SUMMARY
A high prevalence of hepatitis C (HCV) virus infection of up to 80% has been reported for injecting drug users (IDUs) in prison communities. However, there are only very limited data available on the prevalence and course of HCV in young offenders. We performed a study on hepatitis C markers in the largest German Young Offenders’ Institution (YOI), a prison for men (aged 16–24 years). In 2002, all 1176 incoming offenders were asked to participate in the study of whom >95% agreed. Ninety-seven inmates (8·6%) tested positive for anti-HCV or HCV RNA, 79% of whom were viraemic. None of the patients had evidence of cirrhosis at presentation. Interestingly, six individuals (6%) tested positive for HCV RNA in the absence of anti-HCV antibodies, four of whom cleared HCV spontaneously during follow-up without either clinical signs of acute hepatitis or developing HCV antibodies. Hepatitis C markers were significantly more prevalent among immigrants from the former Soviet Union (NIS) than among German inmates (31% vs. 6% respectively, P<0·0001). HIV co-infection was found in five individuals, all of whom were German. In contrast, hepatitis B surface antigen (HBsAg) was detected in five NIS immigrants, one Lebanese and one German inmate. HCV genotypes 2 and 3 were more prevalent in immigrants than in German inmates, while biochemical parameters did not differ significantly between the two groups. In conclusion, the prevalence of hepatitis C was relatively low among inmates of German YOIs although there were significant differences in relation to the country of birth. Our data highlight the need for educational programmes for young offenders in order to prevent the further spread of HCV.
INTRODUCTION
Hepatitis C occurs worldwide, and the WHO estimates that 170 million people are infected [1, 2]. A high prevalence of hepatitis C virus (HCV) infection has been reported in prisons. Several studies from Europe, Australia and the United States indicate hepatitis C prevalence rates in prisons ranging from 20% to 37% [3–14]. Among injecting drug users (IDUs) the prevalence is even higher ranging from 49% to 88%. Drug use is common in prison and needle sharing occurs if there are no needle exchange programmes [15, 16]. However, almost all previous data relate to adult prisons and there are only very limited data available for infected young offenders [13, 17]. There is some evidence that HCV seroprevalence increases in a stepwise fashion with age [18]. In this context, more information on HCV infections in young or first-time inmates would be of importance in exploring the need for prevention programmes in the prison setting. In addition, there is no information about HCV prevalence in the context of migration in Europe. Our study was carried out in the largest German prison for young men [‘young offenders’ institution’ (YOI)]. Data were collected on risk behaviour before and during imprisonment, social and migrational background, and hepatitis and HIV infections including clinical course and medical history.
PATIENTS AND METHODS
In 2002 we asked all 1176 incoming inmates to Hameln YOI who were at least 16 years old to take part in this study to detect and prevent hepatitis C infection. All these inmates were male, and had been sentenced for at least 6 months or were expected to stay for at least 6 months in remand prison. The country of birth of the inmates was divided into three main groups: 729 inmates (62%) were born in Germany, 138 (12%) had migrated to Germany from NIS (New Independent States; equivalent to the former Soviet Union) countries after 1989 and 309 inmates (26%) had migrated from other countries.
A total of 1125 prisoners (96%) gave informed consent for antibody testing for hepatitis A, B, C and HIV as well as for testing for hepatitis B antigens (HBs-Ag) and hepatitis C RNA. These tests were performed at the Department of Virology in the Niedersächisches Landesgesundheitsamt [NLGA (Governmental Institute for Public Health Service of the state of Lower-Saxony, Hannover)] using the following procedures: hepatitis A, B and C (Abbott, Wiesbaden, Germany) AxSYM HAVAB 2.0, HAVAB-M 2.0, AxSYM-HBsAg (V2), AUSAB, core, HBeAg 2.0, Anti-HBe 2.0, HCV version 3.0. The hepatitis C PCR test procedure was an in-house LightCycler HCV PCR (established and validated in the NLGA) with a limit of detection of 102 copies/ml and has been described previously [21]. HCV genotyping was performed at the beginning with INNO-Lipa HCV II (Innogenetics, Ghent, Belgium), and later with Versant™ HCV genotype assay (LiPA; Bayer, Leverkusen, Germany).
Each of the 97 inmates who tested positive for anti-HCV or HCV RNA was then asked for informed consent for additional blood drawing and more detailed data analysis. Seven inmates who had tested positive refused to participate further in the study. Therefore, follow-up information was available for 90 inmates. Hepatitis serology including virus load, subtypes and biochemical parameters were investigated after informed consent was given. Of particular interest were: AST (aspartate aminotransferase), ALT (alanine aminotranferase), bilirubin (total), AP (alkaline phosphatase), GGPT (gamma-glutamyl transpepdidase), LDH (lactic dehydrogenase), albumin, iron (Fe), international normalized ratio (INR), as well as blood counts using standard commercial assays.
After 3 months we performed a detailed interview incorporating a further evaluation of medical history, risk profile, drug consumption, nicotine and alcohol consumption, and subjective and objective health.
In total, 70% of the 90 new incoming HCV patients underwent drug detoxification. Inmates were sent to the detoxification unit immediately after imprisonment. Blood drawing for this study was performed within the first 2 weeks of detoxification and interviewing for risk factors was performed after detoxification.
Statistical analysis
Descriptive statistics were calculated and reported as mean and standard deviation (s.d.) or range unless indicated otherwise using SPSS for Windows XP, version 10.0 (SPSS Inc., Chicago, IL, USA).
The significance of differences in rates or prevalence between prisoners from Germany and from the NIS was assessed by means of χ2 tests. The significance of mean differences of quantitative variables was assessed by using t tests. A P value of <0·05 was considered statistically significant.
RESULTS
Inmates from the NIS were overrepresented by a factor of 4 when compared to the general population of Germany. Whereas, in the age-matched German reference population, 2·6% of individuals had migrated to Germany from NIS countries compared with 12% of offenders from NIS countries, a significantly higher number (P<0·001) [19].
Prevalence of hepatitis C: HCV markers (HCV RNA or HCV antibodies) were found in 97 (8·6%) of the 1125 inmates tested. Seven of the 97 inmates refused to participate further in the study. Therefore, more detailed information was available for only the remaining 90 HCV positive inmates.
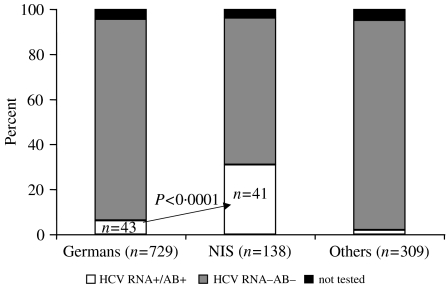
Importantly, hepatitis C markers were significantly more prevalent among immigrants from the NIS than among German inmates (31·1% vs. 6·2% respectively, P<0·0001) (Fig.). Of the 90 inmates who tested positive for anti-HCV or HCV RNA, 43 (47%) were German (of 729 German inmates tested) and 41 (46%) from the NIS (of 138 NIS inmates tested). Another six (7%) positive inmates came from other foreign countries (Poland 2, Turkey 2, Lebanon 1, Yugoslavia 1).
Fig.
Distribution of hepatitis C markers in inmates grouped according to country of birth. Hepatitis C markers were significantly more prevalent among immigrants from the former Soviet Union (NIS) than among German inmates (31·1% vs. 6·2% respectively, P<0·0001).
The 41 (46%) inmates from the NIS with HCV markers had migrated to Germany predominantly from the Russian Federation (n=17) and Kazakhstan (n=17). The other countries of origin were Georgia (n=2), Lithuania (n=2), Latvia (n=1), Moldova (n=1) and Tajikistan (n=1).
Of the 90 inmates who agreed to further testing, 65 (72%) were positive for HCV RNA and anti-HCV antibodies. Six (7%) inmates were only HCV RNA positive without anti-HCV antibodies. Anti-HCV antibodies in the absence of HCV viraemia were detected in 19 individuals (21%). When comparing German inmates and inmates from the NIS a significant difference was not seen in the proportion of inmates with chronic hepatitis and those who were persistently HCV RNA negative (Table 1).
Table 1.
Clinical outcome of young offenders who took part in the study in dependence of HCV RNA and/or HCV-antibody status, for all 90 inmates and for the most important countries of origin
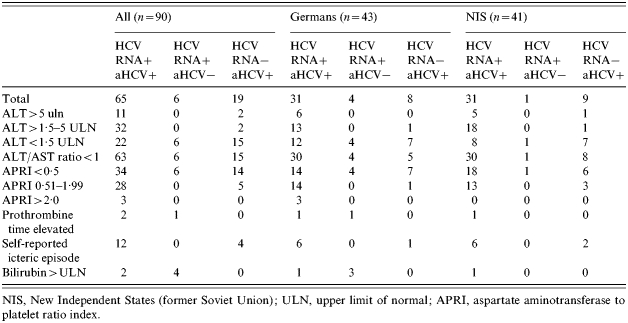
NIS, New Independent States (former Soviet Union); ULN, upper limit of normal; APRI, aspartate aminotransferase to platelet ratio index.
Four of the six HCV RNA-positive/anti-HCV-negative patients cleared HCV RNA from serum spontaneously within 6 months without developing HCV antibodies. Follow-up data of these six individuals are shown in Table 2. Details of these patients will be presented elsewhere (M. F. Meyer et al., unpublished observations).
Table 2.
Virological follow-up of the six HCV RNA-positive/HCV antibodies negative patients over 6 months
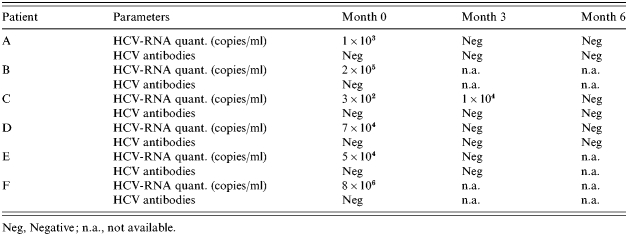
Neg, Negative; n.a., not available.
Risk factor profile
Of the 90 inmates who tested positive for an HCV marker, intravenous (i.v.) drug use was self-reported by 85 inmates (94%). German inmates reported a mean duration of 27·4 months of i.v. drug use, whereas inmates from the NIS reported a mean duration of 38·4 months.
Nearly 70% of incoming HCV-positive offenders underwent opioid and/or benzodiazepine detoxification directly in prison. Before imprisonment most of these young men had already experienced detoxification attempts (mean 4·4, range 1–20 attempts).
Young offenders born in Germany showed a mean of 3·9 attempts (range 0–20), while young offenders from the NIS tended to have had more unsuccessful attempts with a mean of 5·6 attempts (range 0–20, P=0·08).
Drugs such as heroin and/or cocaine were much more common than alcohol in the infected young offenders outside prison. Thirty percent of German inmates and 22% of inmates from the NIS denied consuming alcohol. Daily or almost daily alcohol consumption (>50 g/d) prior to incarceration was reported by 23% of German inmates and by 2% of inmates from the NIS. Combined drug and alcohol use is, therefore, much more prevalent amongst the German group. We did not observe differences relating to the type of alcohol consumed, e.g. beer, wine or other substances such as vodka or schnapps.
Smoking is common in prison. All inmates who took part in this study reported smoking daily. German inmates reported a mean number of pack-years of 8·5 (s.d.=4·8) while inmates from the NIS reported 6·8 (3·9), ranging from 2 to 30. Considering their mean age of only 20·6 (1·6) and 19·9 (1·7) years respectively, they started to smoke at an average age of 11–13 years.
Twenty-nine (43%) of the inmates reported smoking more frequently during imprisonment, while only 15 (22%) prisoners reduced their nicotine intake.
Genotyping was performed on 68 of the HCV RNA-positive individuals: 33 German inmates, 31 inmates from the NIS and four inmates from other countries. In total, genotypes 1 and 3 were most widespread: 34 cases (50%) and 24 cases (35%) respectively. Genotypes 2 and 4 were only present in five (7%) and three (5%) individuals, respectively. Only two inmates had mixed genotypes 1+3 (3%). German inmates were more frequently infected with genotype 1 (64% vs. 32%, P=0·01) while inmates from the NIS were more frequently infected with genotype 3 (27% vs. 48%, P=0·08) (Table 3).
Table 3.
Hepatitis virology for all HCV RNA-positive inmates, grouped according to country of origin
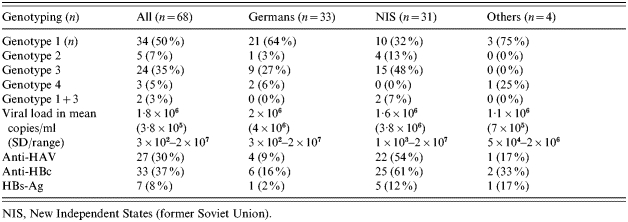
NIS, New Independent States (former Soviet Union).
In the first blood test German inmates showed a mean value of 2×106 copies/ml (range 3×102 to 2×107 copies/ml), while inmates from the NIS had a mean viral load of 1·6×106 copies/ml (range 1×103 to 2×107 copies/ml) (Table 3). None of the HCV-infected inmates received antiviral therapy with pegylated-interferon and/or ribavirin before or during imprisonment.
The frequency of co-infections with HAV, HBV and/or HIV differed in relation to origin. Inmates from the NIS had a higher prevalence of HAV and HBc antibodies, as shown in Table 3. In addition, HBsAg was detected in five (12%) of the NIS inmates, one German (2%) and one Lebanese inmate, who all suffered from chronic hepatitis B infection. HBc antibodies were also more prevalent among NIS inmates (25/41 vs. 7/43, P<0·0001). Similarly, HAV antibodies were detected less frequently in German inmates (22/41 vs. 4/43, P<0·0001). HIV infections were found in five cases, all of whom were German.
Clinical presentation
A presentation the ALT levels were <1·5 times the upper limit of normal (ULN) in 28 out of 71 (39%) viraemic patients, while 11 (16%) individuals showed ALT levels >5 times the ULN. Importantly, all but two of the HCV RNA-positive patients had no biochemical evidence for advanced liver disease since the Child–Pugh score was A in all patients and only two individuals showed an AST/ALT ratio of >1. We also calculated the AST to platelet ratio index (APRI) for individuals. This index is based on AST and platelet levels and has been shown to exclude significant fibrosis (score<0·5) and to predict cirrhosis (score>2·0) with a high accuracy in patients with chronic hepatitis C [20]. Fifty-four inmates showed an APRI score of ⩽0·5 and 27 inmates had scores from 0·51 to 1·5. Six patients had an APRI of >1·5, while only three inmates showed an APRI score of >2·0.
It is worth noting that significantly elevated ALT levels were also present in four of the HCV RNA−/anti-HCV+ individuals, indicating that liver damage was not due to HCV infection but rather due to toxic liver injury caused by drug consumption. However, we cannot exclude completely that there were false-negative HCV RNA results in single cases.
DISCUSSION
Hepatitis C infection is a significant health problem in prisons. A high prevalence of HCV antibodies among prisoners has been reported previously. Overall, 20–37% of inmates were positive for HCV antibodies. However, HCV prevalence was much higher in IDUs ranging from 49% to 88% [3–13]. Few studies have investigated hepatitis C in young inmates. As expected, in our study on prisoners in the 16–24 years age group the 9% prevalence of HCV markers (HCV antibodies or HCV RNA) was lower. This prevalence is about fourfold higher than the 2% reported for young prisoners incarcerated in Washington State, USA [19]. However, only 6% of the subjects in that study had reported i.v. drug use potentially explaining the low HCV prevalence. The relevance of i.v. drug use as the main risk factor for HCV transmission is highlighted by our findings that 94% of anti-HCV-positive individuals had used i.v. drugs before for an average duration of almost 3 years (mean 35 months). This is in line with recent data from Australia on inmates aged 15–18 years showing an anti-HCV prevalence of 21% [13]. Importantly, all but one of the Australian HCV-positive inmates had also reported i.v. drug use. Finally, we have shown previously that the longer inmates have used i.v. drugs, the higher is the chance of anti-HCV-positive individuals becoming persistently HCV RNA positive [21].
One additional potential risk factor for HCV transmission is tattooing. The majority of young inmates had at least one tattoo. Sexual transmission seems to be of minor importance; however 12 of the 90 subjects reported having sexual contact without using condoms although they were aware of being HCV RNA positive (M. F. Meyer et al., unpublished observations). Overall the low prevalence of HCV among young inmates highlights the urgent need for educational programmes on viral hepatitis in prisons. Prevention of further spreading of HCV is likely to be successful only if continuous use of i.v. drugs and needle sharing is avoided [13].
Educational programmes, however, should take the country of origin of inmates into account. While the overall prevalence of hepatitis C among young inmates in Germany was low in our study, about one third of the subgroup of immigrants from the NIS tested positive for hepatitis C markers. In addition, >10% of these suffered from HBV co-infection. In Germany, individuals from the NIS are four times overrepresented in prisons compared with the general population. Nearly half (46%) of HCV infections involved prisoners from the NIS (Fig.). Thus, future educational programmes should focus on this issue. Although most of the immigrants from the NIS lived in Germany for several years, many do not speak German very well and do not interact with German inmates. In addition, inmates from the NIS and Germany significantly differ in their risk behaviour as inmates from the NIS had used i.v. drugs for a longer period and had started i.v. drug use at an earlier age (Table 1). On the other hand, inmates from the NIS reported significant consumption of alcohol less frequently.
Another interesting finding was that, surprisingly, systematic screening not only for anti-HCV but also for HCV RNA identified six HCV RNA-positive/anti-HCV-negative individuals (0·5% of all 1176 individuals tested). Four of whom cleared their infections spontaneously within 6 months without developing HCV antibodies (Table 2). The other two inmates left prison before follow-up data could be determined. It is important to note that all subjects with viraemia in the absence of HCV seroconversion were immunocompetent. Moreover, we cannot exclude a false-positive HCV RNA PCR in single cases, HCV RNA was either detected at two time-points or we have evidence that some of these subjects developed T-cell responses against HCV (M. F. Meyer et al., unpublished observations). It may be of importance that in contrast to patients developing anti-HCV antibodies, three of these four inmates denied any i.v. drug consumption. Thus, it is tempting to speculate that lower levels of the infectious inoculate were present in sporadic HCV transmission compared with i.v. drug-associated transmission. These lower levels of HCV RNA could have contributed to the different outcome of infection and the lack of development of HCV antibodies. Similar findings have been reported recently in prison inmates from Australia [22]. Future studies will have to investigate whether routine HCV RNA testing is useful for all incoming prisoners to detect HCV-viraemic/anti-HCV-negative individuals. These studies should consider the significant costs on one hand and potential prevention of HCV transmission and possible therapeutic consequences on the other.
The clinical course of hepatitis C was rather mild in our cohort. None of the HCV-positive inmates had evidence of cirrhosis as the platelets were normal in all patients and serum markers for liver synthesis were also normal in all but one patient with acute hepatitis. In addition, we calculated the APRI score in all individuals. This index has been shown to be able to exclude cirrhosis with high accuracy [21]. All but three patients had an APRI score of >2 thus excluding cirrhosis. Furthermore, 54 out of 90 patients even had an APRI score of <0·5 indicating the absence of any significant early fibrosis. The mild course of HCV infection in our cohort is in line with several other reports confirming low rates of cirrhosis in children and young adults [23–25] and also highlights the slow speed of progression as our patients had been infected with HCV for only a few years. Thus, large-scale interferon-based therapies in a prison setting for young men may not be needed to prevent severe liver disease. In contrast, successful antiviral therapy could prevent further HCV transmission. However, we did not find any new cases of viral hepatitis after imprisonment (data not shown). Thus, in our view the main task for the management of hepatitis C in prisons for young men will be to overcome drug addiction rather than to treat all HCV RNA-positive patients with an expensive drug with the potential to cause significant side-effects [26]. However, it will be important to reduce potential risk factors for the progression of fibrosis. Smoking is common in prison and all inmates who took part in this study reported smoking daily (mean 8 pack- years). Considering the young age of our patients these results are alarming. In addition, nearly half of the inmates (43%) increased smoking during imprisonment. Smoking has been shown to aggravate the histological activity of chronic hepatitis C and to accelerate progression of fibrosis [27, 28]. Thus, educational programmes should not only focus on hard drugs but also on alcohol and smoking.
In conclusion, significant differences in HCV prevalence among young inmates in Germany can be detected in different migrational backgrounds. However, hepatitis C has a less severe clinical course in young men. Educational programmes and activities to prevent HCV transmission by i.v. drug use are warranted urgently in prisons to prevent further HCV transmission.
ACKNOWLEDGEMENTS
This study was supported in part by a grant from the German Network of Competence on Viral Hepatitis (Hep-Net) to H.W. (core project 10.2.2). We acknowledge the contribution of Dr Armin Baillot from NLGA in performing this study.
DECLARATION OF INTEREST
None.
REFERENCES
- Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002;36:S93–S98. doi: 10.1053/jhep.2002.36389. [DOI] [PubMed] [Google Scholar]
- WHO. Weekly Epidemiological Record. World Health Organization; , No. 49, 10 December 1999, [Google Scholar]
- Macalino GE et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. American Journal of Public Health. 2004;94:1218–1223. doi: 10.2105/ajph.94.7.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillargeon J et al. Hepatitis C seroprevalence among newly incarcerated inmates in the Texas correctional system. Public Health. 2003;117:43–48. doi: 10.1016/s0033-3506(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Weild AR et al. Prevalence of HIV, hepatitis B, and hepatitis C antibodies in prisoners in England and Wales: a national survey. Communicable Disease and Public Health. 2000;3:121–126. [PubMed] [Google Scholar]
- Grupo Noroeste para el estudio de la Hepatitis C en el medio penitenciario. Seroprevalence of hepatitis C virus infection at the time of entry to prison in the prison population in the north-east of Spain. Revista Española de Salud Pública. 1998;72:43–51. [PubMed] [Google Scholar]
- Long J et al. Prevalence of antibodies to hepatitis B, hepatitis C, and HIV and risk factors in entrants to Irish prisons: a national cross sectional survey. British Medical Journal. 2001;323:1209–1213. doi: 10.1136/bmj.323.7323.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backmund M et al. Hepatitis C virus infection in injection drug users in Bavaria: risk factors for seropositivity. European Journal of Epidemiology. 2003;18:563–568. doi: 10.1023/a:1024603517136. [DOI] [PubMed] [Google Scholar]
- Gore SM et al. Prevalence of hepatitis C in prisons: WASH-C surveillance linked to self-reported risk behaviours. QJM. 1999;92:25–32. doi: 10.1093/qjmed/92.1.25. [DOI] [PubMed] [Google Scholar]
- Macalino GE et al. Prevalence and incidence of HIV, hepatitis B virus, and hepatitis C virus infections among males in Rhode Island prisons. American Journal of Public Health. 2004;94:1218–1223. doi: 10.2105/ajph.94.7.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon L et al. Prevalence of HIV, syphilis, hepatitis B, and hepatitis C among entrants to Maryland correctional facilities. Journal of Urban Health. 2004;81:25–37. doi: 10.1093/jurban/jth085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen PB et al. Prevalence and incidence of bloodborne viral infections among Danish prisoners. European Journal of Epidemiology. 2000;16:1043–1049. doi: 10.1023/a:1010833917242. [DOI] [PubMed] [Google Scholar]
- Ogilvie EL et al. Hepatitis infection among adolescents resident in Melbourne Juvenile Justice Centre: risk factors and challenges. Journal of Adolescent Health. 1999;25:46–51. doi: 10.1016/s1054-139x(98)00086-x. [DOI] [PubMed] [Google Scholar]
- Maher L et al. Risk behaviors and antibody hepatitis B and C prevalence among injecting drug users in south-western Sydney, Australia. Journal of Gastroenterology and Hepatology. 2004;19:1114–1120. doi: 10.1111/j.1440-1746.2004.03438.x. [DOI] [PubMed] [Google Scholar]
- Rhodes T, Davis M, Judd A. Hepatitis C and its risk management among drug injectors in London: renewing harm reduction in the context of uncertainty. Addiction. 2004;99:621–633. doi: 10.1111/j.1360-0443.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- Stark K et al. History of syringe sharing in prison and risk of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infection among injecting drug users in Berlin. International Journal of Epidemiology. 1997;26:1359–1366. doi: 10.1093/ije/26.6.1359. [DOI] [PubMed] [Google Scholar]
- Murray KF et al. Prevalence of hepatitis C virus infection and risk factors in an incarcerated juvenile population: a pilot study. Pediatrics. 2003;111:153–157. doi: 10.1542/peds.111.1.153. [DOI] [PubMed] [Google Scholar]
- Baillargeon J et al. Hepatitis C seroprevalence among newly incarcerated inmates in the Texas correctional system. Public Health. 2003;117:43–48. doi: 10.1016/s0033-3506(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Bundesministerium des Inneren. Jahresstatistik. 2003. www.bmi.bund.de/cln_012/nn_165290/Internet/Content/Themen/Vertriebene__Spaetaussiedler/statistiken/jahresstatistik__2005.html www.bmi.bund.de/cln_012/nn_165290/Internet/Content/Themen/Vertriebene__Spaetaussiedler/statistiken/jahresstatistik__2005.html
- Wai CT et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- Lehmann M et al. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. Journal of Medical Virology. 2004;73:387–391. doi: 10.1002/jmv.20103. [DOI] [PubMed] [Google Scholar]
- Post JJ et al. Clearance of hepatitis C viremia associated with cellular immunity in the absence of seroconversion in the hepatitis C incidence and transmission in prisons study cohort. Journal of Infectious Diseases. 2004;189:1846–1855. doi: 10.1086/383279. [DOI] [PubMed] [Google Scholar]
- Wiese M et al. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in germany: a 20-year multicenter study. Hepatology. 2000;32:91–96. doi: 10.1053/jhep.2000.8169. [DOI] [PubMed] [Google Scholar]
- Vogt M et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. New England Journal of Medicine. 1999;341:866–870. doi: 10.1056/NEJM199909163411202. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:21–29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:237–244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- Pessione F et al. Cigarette smoking and hepatic lesions in patients with chronic hepatitis C. Hepatology. 2001;34:121–125. doi: 10.1053/jhep.2001.25385. [DOI] [PubMed] [Google Scholar]
- Hezode C et al. Impact of smoking on histological liver lesions in chronic hepatitis C. Gut. 2003;52:126–129. doi: 10.1136/gut.52.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]