SUMMARY
The prevalence of metallo-β-lactamase (MBL) production among Pseudomonas aeruginosa nosocomial isolates from a Brazilian teaching hospital was determined. A total of 512 P. aeruginosa isolates were recovered from 245 patients during a 10-month period. Ninety-four (38·4%, 95% CI 32·2–44·8%) isolates were MBL producers. Most resistance to β-lactams was mediated by MBL. Forty-one (16·7%) were resistant to all drugs except polymyxin B and 33 (80·5%) of these were MBL producers. Clonal dissemination, documented by DNA macrorestriction, played a major role for the spread of MBL isolates. The blaSPM-1 gene was demonstrated by PCR in 14 randomly selected MBL isolates. The extremely high prevalence of MBL production found challenges the choice of therapeutics for P. aeruginosa, and measures to control horizontal dissemination of MBL producers are urgently required.
Pseudomonas aeruginosa is a leading cause of nosocomial infections throughout the world. This organism has constitutive resistance to many drug classes and readily becomes resistant to all relevant treatments owing to its multiply acquired resistance mechanisms [1].
The β-lactamases are the major resistance mechanism in Gram-negative rods, including P. aeruginosa [2]. In the past, the most common types of β-lactamases, the AmpC types and the extended spectrum β-lactamases, have accounted for much of the β-lactam resistance among Gram-negative nosocomial pathogens, with the exception of carbapenems, which are stable against most of these enzymes [2]. Recently, the Ambler class B β-lactamases, the metallo-β-lactamases (MBLs), have emerged as one of the most potent resistance mechanisms because they inactivate virtually all β-lactams, except aztreonam, and because their genes are carried on highly mobile elements [3]. The prevalence of MBLs has been increasing worldwide, notably among P. aeruginosa, severely limiting therapeutic options for the treatment of patients [3].
β-lactam, including carbapenem, resistance in P. aeruginosa is a significant problem in Latin America, especially in Brazil [4]. In this country, P. aeruginosa producing MBL (MBL-PA), particularly the SPM-1 type, have been reported with increasing frequency [5–7]. The current study was performed to determine the prevalence of MBL production and to assess the impact of these enzymes on β-lactam resistance among P. aeruginosa nosocomial isolates in a tertiary-care teaching hospital in Porto Alegre, Southern Brazil.
From September 2004 to June 2005 all P. aeruginosa isolates recovered from patients admitted >48 h (or from those who had been hospitalized in the last 60 days) to São Lucas Hospital, a 600-bed hospital, were analysed. Only one isolate per patient was included.
Biochemical tests were used to identify P. aeruginosa and included: oxidase production, oxidation of glucose, arginine utilization, nitrate reduction, growth on cetrimide agar, and production of characteristic pigments. Susceptibility was determined by the disk-diffusion method according to NCCLS guidelines [8]. The following antibiotics were tested: amikacin, aztreonam, cefepime, ceftazidime, ciprofloxacin, imipenem, meropenem and piperacillin-tazobactam. Polymyxin B susceptibility was determined using the interpretative criteria (⩾14 mm) proposed elsewhere [9]. All isolates resistant to ceftazidime were screened for MBL production with ceftazidime in the presence of 2-mercaptopropionic acid as previously described [10]. Minimum inhibitory concentrations (MICs) of aztreonam, cefepime, ceftazidime, imipenem, meropenem and piperacillin-tazobactam were determined by E test (AB Biodisk, Solna, Sweden) for MBL-PA isolates.
A sample of randomly selected MBL-PA isolates were tested by PCR for detection of the blaSPM-1 gene as previously described [7]. DNA macrorestriction using SpeI followed by PFGE was performed for molecular typing of randomly selected isolates [11]. Restriction fragment profiles were compared visually and were analysed using the Tenover criteria [12].
Statistical analyses were carried out using SPSS for Windows, version 13.0 (SPSS Inc., Chicago, IL, USA). Prevalence ratios (PRs) and 95% confidence intervals (CIs) were calculated. P values were calculated using χ2 or Fisher’s exact test.
A total of 512 P. aeruginosa isolates were recovered from 245 patients during the study period. MBL-PA isolates were recovered at least once from 94 (38·4%, 95% CI 32·2–44·8%) of these 245 patients. The mean MBL-PA prevalence each month was 36·4%±14·1, ranging from 15·0% in November 2004 to 54·5% in June 2005. MBL-PA isolates were more frequently recovered from respiratory secretions (39·4%), followed by urine (22·3%), blood (11·7%), surgical wound (8·5%), central venous catheter (4·3%), abdominal secretions (4·3%), and other secretions (9·5%). Non-MBL-PA isolates were recovered from respiratory secretions (43·7%), followed by urine (11·3%), surgical wound (10·6%), blood (6·6%), central venous catheter (3·3%), abdominal secretions (3·3%), and other secretions (21·2%). There was no specific site associated with higher frequency of recovery of MBL-PA (P=0·12).
Higher resistance rates were noted for piperacillin-tazobactam (61·2%) followed by ciprofloxacin (59·6%), imipenem (56·7%), cefepime (54·3%), meropenem (52·2%), amikacin (50·6%), ceftazidime (48·6%) and aztreonam (31·8%). All isolates were susceptible to polymyxin B.
As shown in the Table, MBL production was detected in most β-lactam-resistant isolates. MBL production was demonstrated in 33 (80·5%) of 41 isolates susceptible only to polymyxin B, and in 44 (95·7%) of 46 isolates susceptible only to polymyxin B and aztreonam. Resistance to non-β-lactam agents was significantly higher among MBL-PA isolates than non-MBL-PA. Amikacin resistance was noted in 78 (83·0%) of 94 MBL-PA isolates and in only 46 (30·5%) of 151 non-MBL-PA isolates (PR 2·72, 95% CI 2·11–3·53, P<0·001). Ninety-three (98·9%) MBL-PA isolates were resistant to ciprofloxacin, while 53 (35·1%) of non-MBL-PA isolates were resistant to this drug (PR 2·82, 95% CI 2·27–3·51, P<0·001). Aztreonam resistance was also significantly higher among MBL producers (40·4% vs. 26·5%, PR 1·53, 95% CI 1·06–2·19, P=0·03), although most of MBL-PA isolates were intermediately resistant to this drug (median MIC=12 g/l, range 6–16 g/l).
Table.
Resistance to β-lactams and proportion of metallo-β-lactamase (MBL)-mediated resistances in 245 Pseudomonas aeruginosa nosocomial isolates
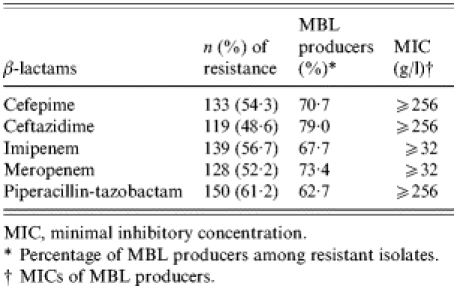
MIC, minimal inhibitory concentration.
Percentage of MBL producers among resistant isolates.
MICs of MBL producers.
Fourteen MBL-PA isolates were tested for the presence of MBL genes and all were positive for blaSPM-1. Of 18 MBL-PA isolates submitted to molecular typing, 17 (94·9%) were clonally related and only one showed a distinct DNA macrorestriction profile.
Our study presents findings of great concern since the prevalence of MBL among all nosocomial isolates was extremely high even considering the lower limit of the confidence interval (32·2%) as the real prevalence in this population. Most β-lactam resistance was mediated by MBL with MICs that indicated a high-level of resistance to these drugs. Moreover, 41 isolates were resistant to all drugs except polymyxin B comprising 16·7% of all P. aeruginosa isolates and 35·5% if we include those 46 isolates which were also susceptible to aztreonam. The great majority of these isolates were MBL producers. Resistance to other non-β-lactam drugs, except polymyxin B, was also significantly higher among MBL producers indicating the presence of other resistance mechanisms. The occurrence of intermediate or full resistance to aztreonam in many isolates points towards the same conclusion.
In summary, this study showed that MBL can emerge in specific settings accounting for broad-spectrum β-lactam resistance, and horizontal transmission has been a major determinant of dissemination and maintenance of the endemic status of MBL-PA at our institution [7]. Since MBLs are proving to be a powerful resistance mechanism either by their spectrum of activity or their capacity to disseminate, and since resistance to polymyxin B will probably increase following the widespread use of this drug, extremely rigid infection control measures must be urgently adopted, otherwise we may be briefly facing untreatable P. aeruginosa nosocomial infections.
Acknowledgments
We are grateful to Patrick Barcelos Gaspareto and Cláudia Meirelles Leite for support with microbiological tests. This study received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES, Ministry of Education, Brazil, and Fundação de Incentivo a Pesquisa e Eventos – FIPE, Hospital de Clínicas de Porto Alegre.
Declaration of Interest
None.
REFERENCES
- Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clinical Infectious Diseases. 2002;34:634–640. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- Jacoby GA, Munoz-Price LS. The new beta-lactamases. New England Journal of Medicine. 2005;352:380–391. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- Walsh TR et al. Metallo-beta-lactamases: the quiet before the storm? Clinical Microbiology Reviews. 2005;18:306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade SS et al. Increasing prevalence of antimicrobial resistance among Pseudomonas aeruginosa isolates in Latin American medical centers: 5 year report of the SENTRY Antimicrobial Surveillance Program (1997–2001) Journal of Antimicrobial Chemotherapy. 2003;52:140–141. doi: 10.1093/jac/dkg270. [DOI] [PubMed] [Google Scholar]
- Gales AC et al. Dissemination in distinct Brazilian regions of an epidemic carbapenem-resistant Pseudomonas aeruginosa producing SPM metallo-β-lactamase. Journal of Antimicrobial Chemotherapy. 2003;52:699–702. doi: 10.1093/jac/dkg416. [DOI] [PubMed] [Google Scholar]
- Sader HS et al. IMPs, VIMs and SPMs: the diversity of metallo-beta-lactamases produced by carbapenem-resistant Pseudomonas aeruginosa in a Brazilian hospital. Clinical Microbiology and Infection. 2005;1:73–76. doi: 10.1111/j.1469-0691.2004.01031.x. [DOI] [PubMed] [Google Scholar]
- Zavascki AP et al. Outbreak of carbapenem-resistant Pseudomonas aeruginosa producing SPM-1 metallo-β-lactamase in a teaching hospital in southern Brazil. Journal of Antimicrobial Chemotherapy. 2005;56:1148–1151. doi: 10.1093/jac/dki390. [DOI] [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards Wayne, PA: NCCLS; 2004. . Performance Standards for Antimicrobial disk Susceptibility Tests. NCCLS Document M100-S10. [Google Scholar]
- Gales AC, Reis AO, Jones RN. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. Journal of Clinical Microbiology. 2001;39:183–190. doi: 10.1128/JCM.39.1.183-190.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa Y et al. Convenient test for screening metallo-beta-lactamase-producing gram-negative bacteria by using thiol compounds. Journal of Clinical Microbiology. 2000;38:40–43. doi: 10.1128/jcm.38.1.40-43.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann ME. Pulsed-field gel electrophoresis. Methods in Molecular Medicine. 1998;15:17–31. doi: 10.1385/0-89603-498-4:33. [DOI] [PubMed] [Google Scholar]
- Tenover FC et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. Journal of Clinical Microbiology. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


