SUMMARY
Nine different arboviruses are known to be transmitted by, or associated with, mosquitoes in Europe, and several (West Nile, Sindbis and Tahyna viruses) are reported to cause outbreaks of human disease. Although there have been no reported human cases in Great Britain (GB), there have been no published in-depth serological surveys for evidence of human infection. This paper investigates the ecological and entomological factors that could influence or restrict transmission of these viruses in GB, suggesting that in addition to West Nile virus, Sindbis and Tahyna viruses could exist in enzootic cycles, and that certain ecological factors could facilitate transmission to humans. However, the level of transmission is likely to be lower than in endemic foci elsewhere in Europe due to key ecological differences related to spatial and temporal dynamics of putative mosquito vectors and presence of key reservoir hosts. Knowledge of the potential GB-specific disease ecology can aid assessments of risk from mosquito-borne arboviruses.
INTRODUCTION
There is currently considered to be no transmission of mosquito-borne arboviruses to humans in Great Britain (GB), despite a number of mosquito-borne arboviruses being endemic in other parts of Europe, where they cause human disease. However, to date no in-depth serological surveys for mosquito-borne viruses in the GB human population have been published, and therefore an assessment of their possible ecology and epidemiology is required to aid understanding as to whether these viruses already exist enzootically in GB and whether they could be associated with human infection.
A number of papers have reviewed the known transmission dynamics of mosquito-borne arboviruses endemic in Europe [1–3], with suggestions that they may occur enzootically in the United Kingdom (UK) [4]. Following the large-scale outbreak of West Nile virus (WNV) in North America and recent outbreaks in Romania, Russia, Israel and France, an investigation into the presence of mosquito-borne viruses in GB was conducted, resulting in evidence of neutralizing antibodies to WNV, Sindbis virus and Usutu virus in British resident birds [5]. Prior to this, the only previous record of a mosquito-borne arbovirus in GB was serological evidence of Tahyna virus in small mammals in Devon [6]. The recent spread of WNV across North America, coupled with seropositivity in British resident birds have led to the development of UK contingency plans for WNV [7] and vector control [8].
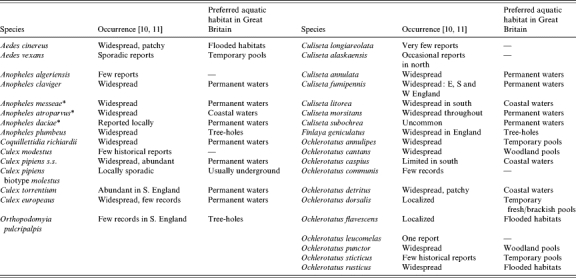
The occurrence and abundance of mosquito vector species, or potential vector species, is a prerequisite for enzootic transmission of mosquito-borne viruses in GB. Thirty-three mosquito species have been recorded in GB (Table 1): six species of Anophelinae (genus Anopheles) and 27 species of Culicinae in seven genera: Aedes (2), Ochlerotatus (11), Finlaya (1), Coquillettidia (1), Culex (4), Culiseta (7), and Orthopodomyia (1). Several species (Ae. vexans, An. algeriensis, Cx. modestus, Cs. longiareolata, Cs. alaskaensis, Oc. communis, Oc. leucomelas, Oc. sticticus and Or. pulcripalpis) are either rare, adopt localized distributions or have only historically occurred in GB and can therefore be generally discounted from eco-epidemiological studies of mosquito-borne arbovirus transmission in GB. An ecological understanding of candidate mosquito vectors of arboviruses is an important aspect of surveillance and aids assessment of likely spatio-temporal dynamics of transmission and associated public health risks. A detailed review of the ecology of candidate WNV mosquito vectors in the British Isles based upon known vector status in continental Europe and host preferences of endemic species has been published [9]. The main aims of this paper are to assess the ecological potential for transmission of other mosquito-borne arboviruses in GB and associated public health concerns and secondly, by incorporating data on distribution from the British mosquito recording scheme to identify areas for possible transmission and more importantly targeted surveillance of potentially medically important arboviruses including WNV†.
Table 1.
Mosquitoes recorded from Great Britain (after [9])
These three sibling species are members of the Anopheles maculipennis s.l. complex.
Mosquito-borne arboviruses in Europe
Up to 1996, eight arboviruses transmitted by, or associated with, mosquitoes had been recorded in Europe (Table 2; [2]) including: members of the Togaviridae (Sindbis), Flaviviridae (West Nile, dengue), and Bunyaviridae (Batai, Inkoo, Lednice, Tahyna and Uukuniemi); Usutu virus has since been added to this list [13]. Five of these arboviruses are generally associated with human disease in Europe: Sindbis, West Nile, Tahyna, Batai and Inkoo. The public health concerns for GB will now be considered in more depth.
Table 2.
List of recorded mosquito-borne arboviruses in Europe
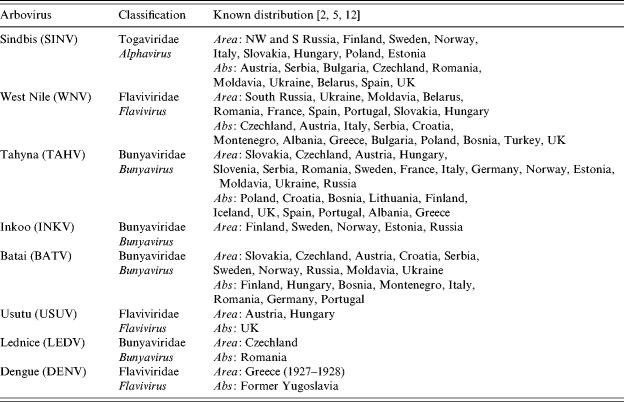
Area, Based on virus isolation or autochthonous disease; Abs, serological evidence only.
Sindbis virus (SINV)
SINV (Togaviridae: Alphavirus) was first isolated from Cx. univittatus near Sindbis village, Egypt in 1952, with the first human case reported in Uganda in 1961. SINV has since been isolated from Africa, Europe, Middle East, Asia and Australia; however, it only appears to be clinically apparent in northern Europe (mainly 60°–64° N) and in South Africa [14]. In Scandinavia, infection with SINV or SIN-like viruses is known by different names in different regions: Ockelbo disease in Sweden, Karelian fever in western Russia, and Pogosta disease in Finland.
There have been no fatal human cases reported and many SIN or SIN-like virus infections are moderate or mild, especially in children and adolescents, so the potential exists for under-reporting and/or under-diagnosis [15]. There appears to be more subclinical infections than clinical cases with reported ratios of 20:1 to 40:1 in Sweden and 17:1 in Finland [16, 17]. However, where clinical disease in humans does occur the infection is characterized by fever, rash and arthritis, with non-pruritic skin lesions beginning as macules, which become papular and progress to central vesicle formation, which are, occasionally, haemorrhagic [18]. Arthralgias occur in large and small joints, particularly ankles, wrists and knees [19] and may be so severe as to be immobilizing [20] with moderate joint pain and stiffness persisting for months or years, with chronic joint (or muscle) problems occurring in ∼20% of cases [20]. Following the first human case in Sweden in the 1960s, Ockelbo disease was responsible for considerable morbidity in Scandinavia during the 1980s. In 1981, the Russian federation and Finland reported 200 and 300 laboratory-confirmed cases of Karelian fever and Pogosta disease respectively [21]. The largest Pogosta disease outbreak occurred in Finland in 1995 with 400 confirmed cases. Annual incidence rates in endemic regions of affected countries range from 2·7/100 000 in Finland, 2·9/100 000 in Sweden to 18/100 000 in northern Karelia [16, 17]. Epidemics can involve hundreds of people, and in these epidemic years, the number of cases may be 10 times higher than in non-epidemic years. The annual number of cases in Finland from 1980 to 1996 ranged from 1 to 1282 [17]. Human disease normally appears at the end of July or beginning of August, peaks in late August with few cases from October [16].
A virus closely related to SINV (Ockelbo virus) was first isolated from mosquitoes of the genus Culiseta in Sweden in 1982 [22] with SINV/SIN-like viruses isolated from Cx. pipiens/Cx. torrentium, Cs. morsitans and Ae. cinereus in Sweden [23], Aedes/Ochlerotatus in Norway and Russia [24, 25], and from Hyalomma marginatum ticks in Italy [26]. During studies in Sweden [23], SIN-like virus was only isolated from mosquitoes collected during July and August with highest ‘minimum field infection rates’ (MFIR) reported in August. The monthly MFIR for Cx. pipiens/Cx. torrentium (5·0/1000 in July, 12·6/1000 in August) was consistently higher than in Cs. morsitans (2·0/1000 in July, 4·1/1000 in August), with Ae. cinereus exhibiting a MFIR of 0·3/1000 in August. This suggests that Cx. pipiens and Cx. torrentium are potential enzootic vectors among birds mainly from late spring to mid-summer, with Cs. morsitans as a potential additional enzootic vector in late summer [27, 28], and Ae. cinereus as a potential bridge vector for transmission from viraemic birds to humans. The relative roles of pipiens and torrentium as enzootic vectors was investigated by orally infecting both species with a range of doses of virus and after an incubation period testing the mosquito’s ability to transmit [29]. Culex torrentium was highly susceptible to Ockelbo virus by the oral route and transmitted the virus effectively to chickens. One of two mosquitoes that ingested a blood meal containing <102 p.f.u./ml became infected, and all mosquitoes that ingested a blood meal containing >104 p.f.u./ml became infected. All 10 re-feeding Cx. torrentium transmitted Ockelbo virus to susceptible chickens. In contrast only one of 28 Cx. pipiens was infected after ingestion of a blood meal of 103·0–3·9 p.f.u./ml, with the frequency of infection increasing to 53% after ingestion of 106 p.f.u./ml. Transmission rates were correlated positively with the virus concentration but were much lower in pipiens than torrentium, and it seems clear that Cx. torrentium is an efficient laboratory vector, and that Cx. pipiens was relatively refractory and a poor vector. Similar competence experiments with SINV/SIN-like virus in Swedish Aedes species showed that Ae. cinereus was relatively susceptible to infection and could transmit the virus [30]. Based on virus occurrence in wild mosquitoes and the results of experimental infection and transmission studies, it is clear that Cx. torrentium is the main vector for transmission among birds in Sweden, and that Ae. cinereus is the main vector for transmission from viraemic birds to humans [31].
The main enzootic vector Cx. torrentium and the potential enzootic mosquito vector Cs. morsitans are present and abundant in GB. The potential, but probably not very important enzootic vector Cx. pipiens is also present and abundant in GB. The two Culex species are almost morphologically indistinguishable, with very few bionomic differences, both being strongly ornithophagic, occupying similar larval habitats such as ponds, marshes, backwaters of streams, hoof prints, pools, ditches and water-containing tree-holes, tanks, butts and discarded tyres [9, 32, 33]. Whilst Cx. pipiens (nominate ornithophagic biotype) is widespread across GB, Cx. torrentium occurs mainly in southern England, where it predominates over Cx. pipiens in peri-domestic habitats. Human biting has rarely been recorded in either species and their roles as bridge vectors is considered negligible. Similarly Cs. morsitans is predominantly ornithophagic, and although there is documented evidence of biting of humans [34], this behaviour is considered rare.
Transmission to humans is due to involvement of mosquitoes of the genus Aedes with less specialized feeding habits. In Sweden, human infection with SINV appears to occur where enzootic cycles with Cx. torrentium exist, and where large numbers of Ae. cinereus can facilitate the transmission to humans. Aedes cinereus has a widespread but patchy distribution across mainland Britain and, where it is locally common, is a troublesome biter of humans [33]. Aquatic sites include areas prone to freshwater summer flooding with females biting a variety of mammals including humans. Other aedine mosquitoes in GB that might be suggested as potential bridge vectors include Oc. cantans and Oc. communis [24, 27]. The latter has only been recorded on a few occasions [10] whereas the former is widespread and patchily abundant in woods and scrublands across most of GB. The ecologies of all six mosquito species are detailed in Table 3 and their known distributions are illustrated in Figure 1 (a, b).
Table 3.
Basic bionomics of candidate enzootic and human bridge vectors of SINV in Great Britain: vector potential inferred from host preference(s)
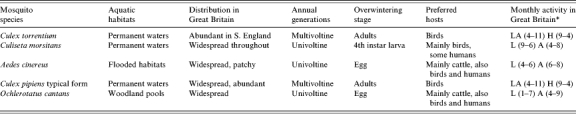
A, Adults; H, hibernating adults; L, larvae.
Months of activity, from January (1) to December (12), in parentheses.
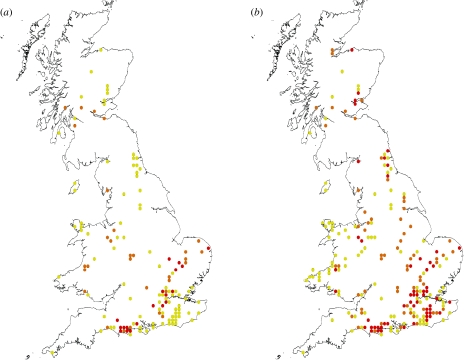
Fig. 1.
Reported distribution of candidate mosquito vectors of SINV depicts (a) 10-km grid squares where the most likely candidate enzootic vectors (Cx. torrentium and Cs. morsitans) and the most likely candidate bridge vector (Ae. cinereus) have been reported, (b) additionally incorporates another possible candidate enzootic vector (Cx. pipiens typical biotype) and candidate bridge vector (Oc. cantans). Red represents bridge and enzootic putative vectors together, orange represents bridge vectors alone, yellow represents enzootic vectors alone.
In Sweden, passeriformes appear to be the principal vertebrate hosts for Ockelbo virus (neutralizing antibodies in 27% passeriformes, 6% galliformes, 4% anseriformes) [35]. In a later study, SINV appeared to infect almost all passerine species with prevalence rates of neutralizing bodies to SINV reported as 6·6% in Erithacus rubecula (robin), 12·8% in Turdus merula (blackbird), 4·2% in Sylvia borin (garden warbler), 5·8% in Sylvia atricapilla (blackcap), 4·3% in Phylloscopus trochilus (willow warbler), 1·9% in Parus major (great tit), 7·3% in Fringilla coelebs (chaffinch), 12·9% in Carduelis chloris (greenfinch), 8·7% in Pyrrhula pyrrhula (bullfinch) and 1·9% in Emberiza citronella (yellowhammer) [36]. The ubiquity of these species and other passerine birds in GB suggests that the lack of reservoir hosts may not be a limiting factor. However, three species of thrush in Sweden reported the highest prevalence rates: 43·3% in Tu. pilaris (fieldfare), 22·2% in Tu. philomelos (song thrush) and 37·0% in Tu. iliacus (redwing), with the conclusion that these three bird species are likely to be the main amplification hosts for SINV in Sweden. The authors concluded that the relative abundance of these three species relative to all other bird species was important in the virus dynamics at a particular site, concluding that the main endemic area for SINV in Europe (i.e. Scandinavia) is closely and positively associated with the abundance of these thrush species. The restricted distribution of clinically apparent disease between 60° N and 64° N may, therefore, be a function of the presence of large numbers of these virus-amplifying hosts during the summer mosquito season, as well as perhaps a climate restriction of the virus.
In Finland however, 30% of game birds had SINV antibodies in 1981–1983 and during the large epidemic of Pogosta disease in 1981, 65% of Tetrao species (black grouse Tetrao tetrix and capercaillie Te. urogallus) were seropositive. Coupled with the high titres in capercaille [35] it is postulated [17] that the epidemic years of Pogosta disease every 7th year in Finland are linked to the 6- to 7-year cycle of tetraonid birds and that cycling of the herd immunity among birds causes the 7-year interval of human SINV epidemics in Finland.
The three bird species implicated as main amplification hosts in Sweden occur in GB but unlike Sweden, neither Tu. pilaris, nor Tu. iliacus breed in GB in any large numbers during the summer months (1–5 and 30–50 breeding pairs respectively [37]). In fact Tu. pilaris and Tu. iliacus are primarily winter visitors to GB arriving from their Scandinavian breeding sites in large numbers (750 000 birds each) from October onwards. The diversity of passerine hosts present in GB suggest that based purely on ecology, and provided suitable mosquito species are in abundance, SINV may occur in enzootic cycles. This is supported by serological evidence from GB [5] with the highest seroprevalence in Tu. merula (blackbird; 56%, n=9), Corvus corone (carrion crow; 62%, n=26), and Pica pica (magpie; 51%, n=45), with evidence of SINV-neutralizing antibodies in sera from a further seven wild and domestic birds from GB collected in 2001 and 2002. However, assuming that a similar involvement of thrushes in virus amplification is required in GB, the absence of large numbers of Tu. pilaris and Tu. iliacus in GB during the summer mosquito season may limit the amplification of the virus in resident birds and hence limit the possibility for large outbreaks of human disease. Where SINV may occur in resident passerines, any subsequent transmission to humans in GB would probably be spatially restricted to areas with large numbers of both Cx. torrentium and Ae. cinereus mosquitoes, with human cases likely to correlate with the seasonality of the main bridge vector, in July and August (areas where these mosquito vectors do appear to coincide is illustrated in Fig. 1). Regarding the possible involvement of tetraonid birds in virus cycles (as in Finland), neither Te. tetrix nor Te. urogallus occur in Britain in any significant numbers (both priority conservation species) and it therefore seems that a similar cycle to that seen in Finland occurring in GB is unlikely. Other members of the family Tetraonidae do occur in GB, for example Lagopus lagopus (red grouse) is abundant in parts of Scotland, however, there is no evidence linking this species to SINV [17].
West Nile virus (WNV)
Recent animal and human WNV outbreaks in the Mediterranean basin and the United States are described in detail elsewhere [38–41]. The background to WNV (Flaviviridae: Flavivirus) epidemiology and detailed ecologies of candidate enzootic and bridge vectors in relation to the British Isles have also been discussed in detail [9, 42] and reference should be made to these articles. However, a summary table of candidate mosquito species that could be implicated in transmission of WNV in GB is provided in Table 4 and the possible spatial dynamics of transmission based upon known records of mosquito distributions are detailed in Figure 2. Regarding seasonality of transmission to humans, due to the large spectrum of potential mosquito vectors, transmission could feasibly occur between May and October, however, it is likely to be elevated during the summer months of July, August and early September.
Table 4.
Basic bionomics of candidate enzootic and human bridge vectors of WNV in Great Britain: vector potential inferred from host preference(s) (after [9])
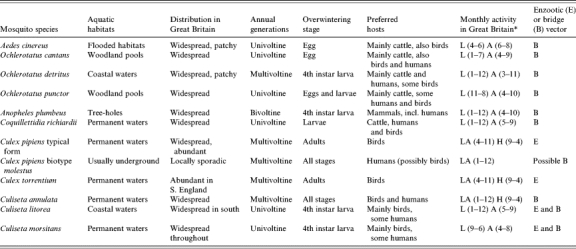
A, Adults; H, hibernating adults; L, larvae.
Months of activity, from January (1) to December (12), in parentheses.
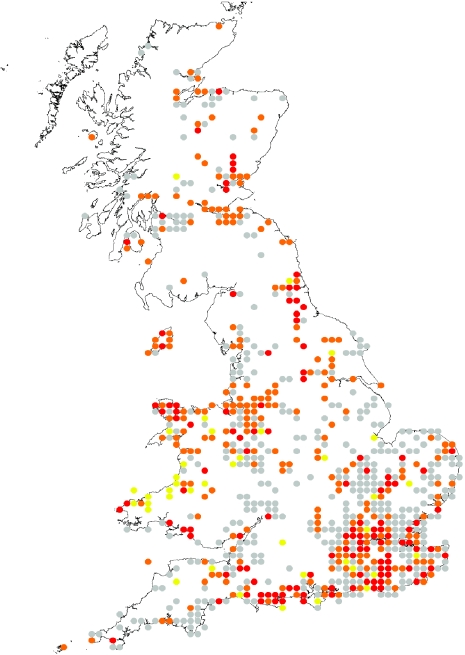
Fig. 2.
Reported distribution of candidate mosquito vectors of WNV: depicts probable areas, based upon known historical distributions of key mosquito species, of candidate enzootic and human bridge vectors of WNV. Red represents areas where both candidate bridge and enzootic mosquito vectors have been recorded together in the same 10-km grid (most probable areas for human transmission), orange represents where candidate bridge vectors occur alone (where transmission to humans might occur but possibly at a lower rate, bridge vectors may also act as enzootic vectors, but in general, they are not classically ornithophagic), and yellow represents where candidate enzootic vectors occur alone. All grid locations represented in grey show the reported distribution of all other mosquito species which although unlikely to be involved in transmission, are included to illustrate coverage of all mosquito distribution data in the database [10].
Tahyna virus (TAHV)
Tahyna virus (Bunyaviridae: Orthobunyavirus) belongs to the California complex of bunyaviruses. TAHV was first isolated in 1958 in Slovakia from Ae. vexans and Oc. caspius [43], and has subsequently been reported from France, Austria, Czechland, Germany, Serbia, Slovenia, Hungary, Romania, Italy, Russia, Ukraine, Moldova and other parts of the former USSR [2, 3, 31, 44], as well in Asia and Africa. Serosurveys of small mammals around Exeter, south-western England [6], found that Apodemus sylvaticus (wood mouse) and Clethrionomys glareolus (bank vole) had antibodies to TAHV.
According to the World Health Organization [45], TAHV-infected humans may present with influenza-like symptoms, and sometimes as meningoencephalitis and atypical pneumonia. No fatal cases have been reported, and many infections are unapparent, despite high antibody prevalences (60–80%) among inhabitants of endemic foci. Symptomatic cases manifest as an acute influenza-like disease (more common in children) with sudden onset of fever lasting 3–5 days, with headache, malaise, conjunctivitis, pharyngitis, myalgia, nausea, gastrointestinal disorders, anorexia and occasional arthralgia [46]. In Slovakia, every seventieth case of febrile illness in children and every fifth case of CNS illness in children is caused by TAHV [47], and in Czechland every seventh influenza case and every fifth case with manifestations of meningoencephalitis in the summer months can be ascribed to this virus [48]. WHO states that ‘inasmuch that TAHV is widespread in Europe and may cause severe disease, it must be considered of public health importance at present, bearing in mind its even greater potential for increased incidence, especially as its vectors are so widespread’ [45].
TAHV vectors are mainly pasture-breeding species of the genera Aedes and Ochlerotatus, with TAHV isolated from several species of mosquito including Ae. vexans, Oc. caspius (caspius/dorsalis), Ae. cinereus, Oc. cantans (cantans/annulipes), Oc. sticticus, Cs. annulata, Cx. modestus, Oc. flavescens, Coquillettidia richiardii, An. maculipennis, and Cx. pipiens [31, 46] with the most virus isolations from Ae. vexans, Oc. caspius/dorsalis, Oc. cantans/annulipes and Ae. cinereus [31]. Aedes vexans is considered to be the most important TAHV vector with 58% of all virus isolations resulting from this species. In Europe it is generally considered that Ae. vexans seems to be a prerequisite for the maintenance of TAHV transmission cycles in an area. However, although the high number of TAHV isolates in Slovakia were from Ae. vexans, this is in contrast to the lack of isolates from this species in Austria where a high number of isolates from Oc. caspius/dorsalis indicates that strains of TAHV may utilize different vector species in different geographical locations. Other Aedes/Ochlerotatus species clearly do contribute to transmission, and Culiseta mosquitoes may be important in the overwintering of TAHV [46], with transovarial transmission occurring in most of these species [49].
All the mosquito species listed above have been recorded in GB, however, the limited number of records of Oc. sticticus and Cx. modestus suggest that neither of these species would contribute significantly to transmission of the virus in GB. Additionally, the rare occurrence of Ae. vexans in GB may, in line with suggestions over its requirement as a prerequisite for TAHV transmission (see above), suggest that TAHV is likely to be less prevalent in Britain compared to other countries where this mosquito species is abundant and is the main vector. However, the isolation of TAHV from other Aedes/Ochlerotatus species including GB resident species and their role as bridge vectors in Europe in place of Ae. vexans, imply that five species could be considered as potential bridge vectors of TAHV in GB. They are Oc. caspius/Oc. dorsalis (coastal saltwater aquatic sites), Oc. cantans, Ae. cinereus and Oc. annulipes (temporary freshwater pools in flooded meadows and woodland pools). Furthermore all are generally widespread (Table 5, Fig. 3), exhibiting preferences for mammal and human biting (Ae. cinereus feeds on mammals including rabbits, and Oc. cantans feeds on woodland animals and rabbits).
Table 5.
Basic bionomics of candidate human bridge vectors of TAHV in Great Britain: vector potential inferred from host-preference(s)

There have been sporadic reports of Aedes vexans, and few historical reports of Oc. sticticus and Cx. modestus.
A, Adults; H, hibernating adults; L, larvae.
Months of activity, from January (1) to December (12), in parentheses.
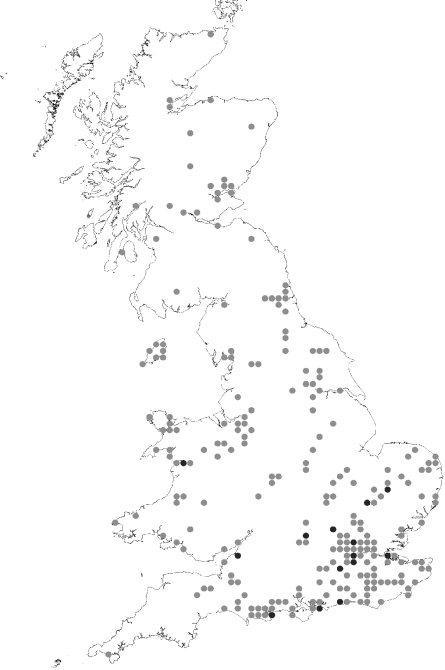
Fig. 3.
Reported distribution of candidate mosquito vectors of TAHV. Includes reported distribution of all candidate mosquito vectors of TAHV: Ae. vexans is represented by black dots, as this is considered, in Europe, to be the principal vector. All other species (Oc. cantans, Oc. caspius, Ae. cinereus, Oc. dorsalis, Oc. annulipes and Cs. annulata) are represented in grey.
The most important vertebrate amplifying hosts of TAHV in central Europe appear to be Lepus europaeus (brown hare) and Oryctolagus cuniculus (European rabbit) [50–52]; both common in GB, the latter abundantly so. Both are considered highly susceptible to TAHV, developing sufficiently high and long-lasting viraemias to infect vectors and are also abundant and attractive hosts for mosquitoes. In addition to L. europaeus and Or. cuniculus, TAHV produced viraemia in almost all mammal species tested [31] including common British mammals Erinaceus europaeus (hedgehog), Vulpes vulpes (red fox) and Meles meles (badger), more restricted British species Mustela putorius (polecat) and Glis glis (fat dormouse), and other non-British wild mammals Cricetus sp. (hamster), Citellus citellus (suslik) and Martes fiona (stone marten). TAHV appears incapable of producing viraemias in bats [53], birds [54, 55], amphibians or reptiles [56].
A number of factors including the occurrence of putative mosquito vectors in GB, the abundance of vertebrate hosts that might sustain viraemias to permit transmission, the reports of TAHV in small mammals in Devon, and public health concerns in other European countries, suggest that further investigation into the potential occurrence, distribution and prevalence of TAHV in GB is required. Furthermore, many of the mosquito species implicated and many of the TAHV outbreaks reported elsewhere in Europe have followed widespread flooding events [57]. The predictions of climate change scenarios suggest wetter milder winters, more frequent storms and flooding in autumn, and although summers are expected to be drier, rainfall is likely to be heavy when it does occur leading to flash flooding. The effect that these predicted weather events might have on endemic mosquito species needs to be considered [58], as well as a greater understanding of the status of Ae. vexans in Britain.
Inkoo virus (INKV)
Inkoo virus (Bunyaviridae: Bunyavirus), a member of the California (CAL) serogroup, is distributed across northern Europe (Norway, Sweden, Finland, Estonia, Russia). Despite neutralization test (NT) antibody surveys in humans showing high prevalences in northern Sweden (84%) and Finland (Lapland 69%), which suggest that INKV is endemic in Scandinavia (prevalence rates are lower in southern Sweden, 26%, and southern Finland, 15%), there has been no clinical evidence of human disease from INKV in either country. In Russia however, CAL viruses (including INKV) produce significant morbidity (involving neurological disease), and the same situation should be considered in western Europe [3, 45].
INKV was first isolated from a pool of Oc. communis/punctor in Finland [59]. In Sweden, INKV has been isolated from both Oc. communis [23] and Oc. punctor [35], and in Norway there have been isolations from Oc. communis and Oc. sticticus [60]. Following studies in Scandinavia, it was concluded [3, 23, 35, 60] that the main vector for INKV is Oc. communis, although Oc. punctor may replace it as a vector in the far north.
Ochlerotatus punctor, Oc. communis and Oc. sticticus have all been reported in GB, with Oc. punctor widely distributed from southern England to northern Scotland, with only two records of Oc. communis (from Nottinghamshire and Jersey [33]) and only a few widely distributed records of Oc. sticticus (from the New Forest to Scotland [32]). Ochlerotatus punctor is a woodland mosquito, exhibiting a univoltine life cycle, with adults active between May and October biting predominantly mammals, including cattle and humans. Biting of humans may be fierce and persistent.
Little information is available on vertebrate INKV hosts. However, seroprevalence rates of INKV NT antibodies have been recorded in northern Finland [59] for bovids (88% in north, 35% in south) and other domestic mammals (89% in north, 31% in south). In other mammals, seroprevalence rates were 89% in Rangifer tarandi (reindeer) and 64% in Alces alces (moose) – both absent or rare in GB – 37% in V. vulpes (red fox) and 5% in L. timidus (snow/mountain hare) – both present in GB, the former abundantly so – with no antibodies found in L. europaeus (brown hare), or various species of vole (Microtus agrestis, Microtus arvalis, Cl. glareolus), mouse (Ap. flavicollis, Mus musculus) or bat (Myotis mystacinus, Myotis daubentoni). In birds INKV NT antibodies were recorded in 0·9% of Bonasia bonasia (hazel grouse), but not in Te. urogallus (capercaillie), Te. tetrix (black grouse) or 13 passerine species tested.
It seems unlikely on current information, that INKV poses a significant risk to human health in GB. The scarcity of human clinical infections in Scandinavia and the seemingly restricted geographical distribution and preference for northerly latitudes may reduce the likelihood of transmission in GB. However, evidence of significant morbidity in Russia from CAL viruses (including INKV) needs to be considered.
Batai virus (BATV)
Batai virus (Bunyaviridae: Orthobunyavirus) was first isolated in Europe from An. maculipennis s.l. as Calovo virus (CVOV) in a village near Calovo, south Slovakia in 1960 [61]. BATV, or closely related viruses, have been identified from several countries in Eurasia and Africa [2], with isolations from Norway, Sweden, Finland, Russia, Ukraine, Czechland, Slovakia, Austria, Hungary, Portugal and Romania, from a number of western European mosquitoes – principally zoophilic An. maculipennis s.l. and An. claviger with isolations also from Oc. communis, Oc. punctor, Ae. vexans and Cq. richiardii [2, 23, 31].
The prevalence of haemagglutination inhibition (HI) antibody to BATV/CVOV in wild and domestic (bovine and ovine) animals ranges from 1% to 46% in studies carried out in Finland, Austria, Slovakia, Portugal, Romania and the former Yugoslavia. Human HI antibody prevalence is generally very low, <1% in Sweden, Finland, Germany, Austria and parts of the former Yugoslavia, with 32% reported in southern Slovakia [3]. Infection with BATV is not generally associated with human disease in western Europe. However, febrile disease, bronchopneumonia, exudative pleurisy, catarrhal and follicular tonsillitis, and acute gastritis have been recorded as clinical signs of BATV infection in the former Czechoslovakia [62]. BATV appears to occur only epidemically in Scandinavia where during an epizootic in the 1960s, neutralizing antibodies were detected in a number of cows and one farmer on coastal farms in Finland where An. maculipennis s.l. was common [63, 64]. Clearly BATV is circulating in western Europe, but due to the very low BATV antibody prevalence in humans, in contrast to the high prevalence of antibodies in other mammals, humans are only rarely involved [3]. This, coupled with the mild clinical signs on the occasions where symptoms have been described, suggests that based on current evidence, BATV is unlikely to constitute a significant public health problem for GB.
Usutu virus (USUV)
Usutu virus (Flaviviridae: Flavivirus) is closely related to important human pathogens such as Japanese encephalitis, Murray Valley encephalitis, dengue, yellow fever, St. Louis encephalitis and WNV. It was first isolated from mosquitoes in South Africa in 1959. Prior to 2001 only two isolations had been reported from mammals, one from Praomys sp. (African soft-furred rat) and one from a man with fever and rash [5]. USUV had not, until recently, been associated with severe or fatal disease in animals or humans, and it had never before been observed outside tropical or subtropical Africa.
However, from August to mid-September 2001, a considerable die-off of Tu. merula (blackbird) was observed around Vienna in Austria and USUV was detected as the cause [12]. Infection was also reported in Strix nebulosa (great grey owl), Hirundo rustica (barn swallow), Passer domesticus (house sparrow) and Parus caeruleus (blue tit) – all abundant in GB except S. nebulosa. USUV was detected in mosquitoes collected in the area, indicating that USUV has established effective transmission cycles between local mosquitoes and birds. The following year the virus re-appeared, confirming fears that the virus could overwinter in Austria, with USUV now having spread to other parts of Austria and to neighbouring Hungary.
However, after the first 2 years of USUV transmission in Austria there had been no evidence of pathogenicity of the Austrian USUV strain for mammals, including humans. In addition there have been no cases of unclear illness including meningoencephalitis in domestic animals or in humans in the endemic areas [12]. Evidence [5] of virus-specific neutralizing antibodies to USUV in British birds suggests that it is being introduced here; however, there have been no significant bird die-offs reported. The lack of pathogenicity of USUV for humans suggests that the risk posed by this virus to humans is very low. However, the concern that closely related flaviviruses have been responsible for large-scale human outbreaks elsewhere suggest that a close watch on the status of this virus or closely related viruses in Europe is required. There is limited information on potential vectors, however, USUV viral RNA has been retrieved by RT–PCR from Cx. pipiens (the main vector), Ae. vexans and Cs. annulata (H. Weissenbock, personal communication). Aedes vexans is rare in GB, the typical biotype of Cx. pipiens is almost exclusively ornithophagic, however, Cs. annulata is a nuisance human-biting mosquito, and has the longest biting season of any British mosquito, responsible for much of the biting reported during late autumn and early spring. It also adopts a wide range of synanthropic natural and artificial aquatic habitats (ponds, ditches, marshes, cisterns and water butts) [32].
Other arboviruses recorded in Europe transmitted by, or isolated from, mosquitoes
Dengue virus (DENV; Flaviviridae: Flavivirus) is not currently endemic to Europe, recorded only historically in Greece where it caused high human mortality in Athens in 1927–1928 [2, 45]. Dengue antibodies have also been recorded sporadically in the former Yugoslavia [44], Spain [65] and Turkey [66], possibly as imported infections. The main vector responsible for DENV transmission globally is Ae. aegypti, which no longer occurs in Europe and does not survive in the British Isles [32]. However, Ae. albopictus, which is considered to transmit DENV [67] has, over the last 20 years, become established in parts of southern Europe, with records from Albania, Italy, France, Montenegro, Switzerland, Spain, Belgium, Holland, Israel and Greece [68]. There have so far been no reports of transmission of viruses to humans by this mosquito in Europe [67], however, its importation and establishment in GB is a possibility [68, 69]. Despite importations of DENV into GB by infected travellers, the current absence of suitable mosquito vectors and the current climatic constraints (∼17°C is a constraining temperature threshold for development [70]) are generally considered as factors limiting transmission in northern latitudes.
Lednice virus (LEDV; Bunyaviridae: Bunyavirus) originally classified as Yaba-1 virus, has only been isolated in Moravia, Czechland [3, 71] from Cx. modestus [72]. Antibodies to LEDV have only been reported in waterfowl (including birds common to GB: mallard Anas platyrhynchos, greylag goose Anser anser, garganey Anas querquedula, teal Anas crecca, gadwall Anas strepera and coot Fulica atra) from Czechland and Romania [3, 73]. There have been no reports of human infection, with serological surveys in both locations eliciting no antibodies to LEDV. The lack of human disease, the presence only of antibodies to LEDV in some migratory birds [73] and the probable absence of key mosquito vectors in GB suggest that LEDV is not currently a public health concern.
Uukuniemi virus (UUKV; Bunyaviridae: Uukuvirus) has a wide distribution across northern and central Europe, transmitted primarily by ticks between forest rodents (including mammals Cl. glareolus, Ap. flavicollis resident to GB) and various ground-feeding passerines. UUKV has been isolated occasionally from various mosquito species (Cx. modestus, Ae. vexans, Oc. punctor and Oc. communis [2]) with only Oc. punctor common in GB. Human disease from UUKV is rare, with only three cases having been reported from southern Russia [74] and therefore public health implications are considered negligible, however, UUKV has been isolated in GB from Ixodes uriae in coastal Scotland [75].
Mosquitoes may also transmit two additional European arboviruses (Sedlec and African horsesickness), with no evidence, however, of human disease [2]. Sedlec virus (SEDV; Bunyaviridae), isolated from warblers and antibodies to it found in a number of reedbelt passerines in Czechland, is possibly transmitted by ornithophilic mosquitoes like Cx. modestus, and African horsesickness virus (AHSV; Reoviridae), is occasionally transmitted by Aedes and Culex, with no records of human disease [2].
DISCUSSION
Nine different mosquito-borne arboviruses are known to be transmitted by, or associated with, mosquitoes in Europe. However, for many our knowledge of the ecology and distribution of these viruses is limited, and for others the occurrence of human infection or human disease is currently considered rare. In the absence of in-depth studies into the epidemiology of these viruses in other parts of Europe, including GB, it may be premature to discount all these viruses from the public health agenda. Indeed, based upon current and growing evidence, a number of arboviruses are known to infect humans and cause disease, and with serological evidence of SINV, WNV, USUV and TAHV in British resident birds and mammals, the possible public health implications of these and similar viruses need to be continually appraised.
Having considered the ecology and epidemiology of each virus in Europe and the possible dynamics in GB, three viruses (WNV, SINV and TAHV) could, based purely on their ecology, occur in enzootic cycles in GB with the potential for human involvement. However, the degree to which this might occur, in comparison to other parts of Europe, is likely to be low. The potential ecology of WNV in GB has not been covered in depth here since it has already been considered in much greater detail elsewhere [9].
Regarding SINV, GB is home to all the key enzootic (Cx. torrentium, Cs. morsitans and Cx. pipiens) and bridge (Ae. cinereus) vector mosquito species that have been implicated in transmission in northern Europe, and to many of the passerine bird hosts for which the virus has been reported. Amplification of the virus in northern Europe appears to be associated with certain bird species and the occurrence and dynamics of these birds appear to correlate with significant outbreaks of Ockelbo disease in Sweden and Pogosta disease in Finland. In Sweden, fledglings of three thrush species are important in amplifying the virus and in the presence of the key mosquito species are associated with the spatio-temporal dynamics of human infection. Two of these species (Tu. pilaris and Tu. iliacus), however, do not regularly occur in GB during the summer breeding season (when fledgling numbers are high) and when mosquitoes are abundant, and it is not until the autumn that these two species visit GB. In Finland, the epidemic years of Pogosta disease appear to be related to cycles in tetraonid birds, and whilst members of the Tetraonidae occur in GB, the species implicated in Finland (Te. urogallus and Te. tetrix) occur in GB in very low numbers. The absence of the two sets of specific eco-epidemiological circumstances described above suggest that, unless an alternative scenario exists to amplify the virus, this may be a limiting factor for large-scale SINV infection in humans in GB. Nevertheless, the possible occurrence of human infection should not be discounted.
TAHV has been reported from a variety of wild mammals with the main amplifying hosts appearing to be L. europaeus and Or. cuniculus, both of which occur in GB, the latter abundantly. The virus is transmitted enzootically and to humans by a number of Aedes and Ochlerotatus species, and in some parts of Europe the presence of Ae. vexans is a prerequisite for TAHV transmission. However, in other parts of Europe different Aedes/Ochlerotatus species appear important, suggesting that different strains of virus might utilize different vector species. Aedes vexans is not common in GB, however, the presence of five other candidate Aedes/Ochlerotatus vector species (Oc. caspius, Oc. dorsalis, Oc. cantans, Ae. cinereus and Oc. annulipes) in GB suggest that TAHV could occur enzootically with possible human involvement.
Determining whether or not any of these viruses, or similar viruses that are discovered, with appropriate eco-epidemiological requirements, occur enzootically or are associated with human infection is clearly reliant upon further field and epidemiological studies. In addition, developing an understanding of the possible ecology and epidemiology of such transmission is dependent upon continued research into British mosquitoes and the development and establishment of databases, organized through the United Kingdom Mosquito Association, on the distribution and abundance of our endemic mosquito fauna. Furthermore, whilst mosquito-borne arboviruses have been isolated from mosquito species in Europe, the ability for British mosquitoes to transmit many of these viruses should be a focus of future research. Isolation of virus from mosquitoes alone is not necessarily evidence of vector status (e.g. Tyuleniy virus), as many mosquito species could become infected with almost any mosquito-borne virus if feeding on a viraemic animal with a high titre viraemia, and therefore the criteria† for incriminating a species as a vector of arboviruses should be considered.
Finally, whilst this paper deals specifically with mosquito-borne arboviruses endemic, or previously endemic, in Europe, the possibility exists for non-European viruses to become introduced. This may occur through movement of infected humans and animals leading to subsequent transmission (e.g. WNV in the United States, USUV in Europe), or as a result of climate change that may promote the abundance and range of endemic or exotic mosquitoes [76] or exotic viruses [4]. The large-scale outbreak of chikungunya virus in the Indian Ocean islands since 2005, and subsequent importation of large numbers of cases into Europe, particularly France [77, 78], raises questions as to whether such exotic viruses could establish in Europe or be transmitted during warmer summers by endemic mosquitoes or by exotic species such as Ae. albopictus. An awareness of the possibility for European transmission of ‘exotic’ viruses by mosquitoes is required, as is a greater understanding of our endemic mosquito fauna, their roles as vectors, as well as the potential for establishment and seasonal activity of exotic mosquito species and the ecological and environmental constraints on the transmission of newly introduced exotic arboviruses.
ACKNOWLEDGEMENTS
The authors thank Dr Zdenek Hubalek, Dr Jan Lundstrom and Emma Kerrod, for their very helpful comments and advice on the manuscript. This work forms part of the Health Protection Agency’s Horizon Scanning activities into emerging vector-borne infections and is funded through the HPA Government Grant-in-Aid.
DECLARATION OF INTEREST
None.
Footnotes
Although currently available distribution maps do not represent a complete picture of the distribution of British mosquitoes [10], they provide, through the combination of recorded distributions of potential vectors, an insight into areas where transmission might occur and therefore assist in targeting surveillance of arboviral transmission. It should be borne in mind, however, that other areas not illustrated on these maps could be suitable for transmission of these arboviruses and the development of geospatial risk maps would be useful. However, incorporating a number of different mosquito species that favour a variety of diverse ecologies would be complex.
The criteria for incriminating a species as a vector of arboviruses must all be met for proven vectorial ability: (1) isolation of a specific virus from specimens collected in nature; (2) demonstration of infection in the mosquito following an experimental feeding on a viraemic host or virus suspension; (3) demonstration of transmission of virus by bite to a vertebrate host or demonstration of virus in expressed salivary fluids; (4) field evidence confirming association of the mosquito species with the vertebrate population in which the virus infection is occurring.
REFERENCES
- 1.Snow KR. Medically important mosquito-borne arboviruses in Europe with special reference to Britain. Antenna. 1991;15:12–20. [Google Scholar]
- 2.Hubalek Z, Halouzka J. Arthropod-borne viruses of vertebrates in Europe. Acta Scientiarum Naturalium Academiae Scientiarum Bohemicae Brno. 1996;30:95. [Google Scholar]
- 3.Lundstrom JO. Mosquito-borne viruses in Western Europe: a review. Journal of Vector Ecology. 1999;24:1–39. [PubMed] [Google Scholar]
- 4.Gould EA et al. Potential arbovirus emergence and implications for the United Kingdom. Emerging Infectious Diseases. 2006;12:549–555. doi: 10.3201/eid1204.051010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley A et al. Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. Journal of General Virology. 2003;84:2807–2817. doi: 10.1099/vir.0.19341-0. [DOI] [PubMed] [Google Scholar]
- 6.Chastel C et al. Arbovirus infections in small mammals in Armorique Park (Brittany) and around Exeter (Great Britain): comparative serological surveys [in French] Bulletin de la Société Française de Parasitologie. 1985:79–82. [Google Scholar]
- 7.Department of Health, UK. http://dh.gov.uk http://dh.gov.uk
- 8.Chartered Institute of Environmental Health, UK. http://www.cieh.npap.org.uk http://www.cieh.npap.org.uk
- 9.Medlock JM, Snow KR, Leach S. Potential transmission of West Nile virus in the British Isles: an ecological review of candidate mosquito bridge vectors. Medical and Veterinary Entomology. 2005;19:2–21. doi: 10.1111/j.0269-283X.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 10.Snow KR, Rees AT, Bulbeck SJ. A Provisional Atlas of the Mosquitoes of Britain. London: 1998. p. 50. [Google Scholar]
- 11.Ashe P, O’Connor JP, Casey RJ. Irish mosquitoes (Diptera: Culicidae): a checklist of the species and their known distribution. Proceedings of the Royal Irish Academy B. 1991;91:21–36. [Google Scholar]
- 12.Weissenböck H et al. Usutu virus activity in Austria, 2001–2002. Microbes and Infection. 2003;5:1132–1136. doi: 10.1016/s1286-4579(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 13.Weissenböck H et al. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, Central Europe. Emerging Infectious Diseases. 2002;8:652–656. doi: 10.3201/eid0807.020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niklasson B., Monath TP. The Arboviruses: epidemiology and ecology. Boca Raton; FL: CRC Press; 1988. pp. 167–176. [Google Scholar]
- 15.Laine M et al. The prevalence of antibodies against Sindbis-related (Pogosta) virus in different parts of Finland. Rheumatology. 2003;42:632–636. doi: 10.1093/rheumatology/keg143. [DOI] [PubMed] [Google Scholar]
- 16.Lundstrom JO et al. Geographic and temporal distribution of Ockelbo disease in Sweden. Epidemiology and Infection. 1991;106:567–574. doi: 10.1017/s0950268800067637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brummer-Korvenkontio M et al. Epidemiology of Sindbis virus infections in Finland 1981–96: possible factors explaining a peculiar disease pattern. Epidemiology and Infection. 2002;129:335–345. doi: 10.1017/s0950268802007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin D.Sindbis virusThe Encyclopedia of Arthropod-transmitted Infections Wallingford, UK: CABI Publishing; 2001469–473. [Google Scholar]
- 19.Espmark A, Niklasson B. Ockelbo disease in Sweden: epidemiological, clinical and virological data from the 1982 outbreak. American Journal of Tropical Medicine and Hygiene. 1984;33:1203–1211. doi: 10.4269/ajtmh.1984.33.1203. [DOI] [PubMed] [Google Scholar]
- 20.Niklasson B, Espmark A, Lundstrom JO. Occurrence of arthralgia and specific IgM antibodies three to four years after Ockelbo disease. Journal of Infectious Diseases. 1988;157:832–835. doi: 10.1093/infdis/157.4.832. [DOI] [PubMed] [Google Scholar]
- 21.Niklasson B, Vene S. Vector-borne viral diseases in Sweden – a short review. Archives of Virology. 1996;11:49–55. doi: 10.1007/978-3-7091-7482-1_6. [DOI] [PubMed] [Google Scholar]
- 22.Niklasson B et al. Association of a Sindbis-like virus with Ockelbo disease in Sweden. American Journal of Tropical Medicine and Hygiene. 1984;33:1212–1217. doi: 10.4269/ajtmh.1984.33.1212. [DOI] [PubMed] [Google Scholar]
- 23.Francy DB et al. Ecologic studies of mosquitoes and birds as hosts of Ockelbo virus in Sweden and isolation of Inkoo and Batai viruses from mosquitoes. American Journal of Tropical Medicine and Hygiene. 1989;41:355–363. [PubMed] [Google Scholar]
- 24.Lvov DK et al. Isolation of Karelian fever agent from Aedes communis mosquitoes. Lancet. 1984;2:399–400. doi: 10.1016/s0140-6736(84)90562-2. [DOI] [PubMed] [Google Scholar]
- 25.Norder H et al. Genetic relatedness of Sindbis virus strains from Europe, Middle East and Africa. Virology. 1996;222:440–445. doi: 10.1006/viro.1996.0441. [DOI] [PubMed] [Google Scholar]
- 26.Gresikova M et al. Identification of a Sindbis virus strain isolated from Hyalomma marginatum ticks in Italy. Acta Virologica. 1978;22:231–232. [PubMed] [Google Scholar]
- 27.Jaenson TGT, Niklasson B. Feeding patterns of mosquitoes (Diptera: Culicidae) in relation to the transmission of Ockelbo disease in Sweden. Bulletin of Entomological Research. 1986;76:375–383. [Google Scholar]
- 28.Jaenson TGT. Vector roles of Fennoscandian mosquitoes attracted to mammals, birds and frogs. Medical and Veterinary Entomology. 1990;4:221–226. doi: 10.1111/j.1365-2915.1990.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 29.Lundstrom JO, Niklasson B, Francy DB. Swedish Culex torrentium and Cx. pipiens (Diptera: Culicidae) as experimental vectors of Ockelbo virus. Journal of Medical Entomology. 1990;27:561–563. doi: 10.1093/jmedent/27.4.561. [DOI] [PubMed] [Google Scholar]
- 30.Turell MJ, Lundstrom JO, Niklasson B. Transmission of Ockelbo virus by Aedes cinereus, Ae. communis and Ae. excrucians (Diptera: Culicidae) collected in an enzootic area in central Sweden. Journal of Medical Entomology. 1990;27:266–268. doi: 10.1093/jmedent/27.3.266. [DOI] [PubMed] [Google Scholar]
- 31.Lundstrom JO. Vector competence of Western European mosquitoes for arboviruses: a review of field and experimental studies. Bulletin of the Society for Vector Ecology. 1994;19:23–36. [Google Scholar]
- 32.Cranston PS Adults, Larvae and Pupae of British Mosquitoes (Culicidae) Freshwater Biological Association; Ambleside, Cumbria: 1987. pp. 1–152. [Google Scholar]
- 33.Snow KR. Mosquitoes. Naturalist’s Handbooks Series. London: Richmond Publishers; 1990. p. 66. [Google Scholar]
- 34.Service MW. The biology of Culiseta morsitans and Culiseta litorea in England. Bulletin of Entomological Research. 1994;84:97–104. [Google Scholar]
- 35.Lundstrom JO, Turell MJ, Niklasson B. Antibodies to Ockelbo virus in three orders of birds (Anserifromes, Galliformes and Passeriformes) in Sweden. Journal of Wildlife Diseases. 1992;28:144–147. doi: 10.7589/0090-3558-28.1.144. [DOI] [PubMed] [Google Scholar]
- 36.Lundstrom JO et al. Prevalence of Sindbis virus neutralizing antibodies among Swedish passerines indicates that thrushes are the main amplifying hosts. Journal of Medical Entomology. 2001;38:289–297. doi: 10.1603/0022-2585-38.2.289. [DOI] [PubMed] [Google Scholar]
- 37.Royal Society for the Protection of Birds. http://www.rspb.org.uk/birds http://www.rspb.org.uk/birds
- 38.Hubalek Z, Halouzka J. West Nile fever: a reemerging mosquito-borne virus disease in Europe. Emerging Infectious Diseases. 1999;5:643–650. doi: 10.3201/eid0505.990505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murgue B et al. West Nile in the Mediterranean Basin: 1950–2000. Annals of the New York Academy of Sciences. 2001;651:114–126. doi: 10.1111/j.1749-6632.2001.tb02690.x. [DOI] [PubMed] [Google Scholar]
- 40.Hayes EB et al. Epidemiology and transmission dynamics of West Nile virus disease. Emerging Infectious Diseases. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayes EB et al. Virology, pathology and clinical manifestations of West Nile virus disease. Emerging Infectious Diseases. 2005;11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higgs S, Snow KR, Gould EA. The potential for West Nile virus to establish outside of its natural range: a consideration of potential mosquito vectors in the United Kingdom. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2004;98:82–87. doi: 10.1016/s0035-9203(03)00004-x. [DOI] [PubMed] [Google Scholar]
- 43.Bardos V, Danielova VL. The Tahyna virus – A virus isolated from mosquitoes in Czechoslovakia. Journal of Hygiene, Epidemiology, Microbiology and Immunology. 1959;111:264–276. [PubMed] [Google Scholar]
- 44.Vesenjak-Hirjan J, Pundapolic V, Dobec M. Geographical distribution of arboviruses in Yugoslavia. Journal of Hygiene and Epidemiology. 1991;35:129–140. [PubMed] [Google Scholar]
- 45.World Health Organization. The vector-borne human infections of Europe: their distribution and burden on public health. 2004. p. 144. [Google Scholar]
- 46.Labuda M.Tahyna virusThe Encyclopedia of Arthropod-transmitted Infections Wallingford, UK: CABI Publishing; 2001482–483. [Google Scholar]
- 47.Bardos VM et al. The clinical picture of Tahyna virus (California Group) – Infection in children [in German] Padriatrie Grenzgebiete. 1980;19:11–23. [PubMed] [Google Scholar]
- 48.Danielová V. Dissemination of arboviruses transmitted by mosquitoes in Czechoslovakia and the epidemiologic consequences [in Czech] Ceskoslovenska Epidemiologie, Mikrobiologie, Immunology. 1990;39:353–358. [PubMed] [Google Scholar]
- 49.Eldridge BF, Edman JD. Medical Entomology: A textbook on public health and veterinary problems caused by arthropods. Dordrecht: Kluwer Academic Publishers; 2000. [Google Scholar]
- 50.Simkova A. Tahyna virus-neutralizing antibodies in naturally infected domestic rabbits and hares. Ceskoslovenska Epidemiologie, Mikrobiologie, Immunologie (Praha) 1966;15:304–306. [PubMed] [Google Scholar]
- 51.Minar J. Food sources of some mosquito species in the natural focus of Tahyna virus in southern Moravia. Folia Parasitologica. 1969;16:81–85. [Google Scholar]
- 52.Bardos V. The role of mammals in the circulation of Tahyna virus. Folio Parasitologica. 1975;22:257–264. [PubMed] [Google Scholar]
- 53.Simkova A. Tahyna virus in bats. Acta Virologica. 1965;9:285. [PubMed] [Google Scholar]
- 54.Simkova A. Tahyna virus in birds. Acta Virologica. 1962;6:190. [PubMed] [Google Scholar]
- 55.Malkova D, Marhoul D. Attempts at experimental infection of pheasants with Tahyna virus. Acta Virologica. 1966;10:375. [PubMed] [Google Scholar]
- 56.Aspock H, Kunz C. Investigation of overwintering of Tahyna and Calovo viruses in amphibians and reptiles [in German] Zentralblatt Bakteriologie. 1971;214:160–173. [PubMed] [Google Scholar]
- 57.Hubalek Z et al. Mosquito-borne viruses, Czech Republic, 2002. Emerging Infectious Diseases. 2005;11:116–118. doi: 10.3201/eid1101.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snow KR, Medlock JM. The potential impact of climate change on the distribution and prevalence of mosquitoes in Britain. European Mosquito Bulletin. 2006;21:1–10. [Google Scholar]
- 59.Brummer-Korvenkontio M. Arboviruses in Finland. V. Serological survey of antibodies against Inkoo virus in human, cow, reindeer and wildlife sera. American Journal of Tropical Medicine and Hygiene. 1973;22:654–661. doi: 10.4269/ajtmh.1973.22.654. [DOI] [PubMed] [Google Scholar]
- 60.Traavik T, Mehl R, Wiger R. Mosquito-borne arboviruses in Norway: further isolations and detection of antibodies to California encephalitis viruses in human, sheep and wildlife sera. Journal of Hygiene. 1985;94:111–122. doi: 10.1017/s0022172400061180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bardos V, Cupkova E. The Calovo virus – the second virus isolated from mosquitoes in Czechoslovakia. Journal of Hygiene, Epidemiology, Microbiology and Immunology. 1962;6:186–192. [PubMed] [Google Scholar]
- 62.Sluka F.Proceedings of the Symposium at Smolenice1966337–339.Bratislava: Slovak Academy of Sciences 1969; 415 [Google Scholar]
- 63.Brummer-Korvenkontio M. Batai (Calovo) arbovirus neutralising antibodies in Finland. Annales Medicinae Experimentalis et Biologiae Fenniae. 1973;51:158–161. [PubMed] [Google Scholar]
- 64.Brummer-Korvenkontio M, Saikku P. Mosquito-borne viruses in Finland. Medical Biology. 1975;53:279–281. [PubMed] [Google Scholar]
- 65.Lozano A., Vesenjak-Hirjan J, Porterfield JS, Arslanagic E. Arboviruses in the Mediterranean Countries. 1980. pp. 143–144. [Google Scholar]
- 66.Serter D., Vesenjak-Hirjan J, Porterfield JS, Arslanagic E. Arboviruses in the Mediterranean Countries. 1980. pp. 155–163. [Google Scholar]
- 67.Gratz N. Critical review of the vector status of Aedes albopictus. Medical and Veterinary Entomology. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 68.Medlock JM et al. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. Journal of Vector Ecology. 2006 doi: 10.3376/1081-1710(2006)31[292:aotpfs]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 69.Snow KR, Ramsdale CD. Mosquitoes and tyres. Biologist. 2002;49:49–52. [PubMed] [Google Scholar]
- 70.Deubel V, Murgue B.DengueThe Encyclopedia of Arthropod-Transmitted Infections Wallingford, UK: CABI Publishing; 2001133–143. [Google Scholar]
- 71.Malkova D et al. Isolation of Yaba 1 arbovirus in Czechoslovakia. Acta Virologica. 1972;16:93. [PubMed] [Google Scholar]
- 72.Voinov IN. Investigation of arbovirus infections in Belarus and other western areas of USSR. Acta Scientarum Naturalium Academiae Scientarum Bohemicae Brno. 1978;1996;30:95. [Google Scholar]
- 73.Hoogstraal H., Vesenjak-Hirjan J, Porterfield JS, Arslanagic E. Arboviruses in the Mediterranean Countries. 1980. pp. 49–63. [Google Scholar]
- 74.Butenko AM et al. Uukuniemia fever: first diagnosed cases of human infections. Arbovirus Infections Exchange. 1995 [Google Scholar]
- 75.Moss SR, Nuttall PA. Isolation of orbiviruses and uukunviruses from puffin ticks. Acta Virologica. 1985;29:158–161. [PubMed] [Google Scholar]
- 76.Snow KR, Medlock JM. The potential impact of climate change on the distribution and prevalence of mosquitoes in Britain. European Mosquito Bulletin. 2006;21:1–10. [Google Scholar]
- 77.Cordel H et al. Imported cases of chikungunya in metropolitan France, April 2005–February 2006. Eurosurveillance. 2006;11:20. doi: 10.2807/esw.11.16.02944-en. [DOI] [PubMed] [Google Scholar]
- 78.Depoortere E, Coulombier D. Chikungunya risk assessment for Europe: recommendations for action. Eurosurveillance. 2006;11:11. doi: 10.2807/esw.11.19.02956-en. [DOI] [PubMed] [Google Scholar]