SUMMARY
Meningococci are regarded as being unlikely to survive outside of their human host although this has possibly been more assumed than demonstrated. Seven strains of meningococci were tested for their ability to survive on glass or plastic while retaining expression of their capsule and important outer membrane proteins. A known number of colony-forming units of each strain were dried onto glass and onto plastic and tested for viability over time. Survival on glass was significantly better than on plastic (P<0·0001). Isolates of the New Zealand epidemic strain, B:4:P1.7-2,4 survived better on glass than all other strains tested. Recovered isolates still expressed their capsules and outer membrane proteins. These findings raise the question of whether meningococci can be transferred from person to person via fomites contaminated with oropharyngeal secretions containing meningococci.
INTRODUCTION
Humans are the only natural host for meningococci. Invasive meningococcal disease generally follows the acquisition of meningococci from an asymptomatic carrier. Public health measures taken at the time of a new case involve the prompt identification and prophylaxis of close contacts of the case, the rationale being to prevent further disease cases by eliminating meningococci from the healthy carrier (shedder). Asymptomatic carriage of meningococcus occurs in ∼5–10% of the general population [1] although higher carriage rates are variously reported in studies of teenagers and young adults [2, 3].
Transfer of droplets shed from the upper respiratory tract is the most likely means by which meningococci are spread from person to person although for individual cases of disease the means of transmission is not usually determined. Pathogen containing particles are frequently emitted through coughing and sneezing. The airborne particles impinge on the mucus-covered surfaces of the oropharynx where they adhere and establish a population [4]. To cause disease a virulent organism must overcome the local defence mechanisms, and become invasive [5]. For the recipient of the transmitted meningococci the outcome is more likely to be asymptomatic nasopharyngeal carriage, with invasive meningococcal disease the uncommon result.
Crowding at parties and bars probably facilitates the transmission of meningococci. Student parties, bars and other situations involving greater interpersonal contact and the sharing of drinks, cigarettes and utensils, may facilitate transmission of oropharyngeal secretions leading to higher rates of carriage and increasing the risk for meningococcal disease [6–8]. Whether indirect transfer can occur via fomites has not been established as only suggestive evidence exists. In a case-control study conducted in New Zealand the sharing of a drink, food, or pacifier (OR 1·6, 95% CI 1·0–2·7) were identified among a number of risks for meningococcal disease [9] and in a study of school-based clusters of meningococcal disease in the United States the sharing of drinking cups or water bottles occurred among the student interactions recorded in relation to several disease clusters [10]. Using a case-control study that controlled for social factors Neal and co-workers [3] found no link between transmission and the sharing of glasses by students. Moreover, in a study of 258 university students Orr et al. [2] recovered few meningococci from saliva leading the authors to suggest that low levels of salivary contact are unlikely to transmit meningococci. In that same study rates of meningococci recovered from nasopharyngeal and throat sites were 32·2% and 19·4% respectively. A more recent study by Tully et al. [8] found intimate kissing with multiple partners a risk factor for carriage and suggested that the risk derives from exchange of oropharyngeal secretions rather than behaviours related to proximity.
Generally it is considered that meningococci do not survive outside of the host [11] although survivability of meningococci on glass for 4 h in ambient temperature has been shown [12], and the use of silica gel to facilitate rapid drying of meningococci on swabs for transportation nationally or internationally has been described [13]. The study we report was set up to determine if pathogenic meningococci do survive on glass and plastics as used for drinking vessels, thereby providing a potential reservoir for the acquisition of meningococci.
METHODS
Strains
Seven isolates from meningococcal disease cases and representing the three most common capsular groups, B (n=5), C (n=1) and W135 (n=1) were obtained from the meningococcal culture collection at the Institute of Environmental Science and Research (ESR). Strain details are listed in the Table. Isolates were stored in glycerol broth (trypticase soy broth, 15% v/v glycerol) at −80°C since receipt.
Table.
Neisseria meningitidis isolates used and their strain types

Preparation of bacterial suspensions
A scraping from the frozen culture was plated onto 5% Columbia sheep blood agar (CBA) and incubated at 36°C in 5% CO2 atmosphere for 18 h before use. Suspensions of meningococci were obtained using the protocol for preparing a set number of colony-forming units for use in serum bactericidal assays [14]. Briefly, 10–20 colonies were spread onto CBA, incubated (36°C, 5% CO2) for 4 h, and the growth obtained used to make a suspension in 3 ml Hanks buffer (without the addition of bovine serum albumin as specified in the protocol [14]). The optical density was measured and adjusted to a cell density equivalent to 3×104 colony-forming units (c.f.u.)/ml in defined medium mucin (DMM) [15]. The actual number of colony-forming units in the suspension was determined by mixing 10 μl DMM suspension with 15 μl Hanks buffer, drizzling the mixture onto CBA and counting the colonies following overnight incubation (36°C, 5% CO2). The average of three such counts was used to determine the actual c.f.u./ml of DMM against which survival was measured. Validation of this method for use in this study was undertaken [16]. All processes using liquid cultures were undertaken in a biological safety cabinet (class II).
Inoculation of fomites
Glass coverslips (18 mm×18 mm) and strips of plastic used to mould the sipper tops of drink bottles (Portola Tech International, Auckland, NZ) were inoculated with 25 μl of the 3×104 c.f.u./ml bacterial DMM suspension. This was undertaken in a biological safety cabinet (class II) and the fomites left there to dry in open Petri dishes. After drying the fomites were then held in a container at existing ambient temperature and humidity.
Recovery of meningococci from fomites
At pre-determined time-points duplicate samples of the respective inoculated fomites (glass and plastic) were pressed onto the surface of New York City (NYC) agar [17] for 10 min. At both 24 h and 48 h of incubation (36°C, 5% CO2) plates were examined for growth and colonies counted. Each colony was assumed to have arisen from a single bacterial cell. Specific time-points varied according to the strain and depended upon the continuing survival of the strain being tested. Time-points were at regular 30-min intervals for the first 3 h on glass, and at hourly intervals afterwards and on successive days. Time-points on the plastic fomite were two-hourly for the first 4 h and four-hourly as appropriate. Each experiment was concluded when at least two successive time-point samples had failed to yield any viable meningococci.
Expression of capsule and outer membrane proteins
Expression of capsular polysaccharide and the porin proteins, PorA and PorB, was detected by serogrouping and serotyping of the recovered bacteria. Serogrouping was undertaken using specific anti-capsular antibodies and expression of the porin proteins was determined using monoclonal antibodies in a whole-cell ELISA [18].
Statistics
All strains, with the exceptions of NZ04/198, H44/76-SL and NZ03/255, were tested at least twice on each fomite and results reported are the average of multiple tests. NZ04/198 was only trialled once on glass, H44/76-SL was trialled only once on both glass and plastic, and NZ03/255 was tested only on glass. Statistical significance between observed differences in survival between organisms was tested using the procedural method proc glm in SAS version 9.1 (SAS Institute, Cary, NC, USA). The statistically analysed differences were those observed between the group B strains, fomite types, and PorA types.
RESULTS
Survival on glass
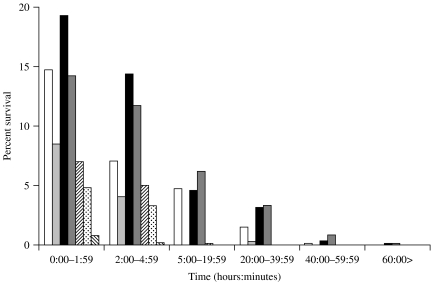
The average number of c.f.u./25 μl of preparation in DMM varied according to the strain used but showed consistency in repetitive testing. For survival measurements, the time-point zero was taken immediately when drying occurred on the fomite. This was usually about 45 min after inoculation. The colony-forming-unit count for all group B strains tested after drying (at time-point zero) on glass averaged between 9% and 20% (60–150 c.f.u.) of the colony-forming units in the original inoculum (Fig. 1). Over time the percentage colony-forming units surviving on the glass coverslips diminished and by 24 h less than 4% (Fig. 1) were viable. It was still possible to recover viable group B meningococci from the coverslips for up to 168 h (6·5 days) with strain NZ03/280 (B:4:P1.7-2,4), for up to 74·5 h (3 days) with strain NZ98/254 (B:4:P1.7-2,4), for up to 50 h (2 days) with strain NZ97/122 (B:1:P1.7-2,4), and up to 21 h with strain Cu00162 (B:4:P1.19,15) (Fig. 1). The other group B strain H44/76-SL (B:15:P1.7,16), which was only tested once, survived at a 4·5% level at 4 h but no colony-forming units were recovered at the next sampling time at 19 h (Fig. 1). In contrast only 4·85% of group C (NZ04/198) survived the first 2 h of drying, 3·32% survived up to 5 h and 0·04% survived from 20–39 h (Fig. 1). Less than 1% of group W135 (NZ03/225) survived drying and no surviving organisms were found after 4 h (Fig. 1).
Fig. 1.
Percentage survival on glass of all seven meningococcal strains by sampling time. □, NZ97/122; , Cu00162; ■, NZ03/280;
, Cu00162; ■, NZ03/280; , NZ98/254;
, NZ98/254;  , H44/76-SL;
, H44/76-SL;  , NZ04/198;
, NZ04/198;  , NZ03/225.
, NZ03/225.
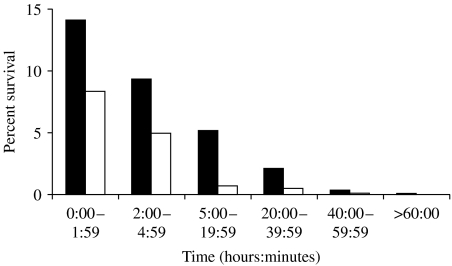
For statistical analysis of the difference in survival between those with the PorA P1.7-2,4 of the NZ epidemic strain and those with an alternative PorA, the model used 169 observations. The model fitted with a variance of R2=0·434412 and P=0·0013 suggesting that there was a significant difference in the percentage survival of the PorA type P1.7-2,4, compared with the other PorA strain types tested (Fig. 2).
Fig. 2.
Comparison of survival times for group B meningococcal strains with PorA type (P1.7-2,4) (■) or an alternative Por A (□).
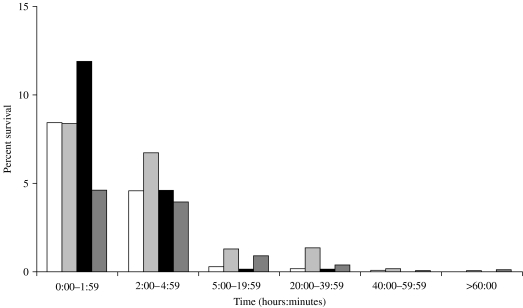
Survival on plastic
As with glass, there was a rapid loss of viable meningococci upon drying on the plastic surface (Fig. 3). All five group B strains were recovered after 1 day but none were viable at the third day of sampling. The group C and group W strains were not tested on plastic.
Fig. 3.
Percentage of survival over time of group B meningococcal strains on plastic. □, NZ03/280; , NZ97/122; ■, NZ98/254;
, NZ97/122; ■, NZ98/254; , Cu00162.
, Cu00162.
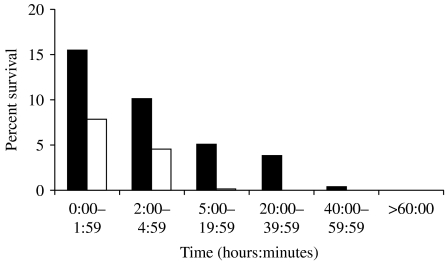
A comparison of survival on glass or plastic (Fig. 4) showed that meningococci survived longer on glass than on plastic, with an average of 14% surviving the first 2 h on glass, but only 8% surviving the first 2 h on plastic. This observed difference occurred at all time-points and was found to be statistically significant (observations=233, R2=0·41887, P<0·0001).
Fig. 4.
The percentage survival of all strains on glass (■) compared with plastic (□).
Testing for continued expression of group polysaccharide and the porin proteins: Meningococci recovered from all time-points continued to express their capsular antigens and their respective PorB and PorA proteins when recovered on NYC agar.
DISCUSSION
Artificial saliva known as DMM is a chemically defined analogue of saliva containing various ions, mucin, vitamins, growth factors and amino acids at concentrations generally similar to those in saliva [15]. Prior testing showed DMM was neither bactericidal nor supportive of meningococcal growth [16]. DMM was chosen as a saliva equivalent because it eliminated uncontrolled confounding factors that could have occurred from diurnal variation, from food-associated factors, or the impact of immunoglobulins in the natural saliva from volunteer subjects. The presence of secretory IgA is considered to be important in limiting colonization by meningococci [19]. The use of artificial saliva also had the advantage of consistency over time, a problem that would have occurred had natural saliva been used. Natural bacterial-free filtered saliva was used by Berger et al. [12] as the suspending solution in their study. Glass beads were placed in the meningococci containing saliva suspension before being allowed to dry for 30 min. These workers recovered meningococci from the glass beads and from inoculated linen up to 4 h and 9 h later respectively [12].
Results showed that that the group B meningococci survived longer than either the group C or the W135 meningococci tested. The survival time for group C was consistent with that measured by Berger et al. [12]. The fact that only 1% of the W135 strain survived the 45 min of drying prior to sampling was surprising given its more frequent association with and persistence in the nasopharynx. The demonstration that there was a statistical difference in survival times (P=0·0013) between strains with the epidemic PorA type P1.7-2,4 and other group B strains with different PorA types (Fig. 2) was a surprising finding and raises pertinent questions in the context of New Zealand’s epidemic. In contrast to glass, no strain survived beyond 20 h on plastic. The reason why meningococci survived for a shorter period on the plastic surface is unclear. Whether this was related to differences in ability to recover meningococci from plastic was not determined.
The findings that meningococci can survive outside of the host on either plastic or glass imply that direct inhalation [4] or intimate kissing [8] may not be the only ways meningococci are transmitted from person to person, particularly in crowded party situations when much sharing occurs and within a short time from deposition. Following an analysis of alcohol consumption and campus bar patronage Imrey et al. [6] suggested that social interaction linked with alcohol consumption facilitated transmission and colonization by meningococci. Other studies have also linked bars, clubs, discotheques and households or dormitories as settings of high risk for transmission of meningococci [3, 7, 20, 21]. However, although Neal et al. [3] showed that frequency of weekly visits to a bar and intimate kissing were independently associated with acquisition of meningococci among university students, they found no association between meningococcal acquisition and the sharing of glasses or cigarettes. It is not known if the actual number of meningococcal colony-forming units deposited by a shedder onto a fomite, such as a drinking vessel, would be sufficient to allow colonization in humans. Bacterial aerosols created by coughing or sneezing have been measured as delivering 104–108 c.f.u./m3 viable organisms [22]. Assuming bacteria in an aerosol are evenly distributed this would equate with delivering between 10 and 1000 c.f.u./cm3 of viable meningococci. The numbers of meningococci per surface area (∼750 c.f.u./cm2) used in this study was within a similar range for deposited bacteria from an aerosol. We showed that at least 80% of the meningococcal load died within 45 min from deposition. Acquisition of sufficient meningococci from contaminated drinking vessels or other fomites is likely to depend on the loading of colony-forming units, the time since deposition, the viability of the meningococci, environmental conditions, and undefined recipient host factors. It is of note that those meningococci that did survive in this study retained expression of their capsule and outer membrane proteins important for interaction with the human host.
The study has produced a surprising finding in that different patient isolates of New Zealand’s epidemic strain (B:4:P1.7-2,4) survived drying better than other group B strain-types. Whether some strains, such as New Zealand’s epidemic strain, are better able to survive transmission by whatever means is uncertain. This needs to be investigated in an appropriate study. While intimate kissing has clearly been shown to be a major risk factor for acquisition of meningococci [3] it cannot be the only method of transfer from host to host. The rapid drying of salivary or respiratory secretions on fomites may prevent the killing action of inhibitory enzymes. Therefore, it is conceivable that transfer of organisms to a new host could occur this way. Until we have better information and understanding about the modes of transfer of meningococci from person to person other than through intimate kissing, the slogans of ‘don’t share drink bottles’ and ‘don’t share spit’ may convey public health messages that at very least do no harm.
ACKNOWLEDGMENTS
We are most grateful to Mike Bryce, Portola Tech International, Auckland for the gift of the plastic samples used in the manufacture of drink bottles and Chris Sissons, Dental Research Group, Wellington School of Medicine, who kindly provided the DMM for use in this study. We thank Ronan O’Toole, School of Biological Sciences, Victoria University of Wellington who was the co-supervisor for Claire Swain’s dissertation.
DECLARATION OF INTEREST
None.
REFERENCES
- Cartwright KA et al. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiology and Infection. 1987;99:591–601. doi: 10.1017/s0950268800066449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr HJ et al. Saliva and meningococcal transmission. Emerging Infectious Diseases. 2003;9:1314–1315. doi: 10.3201/eid0910.030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal KR et al. Changing carriage rate of Neisseria meningitidis among university students during the first week of term: cross sectional study. British Medical Journal. 2000;320:846–849. doi: 10.1136/bmj.320.7238.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe IW. The meningococcus and mechanisms of pathogenicity. Microbiology and Molecular Biology Reviews. 1982;46:162–190. doi: 10.1128/mr.46.2.162-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden BI. Meningococcal infection. Journal of Medical Microbiology. 1988;26:161–187. doi: 10.1099/00222615-26-3-161. [DOI] [PubMed] [Google Scholar]
- Imrey PB et al. Meningococcal carriage, alcohol consumption, and campus bar patronage in a serogroup C meningococcal disease outbreak. Journal of Clinical Microbiology. 1995;33:3133–3137. doi: 10.1128/jcm.33.12.3133-3137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogaert D et al. Epidemiology of nasopharyngeal carriage of Neisseria meningitidis in healthy Dutch children. Clinical Infectious Diseases. 2005;40:899–902. doi: 10.1086/428351. [DOI] [PubMed] [Google Scholar]
- Tully J et al. Risk and protective factors for meningococcal disease in adolescents: matched cohort study. British Medical Journal. 2006;332:445–450. doi: 10.1136/bmj.38725.728472.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M et al. Household crowding a major risk factor for epidemic meningococcal disease in Auckland children. Pediatric Infectious Disease Journal. 2000;19:983–990. doi: 10.1097/00006454-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Zangwill KM et al. School-based clusters of meningococcal disease in the United States. Journal of the American Medical Association. 1997;5:389–395. [PubMed] [Google Scholar]
- Musher DM. How contagious are common respiratory tract infections. New England Journal of Medicine. 2003;348:1256–1266. doi: 10.1056/NEJMra021771. [DOI] [PubMed] [Google Scholar]
- Berger U, Döring MT. The resistance of human Neisseria species against drying out [in German] Archiv fur Hygiene und Bakteriologie. 1969;153:556–559. [PubMed] [Google Scholar]
- Popovic T et al. Evaluation of silica gel packages for transport of Neisseria meningitidis. Journal of Clinical Microbiology. 1998;36:1765–1766. doi: 10.1128/jcm.36.6.1765-1766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DR et al. Validation of the serum bactericidal assay for measurement of functional antibodies against group B meningococcal associated with vaccine trials. Vaccine. 2005;23:2218–2221. doi: 10.1016/j.vaccine.2005.01.070. [DOI] [PubMed] [Google Scholar]
- Wong L, Sissons CH. A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Archives of Oral Biology. 2001;46:477–486. doi: 10.1016/s0003-9969(01)00016-4. [DOI] [PubMed] [Google Scholar]
- Swain CL. The survivability of Neisseria meningitidis in the environment and its relevance to the spread of meningococcal disease [Dissertation] Wellington, NZ: Victoria University of Wellington; 2004. pp. 37–44. , pp. [Google Scholar]
- Knapp JS, Koumans EH, Murray PR. Manual of Clinical Microbiology. 7th edn. Washington, DC: ASM Press; 1999. Neisseria and Branhamella p. 1669. , pp. [Google Scholar]
- Abdillahi H, Poolman J. Whole-cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. Federation of European Micro biological Societies Microbiology Letters. 1987;48:367–371. [Google Scholar]
- Horton RE et al. Influence of age and carriage status on salivary IgA to Neisseria meningitidis. Epidemiology and Infection. 2005;133:883–889. doi: 10.1017/S0950268805004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block C et al. Factors associated with pharyngeal carriage of Neisseria meningitidis among Israel defense force personnel at the end of their compulsory service. Epidemiology and Infection. 1999;122:51–57. doi: 10.1017/s0950268898001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G et al. Carriage of Neisseria meningitidis among household contacts of patients with meningococcal disease in New Zealand. European Journal of Clinical Microbiology and Infectious Diseases. 2001;20:237–742. doi: 10.1007/pl00011260. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Respiratory health hazards in agriculture. American Journal of Respiratory and Critical Care Medicine. 1998;158:S1–S76. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]