1999;159:1891–1897. doi: 10.1164/ajrccm.159.6.9811035. [DOI] [PubMed] [Google Scholar]
- Hawker JI et al. Social deprivation and hospital admission for respiratory infection: an ecological study. Respiratory Medicine. 2003;97:1219–1224. doi: 10.1016/s0954-6111(03)00252-x. [DOI] [PubMed] [Google Scholar]
- Geyer S, Peter R, Siegrist J. Socioeconomic differences in children’s and adolescents’ hospital admissions in Germany: a report based on health insurance data on selected diagnostic categories. Journal of Epidemiology and Community Health. 2002;56:109–114. doi: 10.1136/jech.56.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonesca W et al. Risk factors for childhood pneumonia among the urban poor in Fortaleza, Brazil: a case control study. Bulletin of the World Health Organisation. 1996;74:199–208. [PMC free article] [PubMed] [Google Scholar]
- DiFranza J, Lew R. Morbidity and mortality in children associated with the use of tobacco products by other people. Pediatrics. 1996;97:560–567. [PubMed] [Google Scholar]
- Spencer N et al. Deprivation and bronchiolitis. Archives of Disease in Childhood. 1996;74:50–52. doi: 10.1136/adc.74.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]