SUMMARY
It is estimated that of 50 000 persons in Scotland (1% of the county’s population), infected with the hepatitis C virus (HCV), around 90% injected drugs. This paper reviews data on the prevalence and incidence of HCV, and the methods used to generate such information, among injecting drug users (IDUs), in Scotland. The prevalence estimate for HCV among IDUs in Scotland as a whole (44% in 2000), is comparable with those observed in many European countries. Incidence rates ranged from 11·9 to 28·4/100 person-years. The data have shaped policy to prevent infection among IDUs and have informed predictions of the number of HCV-infected IDUs who will likely progress to, and require treatment and care for, severe HCV-related liver disease. Although harm reduction interventions, in particular needle and syringe exchanges and methadone maintenance therapy, reduced the transmission of HCV among IDUs during the early to mid-1990s, incidence in many parts of the country remains high. The prevention of HCV among IDUs continues to be one of Scotland’s major public health challenges.
INTRODUCTION
Since the mid-1990s, Health Protection Scotland (HPS; formerly the Scottish Centre for Infection and Environmental Health) has coordinated the country’s hepatitis C virus (HCV) infection surveillance programme. The data generated indicate that, as in other resource-rich countries, the population in Scotland most at risk of infection are those who have injected drugs. Scotland, probably, has one of the highest injecting drug use, and HCV among injecting drug users (IDUs), prevalences in Europe; an estimated 89 900 current and former IDUs, of whom 45 500 were HCV infected resided in Scotland during 2005 [1].
Data on the prevalence and incidence of HCV among IDUs are crucial in informing those responsible for developing and evaluating strategies to prevent transmission of infection and services for those infected. In this paper, the authors review the results of, and different methods employed in, surveys undertaken to estimate HCV prevalence and incidence among this population in Scotland. As a country, with a population of 5 000 000, Scotland is culturally and economically typical of many resource-rich countries in the world.
METHODS
Several information sources were accessed to collect data for this review. Studies published between 1990 and 2005 (inclusive) were identified through a computerized search (Medline and EMBASE) using relevant key words and MeSH headings. A search for publications using a list of the names of Scottish researchers known by the authors to be working in the relevant field of work was undertaken. As HPS is often involved directly, or indirectly, in HCV surveillance and research conducted throughout the country, a hand search of the library of papers and unpublished documents to which staff had contributed was performed.
National surveillance data and reports (published and unpublished) on the prevalence and incidence of HCV seropositivity among IDUs in Scotland were reviewed; studies based on self-reported HCV status were excluded. For each study, the following information was recorded: characteristics of the population tested [NHS Board area in which IDUs either (i) resided, (ii) attended health service clinics or (iii) were in prison], the numbers of specimens and the proportion found to be HCV antibody positive, the method used to obtain specimens, the specimen type, the year the specimen was collected and the setting in which the specimen was taken.
RESULTS
Since 1991 when antibody tests to detect HCV became available, 23 scientific articles concerning the epidemiology of HCV in Scotland have been published in peer-review journals; of these, 14 reported studies of HCV prevalence and/or incidence among IDUs. Eight additional relevant sources of information, not commercially published or generally available, were also identified.
Prevalence of diagnosed HCV infection
National surveillance of HCV antibody-positive diagnoses
A database of all persons, known to have been infected with HCV in Scotland, was established by HPS, in association with Scotland’s principal HCV testing laboratories, in 1996 [2]. The database holds the following non-identifying data on all persons in Scotland who have had a positive anti-HCV result: forename initial, soundex code of surname, date of birth, gender, date of earliest positive specimen, source of specimen, area of residence and risk information.
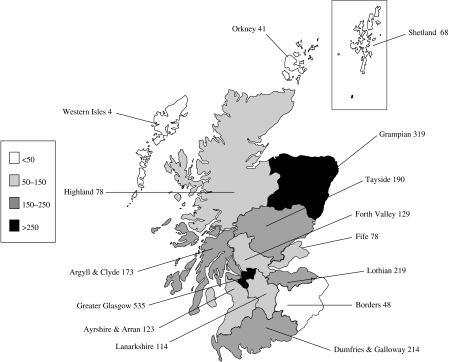
By the end of June 2005, 19 422 persons had been diagnosed with HCV infection [3] of which, approximately 88%, 1 in 341 of Scotland’s population, were alive [4]. Of these, 11 777 (61%) were known to have injected drugs; no risk information was available for 6417 cases. Geographical variations exist with the highest rate of IDU-related diagnosed infection per 100 000 in the Greater Glasgow NHS Board area (535/100 000) and the lowest in the Western Isles NHS Board area (4/100 000) (Fig. 1). The Greater Glasgow, Lothian, Tayside and Grampian NHS Board areas account for three quarters of all diagnosed IDUs.
Fig. 1.
Cumulative cases of diagnosed HCV infection among IDUs (by NHS Board of source of specimen, cumulative 1991 to end June 2005 per 100 000 population).
Prevalence of diagnosed and undiagnosed HCV infection among Scottish IDUs (Table 1)
Table 1.
Prevalence of HCV infection among different injecting drug user (IDU) populations in Scotland
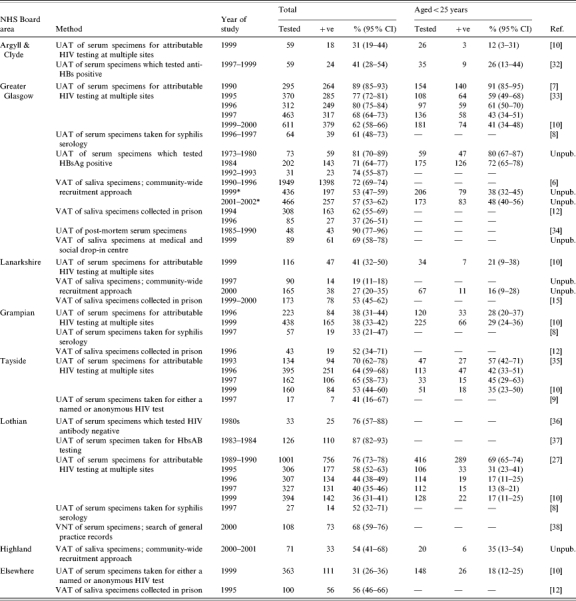
UAT, Unlinked anonymous testing of residual sera from specimens taken for routine clinical purposes (these samples were sourced from laboratories); VAT, voluntary anonymous testing of saliva specimens taken from IDUs specifically for study purposes; VNT, voluntary named testing; CI, confidence interval.
HCV antibody in saliva is a marker of hepatitis C carriage; estimates of hepatitis C carriers have been converted to estimates of HCV antibody-positive cases.
A multiplier of 100/85 has been applied to studies throughout with the exception of the Greater Glasgow Community-wide survey undertaken in 2000–2001 where a multiplier of 100/96 was employed.
IDUs who had commenced injecting since 1990 (for those recruited in 1999) and 1996 (for those recruited in 2001–2002).
The prevalence of HCV among IDUs throughout Scotland has been derived from several retrospective and prospective surveys. Two distinct designs were employed – unlinked anonymous testing of residual sera taken from IDUs undergoing routine clinical tests and voluntary anonymous testing of saliva donated by IDUs participating in surveys.
Unlinked anonymous HCV testing surveys
Unlinked anonymous testing has been used, routinely, as an epidemiological tool in the United Kingdom since 1990. Residues of samples taken from persons belonging to certain populations for routine clinical tests are further tested for HIV, or other infections such as HCV, unless objections to such testing are raised by patients. Patient identifiers are irreversibly unlinked from their corresponding specimens so that a test result cannot be linked to an individual. Selected non-identifying information (gender, age group, source laboratory/geographical area) is retained so that it is impossible to determine the identity of any individual through deductive means. The detail surrounding the method and its ethical basis is recorded elsewhere [5, 6].
In 1997, an unlinked anonymous HCV testing survey was implemented to monitor trends in the prevalence of HCV among IDUs in four NHS Board areas, incorporating the major urban centres (Glasgow, Dundee, Aberdeen and Edinburgh) and covering 50% of the Scottish population; testing was performed, retrospectively, on residual sera from serology specimens voluntarily submitted by IDUs for attributable HIV testing during selected years since 1989 [7, 8]. In 1999–2000, the geographical coverage of the survey was expanded to include IDUs having an attributable HIV test in 12 of the 15 NHS Board areas.
Additional surveys, employing this method, have involved the HCV testing of (i) residual syphilis serology specimens from IDUs attending genitourinary medicine clinics in Glasgow, Edinburgh, Dundee and Aberdeen [9], and (ii) residual rubella serology specimens from IDUs attending antenatal clinics in Dundee [10].
The earliest serological evidence of HCV among IDUs in Scotland was derived from specimens, originally taken between 1973 and 1980, to confirm acute clinical hepatitis B infection (HBsAg+, IgM+) among IDUs in Glasgow; retrospective testing, undertaken in 1993, indicated that 59 of the 73 (81%) were anti-HCV positive (S. Cameron, personal communication). Since then, a total of 11 151 sera have been tested anonymously for HCV; 6432 (57·6%) were antibody positive.
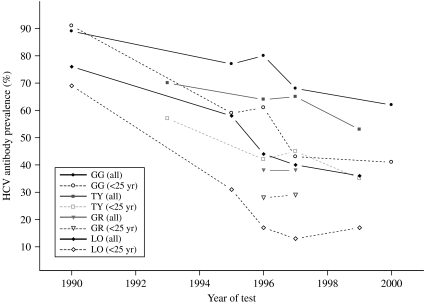
Of 2141 IDUs in Scotland who underwent attributable HIV antibody testing during 1999–2000, 946 (44%) were HCV antibody positive. The highest HCV prevalence (62%) was detected among Greater Glasgow injectors; this was followed by a rate of 53% among those from Tayside and prevalences ranging from 23% to 41% among injectors in other NHS Board areas. HCV prevalence data for Borders and Island NHS Board areas were unavailable because so few injectors had presented there for HIV testing [11]. Serial data from Glasgow, Lothian, Tayside and Grampian NHS Board areas showed that the prevalence of HCV declined in the early to mid-1990s, during the period when harm reduction initiatives were introduced and developed, but remained steady in the late 1990s (Fig. 2) [12]; the decline was particularly evident among those aged <25 years. By examining serial HCV prevalences among young IDUs, the majority of whom commenced injecting drugs within the previous 5 years, potential changes in HCV incidence among this population can be gauged.
Fig. 2.
HCV prevalence among IDUs, in four urban centres in Scotland, who had an attributable HIV test between 1989 and 2000. GG, Greater Glasgow NHS Board; TY, Tayside NHS Board; GR, Grampian NHS Board; LO, Lothian NHS Board.
Voluntary anonymous HCV testing surveys
Cross-sectional surveys of IDUs have been conducted in Scotland since 1990. IDUs were recruited from treatment-agency, needle-exchange and street-site settings using a multi-site, community-wide sampling strategy, to ensure that as representative a sample as possible was achieved. Subject to consent, these respondents were interviewed using a structured questionnaire which included questions on IDU demography, injecting frequency, injecting equipment sharing, access to needle/syringe exchange and methadone maintenance therapy, and imprisonment; further questions have been included on attributable HCV testing (since 1996) and experience and knowledge of, and attitudes to, HCV infection (since 2001). Respondents were also asked to supply a saliva sample for the anonymous testing of HIV and/or hepatitis virus antibodies. The most recent survey, undertaken during 2001–2002 in Glasgow, also included a qualitative component that aimed to understand the reasons why IDUs continued to share injecting equipment in the era of harm reduction.
Voluntary anonymous HCV testing surveys of prisoners, incarcerated in several Scottish prisons between 1994 and 1996, have also been undertaken. Following consent, prisoners’ saliva samples were tested anonymously for HIV and HCV; the test result was linked to a self-completed questionnaire about risk behaviour that included questions on injector status, including whether they had injected while in prison. Questions on other blood exposure, such as having been tattooed, were added to the questionnaires in the later surveys [13].
For the above studies, salivary HCV antibody was initially detected using a modified Monolisa anti-HCV assay (Sanofi Pasteur, Marne-la-Coquette, France); the test had an 85% sensitivity [14]. Since 2001, a modified Murex anti-HCV 4.0 ELISA (Abbot Diagnostics, Maidenhead, UK) assay with a 96% sensitivity has been used. Throughout this paper, a multiplier (100/85 or 100/96) has been applied to convert estimates of HCV antibody in saliva to estimates of HCV antibody in blood.
Among Glasgow injectors recruited to community-wide surveys, there was a significant decline in the unadjusted prevalence of HCV antibody from 67% in 1990 to 56% in 1996 (P=0·02). Data from the 1999 survey (of IDUs who had commenced injecting since 1990), suggested that prevalence remained steady throughout the late 1990s. The most recent studies, undertaken since 2000, reported adjusted HCV antibody prevalences of 57%, 27% and 54% in Greater Glasgow (among IDUs who had commenced injecting since 1996) Lanarkshire and Highland respectively.
The studies undertaken in the prison setting indicated that HCV prevalence was high among prisoners per se, ranging from 17% to 34% (data not shown in Table 1), and increased to 37–66% among prisoners reporting ever having injected drugs; prevalence was slightly higher among injector inmates who reported injecting in prison compared to those who had not [13].
Incidence of HCV among Scottish IDUs (Table 2)
Table 2.
HCV incidence data among different injecting drug user (IDU) populations in Scotland
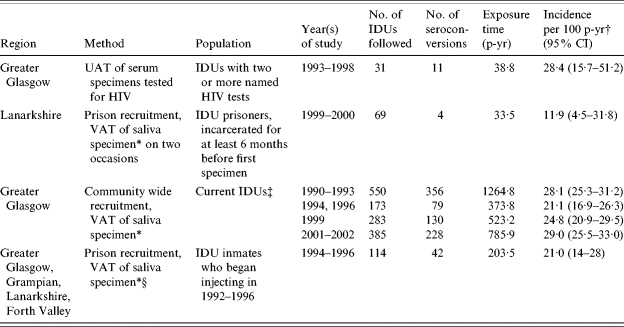
UAT, Unlinked anonymous testing; VAT, Voluntary anonymous testing; p-yr, person-years; CI, confidence interval.
HCV antibody in saliva is a marker of hepatitis C carriage; estimates of hepatitis C carriers have been converted to estimates of HCV antibody-positive cases. A multiplier of 100/85 has been applied to studies throughout with the exception of the Greater Glasgow Community-wide survey undertaken in 2000/2001 where a multiplier of 100/96 was employed.
Incidence calculated using person-years method, where date of seroconversion was taken as the midpoint of the follow-up period, with the exception of the study marked (§) where the date of seroconversion was taken as the end of the follow-up period.
Injected in previous 2 months (1990–1993, 1994 and 1996); injected in previous 6 months (1999 and 2001–2002).
Few estimates of HCV incidence among IDUs in Scotland exist (Table 2). Two published studies used a cohort approach. The first, a retrospective study, involved the unlinked anonymous HCV testing of residual serum specimens from IDUs having two or more voluntary attributable HIV tests during the period 1993–1998; on the basis of 11 seroconversions observed, an incidence of 28·2/100 person-years was reported [15]. The second, a prospective study, involved the voluntary anonymous HCV testing of saliva samples collected from prison inmates on two occasions, 6 months apart; on the basis of four seroconversions among inmates who declared ever having injected drugs, an incidence of 11·9/100 person-years of incarceration was estimated [16]. Further unpublished work undertaken by HPS has estimated incidence rates indirectly by using a combination of HCV prevalence and date of injecting debut data from injectors who had commenced injecting within the previous 6 years and had been recruited to community-wide surveys; this method assumes that interviewed IDUs were HCV antibody negative prior to commencing injecting drugs (HCV prevalence among non-IDUs in Scotland, invariably, is <1%) [17] and that cases had acquired infection midway through their exposure period. Such studies indicate that HCV incidence among injectors in Glasgow declined in the mid-1990s from 28/100 person-years of injecting in 1990–1993 to 21/100 person-years of injecting in 1994–1996, but increased to 25/100 in 1999 and 29/100 in 2001–2002; this trend is consistent with those observed for HCV prevalences generated through unlinked anonymous testing, as above.
DISCUSSION
The methods (including recruitment approach, geographical coverage and periodicity) employed to monitor HCV prevalence and incidence among IDUs in Scotland have been varied; while such differences should be considered when interpreting the data, they provide a relatively clear understanding of the extent and spread of HCV among IDUs throughout the country. A statistical modelling exercise demonstrated that, among IDUs in Glasgow, the estimated HCV incidence was consistent with the estimated HCV prevalence [18].
The population of diagnosed HCV cases is self-selected and as HCV infection, generally, is asymptomatic for many years, the number of diagnoses in Scotland represents only a proportion of the total HCV antibody-positive IDU population. Of the database’s 18 703 records, 11 212 were known to have injected drugs; it is probable, however, that a large proportion of the 6243 cases, for which no risk information was recorded, had injected drugs since 73% (4579/6243) were aged 15–44, the range within which 95% of the 11 252 IDUs belonged. If we assume that these 4579 had injected drugs and an estimated 12% of the HCV-diagnosed IDU population had died, the resulting estimated number of living diagnosed HCV antibody-positive injectors (∼13 900) constitutes less than a third of the estimated 45 500 infected persons in Scotland during 2005, who had ever injected drugs [1].
The approach involving the serial voluntary anonymous testing of IDUs, recruited using a multi-site, community-wide sampling strategy, is considered the optimal one for gauging changes in HCV prevalence among this population; the opportunity to obtain behavioural data which can be linked to HCV test results is an added benefit. Unfortunately, such surveys are expensive, a fact which makes it impossible for them to be performed in many centres. The unlinked anonymous surveys of injectors having an attributable non-HCV (usually HIV) related blood test, however, is a relatively inexpensive and pragmatic way of providing estimates of HCV prevalence among this group nationally. Although such IDUs are not necessarily representative of the total IDU population, real changes in its HCV prevalence can be detected through the use of this approach if it is used consistently over time. Information derived from IDUs aged <25 years, the majority of whom will have started injecting within the previous 5 years, provide compelling evidence of the incidence of infection.
The prevalence estimate for Scotland as a whole (44% in 2000, ranging from 23% to 62% depending on geographical location) is comparable with those observed in many European countries (the EU range is 30–90% [19]) and is similar to that in Australia [20]. In contrast to the prevalence of HCV, that of HIV among injectors in Scotland has remained below 1% since 1998 [21].
In parts of Scotland, particularly Glasgow, the incidence of HCV among current IDU populations decreased in the early to mid-1990s but remains high despite extensive needle/syringe exchange and methadone maintenance provision. HCV continues to spread because of its high prevalence, its high percutaneous infectivity (relative to that for HIV) [22] and because a high proportion of IDUs continue to share injecting equipment; around a third of new injectors, registered with Scotland’s drug misuse database in 2004–2005, had shared needles and syringes within the previous month [23]. Our understanding of the reasons why IDUs continue to share needles, syringes, spoons, filters and water is limited but there is some evidence to indicate that many do not perceive HIV and HCV as major threats because current IDUs infected with the former are so few and those infected with the latter rarely manifest any severe HCV-related morbidity (S. Wadd, personal communication). Further, suboptimal access to sterile injecting equipment promotes ‘sharing’ activity [24]; a survey of needle-exchange services in Scotland in 1997 found that the level of needle and syringes distributed was insufficient to provide a sterile needle and syringe per injecting episode for all injectors [25]. This issue was addressed in 2002, when the Lord Advocate (the principal Law Officer of the Crown in Scotland), relaxed the restriction on the numbers of needles and syringes that could be given to an IDU on any single visit to an exchange (a maximum of 15 has been increased to 60). The impact of this and other initiatives such as the transfer of needle and syringe exchanges from dedicated health service sites to pharmacy-based ones and increased access to methadone maintenance therapy will be evaluated by continuously monitoring the prevalence of HCV among, particularly young, IDUs. It remains to be seen if the Scottish Executive Health Department target of a 20% reduction of HCV prevalence among IDUs between 2000 and 2005 has been met [26].
The data generated by the surveillance programme have been referenced in reports to support HCV public health policy statements at local [27], Scotland [28], United Kingdom [29, 30] and European Union [31] levels. In addition, the above data have been incorporated into statistical models designed to determine the number of HCV-infected IDUs who are likely to progress to severe HCV-related liver disease and thus require treatment and care in the future [1]; accordingly, the data will inform the local and central planning of health services regarding HCV disease.
ACKNOWLEDGEMENTS
We acknowledge the contribution of survey participants and are also grateful to the many people (virologists, clinicians, clerical workers and public health physicians) who have made important contributions to Scotland’s HCV surveillance programme.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Hutchinson SJ, Bird SM, Goldberg DJ. Modelling the current and future disease burden of hepatitis C among injecting drug users in Scotland. Hepatology. 2005;42:711–723. doi: 10.1002/hep.20836. [DOI] [PubMed] [Google Scholar]
- 2.Shaw L et al. Establishment of a database of diagnosed HCV-infected persons in Scotland. Communicable Disease & Public Health. 2003;6:305–310. [PubMed] [Google Scholar]
- 3.Anon. Diagnosis of hepatitis C virus in Scotland: data to December 2003. SCIEH Weekly Report. 2004;38:150–155. [Google Scholar]
- 4.Hutchinson SJ et al. Hepatitis C virus infection in Scotland: epidemiological review and public health challenges. Scottish Medical Journal. 2006;51:8–15. doi: 10.1258/RSMSMJ.51.2.8. [DOI] [PubMed] [Google Scholar]
- 5.Nicoll A et al. The public health applications of unlinked anonymous seroprevalence monitoring for HIV in the United Kingdom. International Journal of Epidemiology. 2000;29:1–10. doi: 10.1093/ije/29.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Heptonstall J, Gill ON. The legal and ethical basis for unlinked anonymous HIV testing. Communicable Disease Report. 1989;48:3–6. [Google Scholar]
- 7.Taylor A et al. Prevalence of hepatitis C virus infection among injecting drug users in Glasgow 1990–1996: are current harm reduction strategies working. Journal of Infection. 2000;40:176–183. doi: 10.1053/jinf.2000.0647. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg D, Cameron S, McMenamin J. Hepatitis C virus antibody prevalence among injecting drug users in Glasgow has fallen but remains high. Communicable Disease & Public Health. 1998;1:95–97. [PubMed] [Google Scholar]
- 9.Goldberg D et al. Hepatitis C virus among genitourinary clinic attenders in Scotland: unlinked anonymous testing. International Journal of STD & AIDS. 2001;12:17–21. [PubMed] [Google Scholar]
- 10.Goldberg D et al. Hepatitis C virus among high and low risk pregnant women in Dundee: unlinked anonymous testing. British Journal of Obstetrics & Gynaecology. 2001;108:365–370. doi: 10.1111/j.1471-0528.2001.00089.x. [DOI] [PubMed] [Google Scholar]
- 11.Hay G, McKeganey N, Hutchinson S. Edinburgh, ISO: 2001. [Google Scholar]
- 12.Hutchinson SJ et al. Prevalence of hepatitis C among injectors in Scotland 1989–2000: declining trends among young injectors halt in the late 1990s. Epidemiology and Infection. 2002;128:473–477. doi: 10.1017/s0950268802006945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gore SM et al. Prevalence of hepatitis C in prisons: WASH-C surveillance linked to self-reported risk behaviours. Quarterly Journal of Medicine. 1999;92:25–32. doi: 10.1093/qjmed/92.1.25. [DOI] [PubMed] [Google Scholar]
- 14.Cameron SO et al. Detection of antibodies against hepatitis C virus in saliva: a marker of viral replication. Journal of Viral Hepatitis. 1999;6:141–144. doi: 10.1046/j.1365-2893.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 15.Roy KM et al. A method to detect the incidence of hepatitis C infection among injecting drug users in Glasgow 1993–98. Journal of Infection. 2001;43:200–205. doi: 10.1053/jinf.2001.0908. [DOI] [PubMed] [Google Scholar]
- 16.Champion J et al. The incidence of hepatitis C virus infection and associated risk factors among Scottish prisoners: a cohort study. American Journal of Epidemiology. 2004;159:514–519. doi: 10.1093/aje/kwh061. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson S et al. Hepatitis C among childbearing women in Scotland. Gut. 2004;53:593–598. doi: 10.1136/gut.2003.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutchinson SJ et al. Modelling the spread of hepatitis C virus infection among injecting drug users in Glasgow: implications for prevention. International Journal of Drug Policy [Google Scholar]
- 19.European Monitoring Centre for Drugs and Drug Addiction. Lisbon: 2003. [Google Scholar]
- 20.Dore GJ et al. Epidemiology of hepatitis C virus infection in Australia. Journal of Clinical Virology. 2003;26:171–184. doi: 10.1016/s1386-6532(02)00116-6. [DOI] [PubMed] [Google Scholar]
- 21.Anon. HIV prevalence among non-IDU heterosexuals, IDUs and men who have sex with men who undergo named HIV testing in Scotland: 2003 update. SCIEH Weekly Report. 2004;38:181–182. [Google Scholar]
- 22.Coutinho RA. HIV and hepatitis C among injecting drug users. British Medical Journal. 1998;317:424–425. doi: 10.1136/bmj.317.7156.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Information Services Division. Edinburgh: 2005. [Google Scholar]
- 24.Hutchinson SJ et al. Factors associated with injecting risk behaviour among serial community-wide samples of injecting drug users in Glasgow 1990–94: implications for control and prevention of blood-borne viruses. Addiction. 2000;95:931–940. doi: 10.1046/j.1360-0443.2000.9569319.x. [DOI] [PubMed] [Google Scholar]
- 25.Parsons J et al. Over a decade of syringe exchange: results from 1997 UK survey. Addiction. 2002;97:845–850. doi: 10.1046/j.1360-0443.2002.00128.x. [DOI] [PubMed] [Google Scholar]
- 26.Scottish Executive Health Department. Edinburgh: 2001. [Google Scholar]
- 27.Greater Glasgow Health Boards. Glasgow: 2000. [Google Scholar]
- 28.Office for Public Health in Scotland. Hepatitis C. Glasgow: 2000. [Google Scholar]
- 29.Department of Health. London: 2002. [Google Scholar]
- 30.British Liver Trust. London: 2002. [Google Scholar]
- 31.Wessing L, Jager J, Limburg W, Kretzschmar M, Postma M, Wiessing Let al. Surveillance of hepatitis C infection among injecting drug users in the European UnionHepatitis C and Injecting Drug Use: impact, costs and policy option Belgium: European Monitoring Centre for Drugs & Drug Addiction (EMCDDA)200491–135. [Google Scholar]
- 32.Stevenson J et al. An outbreak of acute hepatitis B infection among injecting drug users in Inverclyde, Scotland. Communicable Disease and Public Health. 2001;4:60–63. [PubMed] [Google Scholar]
- 33.Goldberg D et al. Trends in HCV prevalence among injecting drug users in Glasgow and Edinburgh during the era of needle/syringe exchange. Scandinavian Journal of Infectious Disease. 2001;33:457–461. doi: 10.1080/00365540152029936. [DOI] [PubMed] [Google Scholar]
- 34.McCruden EAB et al. Hepatitis virus infection and liver disease in injecting drug users who died suddenly. Journal of Clinical Pathology. 1996;49:552–555. doi: 10.1136/jcp.49.7.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIntyre PG et al. Prevalence of antibodies to hepatitis C virus, HIV and human T-cell leukaemia/lymphoma viruses in injecting drug users in Tayside, Scotland, 1993–1997. Epidemiology and Infection. 2001;126:97–101. doi: 10.1017/s0950268801005040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson HG et al. Use of several second generation serological assays to determine the true prevalence of hepatitis C infection in haemophiliacs treated with non-virus inactivated factor VIII and XI concentrates. British Journal of Haematology. 1992;80:514–518. doi: 10.1111/j.1365-2141.1992.tb04566.x. [DOI] [PubMed] [Google Scholar]
- 37.Gore SM et al. Pilot study to estimate survivors to 1995 of 1983–1984 prevalent hepatitis C infections in Lothian patients who tested positive or negative for hepatitis B surface antigen in 1983–1984. Journal of Infection. 1998;37:159–165. doi: 10.1016/s0163-4453(98)80171-0. [DOI] [PubMed] [Google Scholar]
- 38.Peat M et al. Audit of bloodborne virus infections in injecting drug users in general practice. Communicable Disease and Public Health. 2000;3:244–246. [PubMed] [Google Scholar]