Abstract
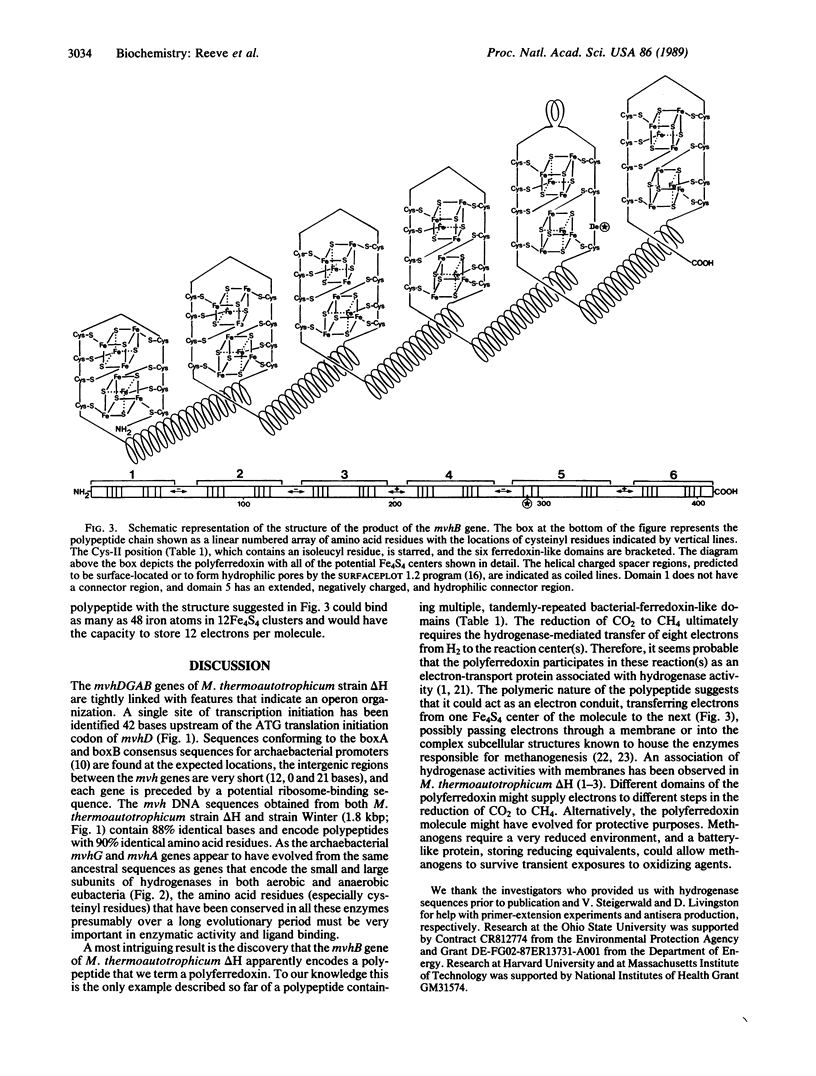
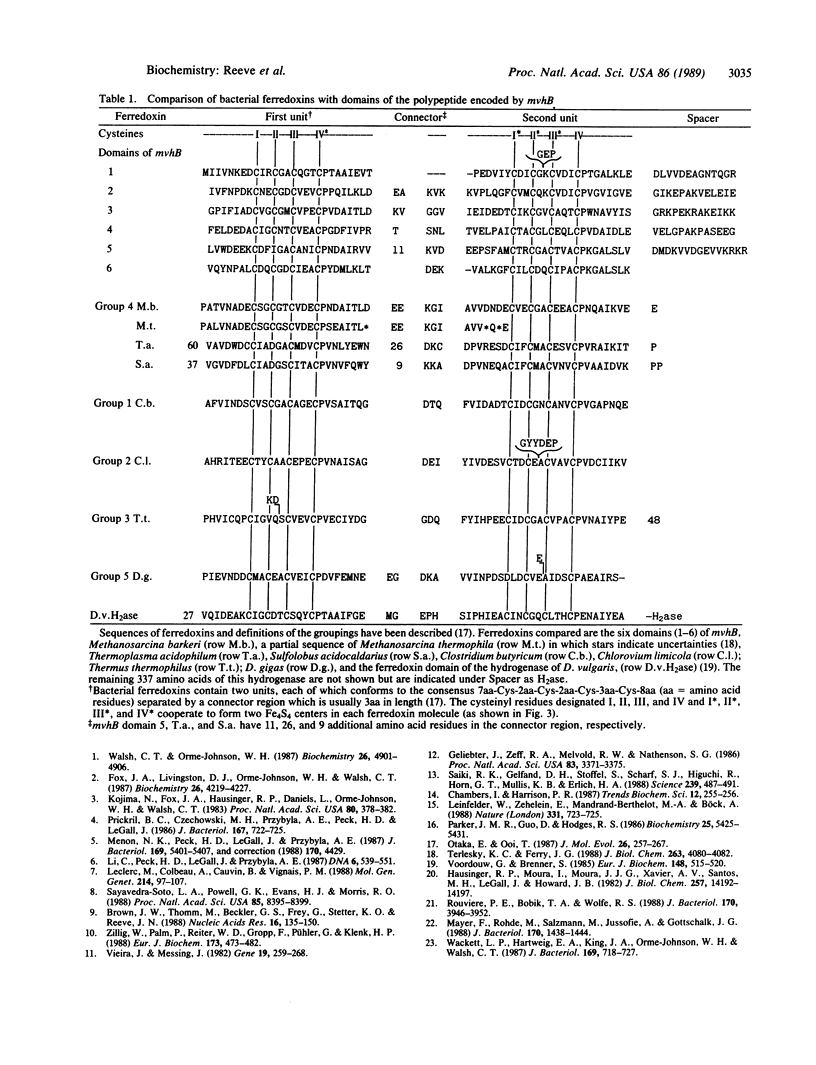
The genes mvhDGA, which encode the subunit polypeptides of the methyl viologen-reducing hydrogenase in Methanobacterium thermoautotrophicum strain delta H, have been cloned and sequenced. These genes, together with a fourth open reading frame designated mvhB, are tightly linked and appear to form an operon that is transcribed starting 42 base pairs upstream of mvhD. The organization and sequences of the mvhG and mvhA genes indicate a common evolutionary ancestry with genes encoding the small and large subunits of hydrogenases in eubacterial species. The product of the mvhB gene is predicted to contain six tandomly repeated bacterial-ferredoxin-like domains and, therefore, is predicted to be a polyferredoxin that could contain as many as 48 iron atoms in 12 Fe4S4 clusters.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. W., Thomm M., Beckler G. S., Frey G., Stetter K. O., Reeve J. N. An archaebacterial RNA polymerase binding site and transcription initiation of the hisA gene in Methanococcus vannielii. Nucleic Acids Res. 1988 Jan 11;16(1):135–150. doi: 10.1093/nar/16.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. A., Livingston D. J., Orme-Johnson W. H., Walsh C. T. 8-Hydroxy-5-deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum: 1. Purification and characterization. Biochemistry. 1987 Jul 14;26(14):4219–4227. doi: 10.1021/bi00388a007. [DOI] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausinger R. P., Moura I., Moura J. J., Xavier A. V., Santos M. H., LeGall J., Howard J. B. Amino acid sequence of a 3Fe:3S ferredoxin from the "archaebacterium" Methanosarcina barkeri (DSM 800). J Biol Chem. 1982 Dec 10;257(23):14192–14197. [PubMed] [Google Scholar]
- Kojima N., Fox J. A., Hausinger R. P., Daniels L., Orme-Johnson W. H., Walsh C. Paramagnetic centers in the nickel-containing, deazaflavin-reducing hydrogenase from Methanobacterium thermoautotrophicum. Proc Natl Acad Sci U S A. 1983 Jan;80(2):378–382. doi: 10.1073/pnas.80.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc M., Colbeau A., Cauvin B., Vignais P. M. Cloning and sequencing of the genes encoding the large and the small subunits of the H2 uptake hydrogenase (hup) of Rhodobacter capsulatus. Mol Gen Genet. 1988 Sep;214(1):97–107. doi: 10.1007/BF00340186. [DOI] [PubMed] [Google Scholar]
- Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988 Feb 25;331(6158):723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- Li C., Peck H. D., Jr, LeGall J., Przybyla A. E. Cloning, characterization, and sequencing of the genes encoding the large and small subunits of the periplasmic [NiFe]hydrogenase of Desulfovibrio gigas. DNA. 1987 Dec;6(6):539–551. doi: 10.1089/dna.1987.6.539. [DOI] [PubMed] [Google Scholar]
- Mayer F., Rohde M., Salzmann M., Jussofie A., Gottschalk G. The methanoreductosome: a high-molecular-weight enzyme complex in the methanogenic bacterium strain Gö1 that contains components of the methylreductase system. J Bacteriol. 1988 Apr;170(4):1438–1444. doi: 10.1128/jb.170.4.1438-1444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon N. K., Peck H. D., Jr, Gall J. L., Przybyla A. E. Cloning and sequencing of the genes encoding the large and small subunits of the periplasmic (NiFeSe) hydrogenase of Desulfovibrio baculatus. J Bacteriol. 1987 Dec;169(12):5401–5407. doi: 10.1128/jb.169.12.5401-5407.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaka E., Ooi T. Examination of protein sequence homologies: IV. Twenty-seven bacterial ferredoxins. J Mol Evol. 1987;26(3):257–267. doi: 10.1007/BF02099857. [DOI] [PubMed] [Google Scholar]
- Parker J. M., Guo D., Hodges R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986 Sep 23;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Prickril B. C., Czechowski M. H., Przybyla A. E., Peck H. D., Jr, LeGall J. Putative signal peptide on the small subunit of the periplasmic hydrogenase from Desulfovibrio vulgaris. J Bacteriol. 1986 Aug;167(2):722–725. doi: 10.1128/jb.167.2.722-725.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., Bobik T. A., Wolfe R. S. Reductive activation of the methyl coenzyme M methylreductase system of Methanobacterium thermoautotrophicum delta H. J Bacteriol. 1988 Sep;170(9):3946–3952. doi: 10.1128/jb.170.9.3946-3952.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sayavedra-Soto L. A., Powell G. K., Evans H. J., Morris R. O. Nucleotide sequence of the genetic loci encoding subunits of Bradyrhizobium japonicum uptake hydrogenase. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8395–8399. doi: 10.1073/pnas.85.22.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlesky K. C., Ferry J. G. Purification and characterization of a ferredoxin from acetate-grown Methanosarcina thermophila. J Biol Chem. 1988 Mar 25;263(9):4080–4082. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Voordouw G., Brenner S. Nucleotide sequence of the gene encoding the hydrogenase from Desulfovibrio vulgaris (Hildenborough). Eur J Biochem. 1985 May 2;148(3):515–520. doi: 10.1111/j.1432-1033.1985.tb08869.x. [DOI] [PubMed] [Google Scholar]
- Wackett L. P., Hartwieg E. A., King J. A., Orme-Johnson W. H., Walsh C. T. Electron microscopy of nickel-containing methanogenic enzymes: methyl reductase and F420-reducing hydrogenase. J Bacteriol. 1987 Feb;169(2):718–727. doi: 10.1128/jb.169.2.718-727.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. T., Orme-Johnson W. H. Nickel enzymes. Biochemistry. 1987 Aug 11;26(16):4901–4906. doi: 10.1021/bi00390a001. [DOI] [PubMed] [Google Scholar]
- Zillig W., Palm P., Reiter W. D., Gropp F., Pühler G., Klenk H. P. Comparative evaluation of gene expression in archaebacteria. Eur J Biochem. 1988 May 2;173(3):473–482. doi: 10.1111/j.1432-1033.1988.tb14023.x. [DOI] [PubMed] [Google Scholar]


