SUMMARY
From 1996 to 2003, four 12-month population-based surveys were performed in FoodNet sites to determine the burden of diarrhoeal disease in the population. Acute diarrhoeal illness (ADI) was defined as ⩾3 loose stools in 24 hours with impairment of daily activities or duration of diarrhoea >1 day. A total of 52 840 interviews were completed. The overall weighted prevalence of ADI in the previous month was 5·1% (95% CI±0·3%), corresponding to 0·6 episodes of ADI per person per year. The average monthly prevalence of ADI was similar in each of the four survey cycles (range 4·5–5·2%). Rates of ADI were highest in those age <5 years. Of those with ADI, 33·8% (95% CI±2·7%) reported vomiting, 19·5% (95% CI±2·1%) visited a medical provider, and 7·8% (95% CI±1·4%) took antibiotics. Rates of ADI were remarkably consistent over time, and demonstrate the substantial burden placed on the health-care system.
INTRODUCTION
Acute diarrhoeal illness (ADI) is very common worldwide. Diarrhoeal disease is estimated to account for 2·5 million childhood deaths annually, predominantly in developing countries [1]. Even in developed countries, the burden of diarrhoeal disease remains substantial. It has been estimated that approximately 375 million episodes of acute diarrhoea occur in the United States each year [2]. Diarrhoea accounts for 4% of hospital admissions among children [3], and foodborne diseases alone cause 5000 deaths per year in the United States [4].
Accurately determining the burden of diarrhoeal disease in the general population is important for guiding public health decision-making, yet precisely determining the incidence of diarrhoeal disease is challenging. Previous estimates of the incidence of diarrhoea in the United States have been based on small studies conducted more than 40 years ago [5, 6] or on data collected over a limited time period [2, 7]. Public health surveillance data, which often rely on passive reporting of laboratory-confirmed disease, are not adequate to precisely determine the incidence of diarrhoeal disease in the community. This is because most diarrhoeal illness in the community is undiagnosed [4, 8–10]. Many people with diarrhoeal disease will not seek medical attention [2, 7, 8], most of those who do will not have stool cultures performed [2, 7, 8], the large majority of stool cultures do not identify a pathogen [11, 12], and many notifiable diseases that are identified are not reported to the health department [9, 13, 14].
In the absence of adequate surveillance data, telephone surveys have been used to determine the prevalence of diarrhoea. Telephone surveys in 1996–1997 and 1998–1999 estimated that US residents experienced approximately 0·7 episodes of ADI (lasting >1 day or impairing daily activities) per person-year, with the highest rates among persons aged <5 years, and the lowest rates among persons aged ⩾65 years [2, 7]. We examined data from these and two additional large population-based surveys performed during 2000–2003 to describe the current epidemiology of ADI in the United States.
METHODS
From June 1996 to February 2003 the Foodborne Diseases Active Surveillance Network (FoodNet) administered four 12-month cycles of a population-based telephone survey to determine the burden of ADI [15]. FoodNet is a collaborative project involving the Centers for Disease Control and Prevention (CDC), state health departments, academic partners, the Food and Drug Administration and the U.S. Department of Agriculture, closely monitoring foodborne disease in participating sites. During the study period the FoodNet surveillance area expanded from five sites (Minnesota, Oregon and selected counties in California, Connecticut and Georgia) included in cycles 1–9 sites (Connecticut, Georgia, Minnesota, Oregon and selected counties in California, Colorado, Maryland, New York and Tennessee) in cycle 4. In 2003 the study covered a population of 37·6 million persons (13·8% of the US population) (Table 1). For each cycle of the telephone survey, approximately 150 persons per site were interviewed each month, based on calculations at the inception of the study to determine a survey population sufficient to make meaningful observations stratified by state.
Table 1.
Demographic characteristics of respondents to each of the four 12-month cycles of the FoodNet population survey, and all four cycles combined

Interviews were conducted using a single-stage random-digit dialling methodology after screening to remove non-residential numbers [16], for a disproportionate stratified random sample stratified by state. At least 15 attempts were made to each number, over at least three occasions on various days and times. A member of the household was selected using the Computer Assisted Telephone Interviewing (CATI) method based on the Kish grid of random numbers. Only English-speaking persons were interviewed until the fourth cycle, during which Spanish-speaking persons were also included. Respondents aged ⩾12 years were interviewed directly, and an adult carer was interviewed regarding participants aged <12 years. Verbal informed consent was obtained from all participants and parents of children aged <18 years, and the study was approved by the CDC Institutional Review Board.
Respondents were asked extensive questions regarding demographic characteristics, health status, diarrhoeal illness and associated symptoms, and contact with health-care services. In the first two cycles of the survey respondents were asked about symptoms experienced in the 4 weeks before the interview, and in the final two cycles they were asked about the month prior (i.e. from the date 1 month before interview to the date of interview). Questions regarding diarrhoea associated with vomiting varied slightly between cycles. In cycle 1, for example, the question was: ‘The next set of questions is about diarrhoea, that is 3 or more loose stools in any 24 hour period. In the last 4 weeks have you had diarrhoea?’ This was followed by questions pertaining to maximum number of stools, activity limitations, duration of diarrhoea, etc. In cycle 2, the phrasing was: ‘The next set of questions is about gastrointestinal illness. In the past 4 weeks, have you had any of the following symptoms?’, with one choice being ‘diarrhoea, defined as 3 or more loose stools or bowel movements in any 24-hour period’, again followed by additional related questions. In cycles 3 and 4, the question was asked: ‘In the past month, have you had either vomiting or diarrhoea?’ If the answer was yes, detailed questions were asked about individual symptoms, including diarrhoea. In all cycles, the same case definition was applied.
There were also minor differences in the wording of questions regarding use of antimicrobials. In cycle 1 survey respondents were asked whether they had taken ‘any antibiotics prescribed for this illness’; in cycles 2 and 3 they were asked: ‘Did you take any antibiotics for this illness?’, and in cycle 4 the question was: ‘Did you take any antibiotics for this illness such as Bactrim or ciprofloxacin?’ and in that cycle they were asked to name the antibiotic. In cycles 1, 2 and 4 an additional question was asked about other prescription or over-the-counter anti-diarrhoeal medications.
We defined a ‘diarrhoeal episode’ as ⩾3 loose stools in a 24-h period, and ‘acute diarrhoeal illness’ (ADI) as ⩾3 loose stools in 24 h with either impairment of daily activities (missing any time from work, or preventing school, recreation or vacation activities) or duration of diarrhoea >1 day. Persons with a chronic illness in which diarrhoea was a major symptom (such as colitis or irritable bowel syndrome) were excluded from the analysis. We created a single variable for race and ethnicity; respondents who reported Hispanic ethnicity were classified as Hispanic regardless of race. Residential setting (urban, suburban, town or rural) was classified by self-report.
Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA). Response rates were defined according to standard formulas of the Council of American Survey Research Organizations (CASRO). The CASRO rate is a measure of respondent cooperation defined as the proportion of all eligible respondents in the sample for whom an interview was completed [17]. Proportions were weighted to compensate for unequal probabilities of selection and to reflect the surveillance population by age and sex, using a method similar to the Behavioral Risk Factor Surveillance System [7, 18]. Relative risks were estimated from odds ratios calculated on logistic regression using published methods [19].
RESULTS
The number of respondents in each 12-month survey cycle increased from 9003 in cycle 1 to 16 435 in cycle 4 (Table 1). The four cycles provided a combined total of 52 840 completed interviews. The CASRO response rate declined over time from 41% in the second cycle to 27% in the fourth cycle. Response-rate data were not available for cycle 1. Demographic characteristics of respondents were similar to each other across the four cycles.
Overall, 3·9% (range 2·8–5·2% in each cycle) of respondents reported an underlying chronic illness associated with diarrhoea. After excluding these respondents, the overall prevalence of a self-reported diarrhoeal episode in the month before interview was 7·7% (95% CI±0·3%). The prevalence was 9·7% in cycle 1, 7·3% in cycles 2 and 3, and 7·2% in cycle 4. Overall, the prevalence of ADI (with impairment of daily activities or duration of diarrhoea >1 day) in the month before interview was 5·1% (range 4·5–5·2%). The prevalence of ADI was similar in all four cycles (Table 2). The only variation was that the rate of ADI reported in cycle 2 was slightly lower than the rate in cycle 4 (P=0·046). The overall rate of ADI corresponds to 0·6 episodes/person per year.
Table 2.
Weighted prevalence of acute diarroheal illness* in the month before interview, among respondents with no underlying chronic diarrhoeal disease, by demographic characteristic in the four FoodNet population surveys, 1996–2003

Three or more loose stools with impairment of daily activities or duration of diarrhoea >1 day, in the month prior to interview.
Reference group for statistical comparison.
Statistically different from comparison group within that cycle, P<0·05.
There were no consistent significant differences in rates of ADI among sites within survey cycles, or among the original survey sites between cycles (data not shown); therefore data from all sites were combined. Rates of ADI by age, sex, race, education, residence and insurance status are shown in Table 2. Overall, persons aged ⩾55 years and 5–17 years reported rates of ADI lower than the 36–54 years age group; respondents aged <5 years reported significantly higher rates than this comparison group [relative risk (RR) 1·6, 95% confidence interval (CI) 1·3–2·0). Rates of ADI among females was higher than males (RR 1·2, 95% CI 1·1–1·4). The overall rate of ADI was lower among black respondents than whites (RR 0·7, 95% CI 0·6–0·8). The rate reported by adults with less than a high school education was lower than that of high school graduates (RR 0·8, 95% CI 0·6–1·0). Overall, the rate of ADI among rural residents was higher than urban residents (RR 1·2, 95% CI 1·0–1·5); medically uninsured respondents reported higher rates than the insured (RR 1·3, 95% CI 1·0–1·5). Sex, race, age and insurance status remained significant on multivariable logistic regression, while education and residence did not.
ADI was associated with fever, vomiting and cough in substantial proportions of respondents, while bloody diarrhoea was uncommon (3·5%) (Table 3). Among persons with ADI, data on duration of their most recent diarrhoeal illness was available for 92%, with a median duration of 2 days (mean 3 days); 82% reported a duration of ⩽3 days and 94% reported a duration of ⩽7 days. Overall 19·5% (range 12·2–22·9%) of respondents sought medical care for their ADI, although only 3·7% submitted a stool specimen for laboratory testing and 1·9% were admitted to a hospital. Of persons with ADI 7·8% overall (range 3·9–10·8%) reported taking antibiotics and 34·4% (range 33·3–34·7%) reported taking either prescription or over-the-counter anti-diarrhoeal medications.
Table 3.
Weighted prevalence of acute diarroheal illness* and associated symptoms and care-seeking, among respondents with no underlying chronic diarrhoeal disease, in the four cycles of the FoodNet population survey, and all cycles combined

n.a., Data not available for that survey cycle.
Three or more loose stools with impairment of daily activities or duration of diarrhoea >1 day, in the month prior to interview.
Indicates statistically significant difference compared to cycle 4, P<0·05.
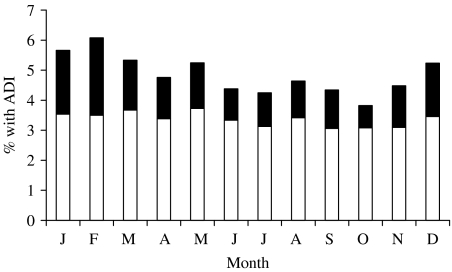
The proportion of persons with diarrhoea varied somewhat by season, driven primarily by the proportion of persons experiencing concomitant vomiting (Fig.). Similar seasonal variation in ADI rates are seen in both children and adults, and in both males and females.
Fig.
Among survey respondents without a chronic diarrhoeal illness in all cycles combined (n=50 757), proportion reporting acute diarrhoeal illness (ADI) in the month prior to interview, by month of interview. ■, With vomiting; □, without vomiting.
DISCUSSION
Acute diarrhoea is common and represents an important health-care burden in the United States. The overall rate of ADI substantial enough to impair daily activities or lasting >1 day was 0·6 episodes/person per year in these surveys. Reported rates of ADI were remarkably consistent over time and are similar to estimates from studies conducted in the United States in previous decades [5–7, 5–7, 20, 21]. These findings demonstrate the ongoing burden ADI places on individuals and the health-care systems they use, and highlight the importance of better understanding the specific causes of and effective methods for preventing these illnesses. Reliable estimates of the burden of ADI are critical to efforts to assess the prevalence of disease due to specific pathogens [4], and to guide development of preventive measures.
While overall rates of ADI were quite stable over time, there was some variability among subgroups. While there were minor changes in the way some questions were asked, it is not immediately obvious that this would explain significant differences in results. Other studies have noted that a large proportion of persons reporting ADI have concomitant respiratory symptoms [22], although only the last two survey cycles in this series enquired about such symptoms specifically. The association of vomiting with diarrhoea had a significant impact on differences in rates of ADI observed in a recent international comparison [23]. The effect of respiratory and upper gastrointestinal (i.e. vomiting) symptoms on interpretation of diarrhoeal disease rates is the subject of ongoing investigation within FoodNet. The contribution of vomiting-associated illnesses to seasonal variations in ADI, for example, may reflect an increase in norovirus infections during the winter months [24]. Lack of readily accessible diagnostic confirmation of such viral illnesses has until relatively recently hindered the development of a better understanding of such patterns [25].
We observed significantly lower rates of diarrhoeal disease in older adults, similar to findings in other studies [26]. It is an important distinction that while older persons may suffer from disproportionately high morbidity and mortality when infected [27], rates of infection are not higher. The association of residence with rates of ADI showed an inconsistent pattern and was not significant on multivariate analysis. Rural residence is probably associated with differences in access to medical care and other socioeconomic indicators which would influence these observations.
The proportion of persons with ADI reporting the use of antibiotics is substantial. Some variation in the use of antimicrobials over the study period may be explained by differences in the wording of that question between cycles. In the first three cycles the name of the antimicrobial agent was not recorded, and it is possible that people mistakenly reported taking an antibiotic when they were instead taking other medications, leading to an overestimate. In the fourth cycle, approximately 10% of persons reporting taking an antimicrobial subsequently named medications which were not antibiotics. Because more persons were treated with antibiotics than submitted a stool specimen, and <10% of routine stool cultures identifed a bacterial pathogen [12], it is clear that the large majority of persons treated with antibiotics are treated empirically based on clinical judgment without laboratory confirmation of the aetiology of the diarrhoea. While certain diarrhoeal diseases with a bacterial aetiology benefit from antimicrobial therapy, many others do not, and empiric treatment of some pathogens can lead to detrimental effects [27]. In many individual cases culturing of stool specimens may not be warranted. For public health purposes, however, such as investigation of suspected outbreaks and understanding the epidemiology of specific pathogens, identifying an aetiology is critical. These data suggest that it is important to better understand how and when empirical antimicrobial therapy is used for ADI and encourage adherence to national guidelines for its management [27].
Comparison of diarrhoeal disease rates between countries has often been hindered by variations in methods and definitions used in different studies [28]. For example, a study in England found rates of diarrhoea comparable to ours using retrospective estimates, but much lower rates using prospective weekly mailed cards associated with stool specimen collection [29]. A prospective population-based study in The Netherlands included cases with vomiting but no diarrhoea (as well as those with diarrhoea or both symptoms) and involved stool specimen collection [26]. Recent coordination of studies has allowed comparison of rates in four developed nations [23], and expansion of these efforts to other sites is under way. Studies such as this, using detailed, comparable definitions are critical for comparing rates between sites and assessing surveillance systems and prevention methods in very different social contexts, which is an important goal of international public health organizations.
This study has several limitations. These surveys do not allow identification of the relative contributions of specific pathogens, outbreaks, or regional differences in reporting habits and perceptions of disease to the observed variations in rates. For the purposes of estimating annual rates of ADI per person-year, we considered the survey periods of the first two cycles (28 days prior to the interview) and the last two cycles (1 month prior to interview) to be equivalent. While no studies have been performed specifically comparing the accuracy of reporting using such intervals, the well-described (and opposite) effects of omission and telescoping on retrospective surveys [30] probably outweigh any imprecision introduced with this assumption. Prospective surveys have demonstrated lower estimates of diarrhoeal disease than retrospective telephone surveys worldwide [26, 29]. Estimates of annual rates of ADI per person-year are based on the conservative assumption of a single illness per respondent during the recall period, which could contribute to underestimation of true rates. Recall bias, or the tendency to ‘telescope’ recollection of illness into the recent past could contribute to overestimation of rates. We excluded persons self-reporting a chronic illness in which diarrhoea was a major symptom. Because persons with chronic illness may also experience ADI, and because we do not have additional information on the nature of the self-reported chronic diseases, this conservative assumption also probably led to the underestimation of true rates of ADI. Response rates declined markedly over time between these survey cycles. A widespread decline in response rates for all types of surveys has been of increasing concern to researchers in many fields [31–34], although several recent studies suggest that this pervasive trend does not necessarily increase study bias [32]. The response rate in the latest cycle of this survey was much higher than the mean response rate of similar recent random-digit dialling telephone surveys [34]. While random-digit dialling was incorporated in the methodology, the use of published telephone directories probably excluded most persons with only cellular telephones, which is a growing population. Despite these limitations, the population and time period covered by this study make it one of the most comprehensive assessments of ADI in the United States currently available.
ADI remains an important cause of morbidity in the United States. The challenges involved in addressing this problem in developed countries are quite different from those in areas where improvements in basic sanitation and hygiene will have a dramatic effect. Many of the findings in this analysis suggest important avenues for further investigation. Better understanding of the determinants of care-seeking behaviour, antimicrobial prescription and submission of specimens for laboratory analysis will have important implications for addressing the burden to the health-care system of ADI. The proportion of persons reporting ADI associated with respiratory symptoms, which are not typically considered part of most foodborne gastrointestinal syndromes, is noteworthy. Identifying the aetiologies of ADI has important implications for its management and prevention [35]. Additional efforts to identify the proportion of ADI attributable to foodborne and other sources will also be important for appropriately targeting prevention measures.
ACKNOWLEDGEMENTS
This study was supported by Cooperative Agreement funding for the Emerging Infections Program, Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.
Footnotes
The views expressed in this paper are those of the authors, and may not be attributed to the Economic Research Service or the U.S. Department of Agriculture.
REFERENCES
- Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet. 2004;363:641–653. doi: 10.1016/S0140-6736(04)15599-2. [DOI] [PubMed] [Google Scholar]
- Herikstad H et al. A population-based estimate of the burden of diarrhoeal illness in the United States: FoodNet, 1996–7. Epidemiology and Infection. 2002;129:9–17. doi: 10.1017/s0950268801006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CM et al. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatric Infectious Diseases Journal. 2001;20:14–19. doi: 10.1097/00006454-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Mead PS et al. Food-related illness and death in the United States. Emerging Infectious Diseases. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto AS, Koopman JS. The Tecumseh Study. XI. Occurrence of acute enteric illness in the community. American Journal of Epidemiology. 1980;112:323–333. doi: 10.1093/oxfordjournals.aje.a112998. [DOI] [PubMed] [Google Scholar]
- Dingle JH, Badger GF, Jordan WS. Illness in the Home: A study of 25,000 illnesses in a group of Cleveland families. The Press of Western Reserve University; 1964. p. 189. , pp. [Google Scholar]
- Imhoff B et al. Burden of self-reported acute diarrheal illness in FoodNet surveillance areas, 1998–1999. Clinical Infectious Diseases. 2004;38:S219–S226. doi: 10.1086/381590. [DOI] [PubMed] [Google Scholar]
- Chalker B, Blaser MJ. A review of human salmonellosis: III. Magnitude of Salmonella infection in the United States. Review of Infectious Diseases. 1988;10:111–124. doi: 10.1093/clinids/10.1.111. [DOI] [PubMed] [Google Scholar]
- Standaert S et al. The reporting of communicable diseases: a controlled study of Neisseria meningitidis and Haemophilus influenzae infections. Clinical Infectious Diseases. 1995;20:30–36. doi: 10.1093/clinids/20.1.30. [DOI] [PubMed] [Google Scholar]
- Barrett S, Lau Y. Incompleteness of statutory notification of bacterial gastro-intestinal infection. Public Health. 1997;111:183–185. doi: 10.1016/s0033-3506(97)00580-5. [DOI] [PubMed] [Google Scholar]
- Voetsch AC et al. Laboratory practices for stool-specimen culture for bacterial pathogens, including Escherichia coli O157:H7, in the FoodNet sites, 1995–2000. Clinical Infectious Diseases. 2004;38:S190–S197. doi: 10.1086/381586. (Suppl. 3): [DOI] [PubMed] [Google Scholar]
- Tauxe RV, Cohen ML, Blaser MJ. Infections of the Gastrointestinal Tract. New York: Raven Press; 1995. Epidemiology of diarrheal diseases in developed countries pp. 37–52. , pp. [Google Scholar]
- Konowitz PM, Petrossian GA, Rose DN. The underreporting of disease and physicians’ knowledge of reporting requirements. Public Health Reports. 1984;99:31–35. [PMC free article] [PubMed] [Google Scholar]
- Voetsch AC et al. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clinical Infectious Diseases. 2004;38:S127–S134. doi: 10.1086/381578. (Suppl 3): [DOI] [PubMed] [Google Scholar]
- Allos BM et al. Surveillance for sporadic foodborne disease in the 21st century: the FoodNet perspective. Clinical Infectious Diseases. 2004;38:S115–S120. doi: 10.1086/381577. (Suppl. 3): [DOI] [PubMed] [Google Scholar]
- Remington PL et al. Design, characteristics and usefulness of state-base behavioral risk factor surveillance: 1981–87. Public Health Reports. 1988;103:366–375. [PMC free article] [PubMed] [Google Scholar]
- CDC Atlanta, GA: Centers for Disease Control and Prevention; . Behavioral Risk Factor Surveillance System 2003. Year-to-Date Data Quality Report Handbook Version 3.0.0, 2004. [Google Scholar]
- National Center for Chronic Disease Prevention and Health Promotion Centers for Disease Control and Prevention; Atlanta, GA: 2004. . Behavioral Risk Factor Surveillance System (BRFSS): Turning information into health. ( [Google Scholar]
- Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. Journal of the American Medical Association. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- Hughes JM et al. Acute gatrointestinal illness in Charlottesville: a prospective family study [Abstract] Clinical Research. 1978;26:28A. [Google Scholar]
- Garthright EE, Archer DL, Kvenberg JE. Estimates of incidence and costs of intestinal infectious diseases in the United States. Public Health Reports. 1988;103:107–116. [PMC free article] [PubMed] [Google Scholar]
- Majowicz SE et al. Magnitude and distribution of acute, self-reported gastrointestinal illness in a Canadian community. Epidemiology and Infection. 2004;132:607–617. doi: 10.1017/s0950268804002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan E et al. Prevalence of diarrhea in the community in Australia, Canada, Ireland and the United States. International Journal of Epidemiology. 2004;34:454–460. doi: 10.1093/ije/dyh413. [DOI] [PubMed] [Google Scholar]
- Mounts A et al. Cold weather seasonality of gastroenteritis associated wtih Norwalk-like viruses. Journal of Infectious Diseases. 2000;181:S284–S287. doi: 10.1086/315586. (Suppl. 2): [DOI] [PubMed] [Google Scholar]
- Glass RI et al. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. Journal of Infectious Diseases. 2000;181:S254–S261. doi: 10.1086/315588. (Suppl. 2): [DOI] [PubMed] [Google Scholar]
- deWit MAS et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. American Journal of Epidemiology. 2001;154:666–674. doi: 10.1093/aje/154.7.666. [DOI] [PubMed] [Google Scholar]
- Guerrant RL et al. Practice guidelines for the management of infectious diarrhea. Clinical Infectious Diseases. 2001;32:331–350. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- World Health Organization Geneva, Switzerland: WHO; 2002. pp. 1–26. . Department of Communicable Disease Surveillance and Response. Method for Foodborne Disease Surveillance in Selected Sites – Report of a WHO Consultation. , pp. [Google Scholar]
- Wheeler JG et al. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. British Medical Journal. 1999;318:1046–1050. doi: 10.1136/bmj.318.7190.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradburn NM, Rossi PH, Wright JD, Anderson AB. Handbook of Survey Research. New York: Academic Press; 1983. Response effects; pp. 289–328. , pp. [Google Scholar]
- Tortora RD. Response trends in a national random digit dial survey. Metodoloski zvezki. 2004;1:21–32. [Google Scholar]
- Tourangeau R. Survey research and societal change. Annual Review of Psychology. 2004;55:775–801. doi: 10.1146/annurev.psych.55.090902.142040. [DOI] [PubMed] [Google Scholar]
- Atrostic BK et al. Nonresponse in U.S. government household surveys: consistent measures, recent trends and new insights. Journal of Official Statistics. 2001;17:209–226. [Google Scholar]
- Steeh C et al. Are they really as bad as they seem? Nonresponse rates at the end of the twentieth century. Journal of Official Statistics. 2001;17:227–247. [Google Scholar]
- Hedberg CW. Food-related illness and death in the United States [Letter] Emerging Infectious Diseases. 1999;5:840. doi: 10.3201/eid0506.990624. [DOI] [PMC free article] [PubMed] [Google Scholar]