SUMMARY
Salmonella enterica serotype Abortusovis, an ovine host-specific serotype, rare in most countries, is responsible for epidemic abortion episodes in Spain. With the aim of surveillance and detection of the spread of specific clones, 55 Abortusovis isolates collected during 1996–2001 from flocks in 11 provinces, were studied using XbaI–PFGE. Despite the fact that the strains were geographically and spatially related, PFGE demonstrated an epidemiologically acceptable discriminating power, identifying 20 clones (similarity, 52–96%). Clones Sabv6, 1, 5 were disseminated in seven, five and two areas respectively, while another 17 clones appeared in single places. Clones from nearby geographic regions showed a high relatedness (one band of difference in the PFGE profile) Sabv1-2-3, Sabv5-6, Sabv7-8, and Sabv13-14, suggesting a common ancestor. Co-isolation in the same flock (Sabv5-6, Sabv1-3, Sabv1-6) was detected. PFGE surveillance detected the predominance and widespread distribution of clone Sabv6 in 21 out of the 55 Abortusovis serotype episodes studied in Spain.
INTRODUCTION
Salmonella enterica subsp. enterica ser. Abortusovis is an ovine host-specific serotype, and is the most frequent cause of abortion outbreaks, stillbirths, and illness in lambs infected at birth in ovine flocks [1]. Available epidemiological data indicated that Abortusovis serotype is rare in most countries and regions of the world except in Europe, where it is particularly common, with reported cases in France, Spain, Germany, Cyprus, Italy, Switzerland, Russia, and Bulgaria, southwest England and Wales and also in Western Asia [2, 3].
The infection is introduced into flocks by means of animal carriers such as new sheep replacements, contact with other animals in seasonal migration, wild and carrion birds, rodents [4]. The oral route is the main route of infection, from food and water contaminated by vaginal discharges, placenta, aborted fetuses (liver and stomach contents), and infected newborn. Furthermore, in some conditions, faeces, milk and respiratory secretions can correspond to infectious material. Other routes of acquisition include respiratory and conjunctival routes [5]. Of minor importance, but also reported, is venereal spread from contact with a contaminated sheep, the male ovine introduces the infection into the flock [3].
From the third month of pregnancy, this pathogen induces abortion, in the absence of other clinical symptoms [5], but sometimes preceded by depression, uncertain walking, mucous vaginal discharge and diarrhoea. Following this, ewes seemed to be healthy or showed transient fever [6]. Sometimes, ewe mortality occurs from septicaemic complications and anorexia, acute metritis, enteritidis and peritonitis resulting from placental retention (5–7% of cases) [7], differing from infection causes by Dublin and Typhimurium serotypes. In addition, neonatal mortality of lambs is frequent with living lambs at term which are non-viable and die within a few hours of birth from septicaemia. Occasionally, lambs appear to be healthy but die during the first month, showing signs of enteritis, pneumonia or polyarthritis. Conversely, the infection is asymptomatic in non-pregnant ewes and in rams [3, 6].
Few epidemiological surveys have been able to be carried out owing to the low number of available Abortusovis isolates. Ribotyping, plasmid profiling, and IS200 fingerprinting [8–11], were performed as molecular characterization techniques with success to identify different and predominant Abortusovis genotypes.
To date, the highly discriminatory method of pulsed-field gel electrophoresis (PFGE) has not been used as a genotypic marker in Abortusovis serotype. The aim of this study was to survey and to detect the spread of specific clones causing epidemic abortions in ewe flocks at different geographical areas in Spain during a 5-year period by using PFGE typing. Susceptibility testing was also done, to provide insight into the antimicrobial resistance of this serotype in Spain.
MATERIAL AND METHODS
Bacterial strains
Fifty-five field strains of Salmonella enterica serotype Abortusovis, collected from epidemic abortions or neonatal mortality episodes affecting different ovine flocks from Spanish provinces during the 1996–2001 period (Table, Fig. 1), were submitted to the Salmonella and Shigella Spanish National Reference Laboratory (LNRSSE), for serotype confirmation. Strains were isolated from pathological samples such as vaginal discharges (n=17), placentas and aborted foetus or dead lambs' tissues (liver and stomach contents) (n=38). Biochemical identification was performed by conventional methods and serotyping by slide agglutination using commercial Salmonella antisera (Staten Serum Institut, Copenhagen, Denmark). Escherichia coli ATCC 25922 was used as a control strain in the disk diffusion study, while the S. enterica serotype Braenderup reference strain was used as a control strain included in each gel of the PFGE as a global reference pattern (as in Salm-gene) [12].
Table.
Epidemiological characteristics of 55 Abortusovis strains isolated during a 5-year period in Spanish ovine flocks

Vaginal d., vaginal discharge; S, streptomycin; Sh, spectinomycin; Su, sulphametoxazole; SXT: trimethoprim–sulphamethoxazole.
Fig. 1.
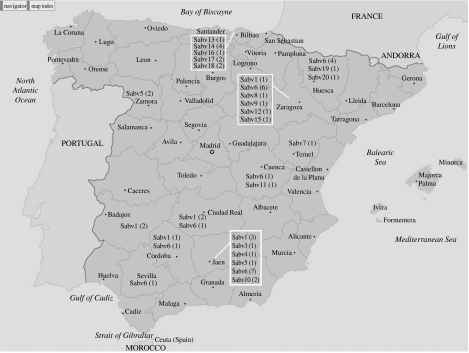
Map of Spain indicating the geographic origin of the epidemic episodes of Abortusovis serotype in ovine flocks, the different clones identified in each area (Sabv), and the number of isolates collected of each clone.
Antimicrobial susceptibility testing
All strains were screened for resistance to 18 antimicrobial agents by the disk diffusion method according to CLSI guidelines [13] on Mueller–Hinton agar (BD Diagnostic Systems, Heidelberg, Germany). The antimicrobial agents tested were purchased by Oxoid (Unipath, Basingstoke, Hants., UK) and were as follows: ampicillin (10 μg/disk), amoxicillin-clavulanate (30 μg/disk), ticarcillin (75 μg/disk), cephalothin (30 μg/disk), cefotaxime (30 μg/disk), spectinomycin (Sh, 10 μg/disk), streptomycin (S, 10 μg/disk), gentamicin (10 μg/disk), tobramycin (10 μg/disk), apramycin (15 μg/disk), nalidixic acid (30 μg/disk), ciprofloxacin (5 μg/disk), enrofloxacin (5 μg/disk), tetracycline (30 μg/disk), sulphametoxazole (Su, 300 μg/disk), trimethoprim–sulphamethoxazole (SXT, 1·25 and 23·75 μg/disk), chloramphenicol (30 μg/disk), and polimixin B (500 μg/disk). In addition, trimethoprim–sulphamethoxazole resistance was confirmed by the E-test (AB Biodisk, Solna, Sweden). CLSI standard break-points for Gram-negative enteric organisms were used as interpretative criteria, and intermediate categories were considered as resistant [13].
IS200 detection by PCR
Genomic DNA extractions of all strains for PCR assay were obtained by the ‘boiling method’ [14]. Primers and amplification conditions of DNA carrying an IS200 fragment were as previously described by Beuzón et al. [15]. Marker X (Roche Diagnostics, Barcelona, Spain) was used as a molecular weight marker.
PFGE and phylogenetic analysis
The PFGE technique was performed as outlined by the Salm-gene protocol [12] by using the CHEF-DRII System (Bio-Rad Laboratories, Hercules, CA, USA) in 0·5× Tris–borate–EDTA at 14°C and a 6 V/cm gradient with pulse ramping from 2 to 64 s over 22 h. XbaI-digested fragments were visually compared and interpreted in accordance with established criteria [16]. Only strains with an identical profile were considered to represent a single clone.
To analyse PFGE profiles, computer-monitored fingerprinting was performed with Gel Compar (Bio-Rad, Hemel Hempstead, Herts., UK) with TIFF files. Band patterns were scored for the presence and absence of bands at given molecular weights, and strains that differed by one band were assigned different PFGE profiles that were encoded by a number preceded by ‘Sabv’ (Salmonella Abortusovis serotype), after approval by HPA (Health Protection Agency, Colindale, UK). Cluster analyses using the Dice coefficient [17] for band matching with 0·8% tolerance position and a hierarchic unweighted pair-group method with averaging algorithm (UPGMA) were used to generate a dendogram describing the relationship among Abortusovis pulse types. The discrimination index was determined applying Simpson's diversity coefficient [18].
RESULTS
Antimicrobial susceptibility testing
All strains showed full streptomycin and spectinomycin resistance, remaining susceptible to gentamicin and tobramycin. Although 23 out 55 strains were sulphamethoxazole resistant, only four of them also expressed resistance to trimethoprim–sulphamethoxazole. When minimum inhibitory concentrations were determined by the E-test for this combination, the four strains, 857/97, 24/98, 1/97, and 6/97 showed a high level of resistance with values of 456:24, 456:24, 456:24, and >608:32 μg/ml. No β-lactams, quinolones, tetracycline or chloramphenicol resistance were observed in any of the studied strains. The antimicrobial resistance phenotypes found were: SSh phenotype (32 isolates), SShSu phenotype (19 isolates), and SShSuSXT phenotype (4 isolates). The epidemiological characteristics, and susceptibility profile of each strain, grouped by geographical origin, are shown in the Table.
IS200 detection
All strains resulted in an IS200 amplification DNA fragment of ∼900 bp. Larger fragments were not identified in any of the Abortusovis strains.
PFGE and phylogenetic analysis
PFGE was used to characterize 55 Salmonella Abortusovis serotype strains recovered from ovine flocks. Under XbaI restriction, the number of bands originated for this serotype ranged from 11 to 20 (Fig. 2), with 20 different PFGE profiles (PFPs), Sabv1-20, as shown in the Table. Taking all isolates into account (55 related strains) Simpson's diversity index reached a value of 0·865. Among the 20 PFPs, a wide genetic similarity coefficient value range was obtained, 52–96%. It was observed in the generated dendogram (Fig. 2) that several of these PFPs showed a higher genetic similarity (96%), with only one band difference, as occurs with Sabv1 and 2, Sabv5 and 6 (Fig. 3), and Sabv13 and 14. Differing also by one band, Sabv7 and 8, Sabv3 with respect to Sabv1 (Fig. 3), displayed 92% and 90% of relatedness respectively. Two clusters can be distinguished with nearly 50% similarity, a major cluster, termed A, which grouped the majority of the strains (n=49) and 15 PFPs (Sabv1-15), and the minor cluster B, with seven isolates and five PFPs (Sabv16-20). Some PFPs or clones, Sabv6, Sabv1, Sabv14, Sabv5, appeared at a higher frequency, and were observed in 21, seven, five, and four strains respectively (Fig. 3). Meanwhile 13 PFPs (23·6% of strains), were unique.
Fig. 2.
Phylogenetic relationship among Spanish 55 isolates of Salmonella enterica serotype Abortusovis. The 20 identified pulsotypes are listed adjacent to the corresponding XbaI–PFGE banding pattern. Clustering (A and B) and subclustering (A1 and A2) were observed when an approximate 50% and 60% of genetic similarity coefficient was considered, respectively. DNA molecular-weight scale derived from Braenderup Salm-gene reference control.
Fig. 3.
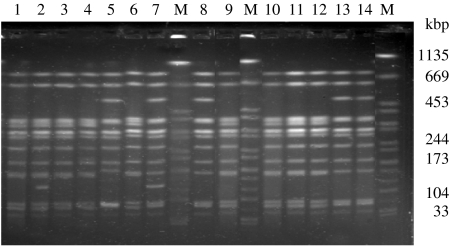
PFGE profiling of Abortusovis strains. Lanes 1, 2, co-isolated strains no. 4/97 (Sabv6), 5/97 (Sabv5); lanes 3–5, 14/98 and 6/97 (Sabv6), 8/97 (Sabv4); lanes 6–8, co-isolated strains 9/97 (Sabv6), 10/97 (Sabv3), and 11/97 (Sabv1); lanes 9–11, 14, strains 15/98, 4966/00 and 4979/00 (Sabv6), 42/99 (Sabv1); lanes 12, 13, co-isolated strains 29/98 (Sabv6) and 30/98 (Sabv1). The control strain Braenderup serotype is used as molecular marker in lane M.
DISCUSSION
In contrast to other serotypes, such as Typhimurium and Enteritis, that infect a wide range of hosts, causing a relatively mild enteric disease, some Salmonella serotypes are very host-restricted causing severe systemic disease, such as Typhi in humans and Abortusovis in sheep. The evolution of host specifity of Salmonella serotypes is linked with an increase in pathogenicity for the specific host [19]. Ovine salmonellosis may occur with a range of different symptoms of variable severity [5], depending mainly on the particular serotype involved, Abortusovis, Dublin and Typhimurium. The former is the main cause of ovine abortions in Europe and western Asia [4].
This serotype maintains the virulence for fetal and newborn lambs, and liver, spleen, brain and stomach are the main sites of multiplication, with reduced virulence in adult sheep, evident during abortion [5]. By this route, the pathogen can disseminate in high numbers into the environment and infect new hosts [20]. The avoidance of an induced inflammatory response and mucosal damage by the highly host-restricted Abortusovis serotype may facilitate the systemic spread, invading the intestinal mucosa in low numbers [3]. The induction of abortion and the mortality of newborn lambs constitute a major economic problem in geographical areas with a sheep-based economy [21]. In endemic areas, this occurs in 30–60% of the ovine flock, mainly during the final stages of gestation of the first pregnancy, with a cyclic rhythm [4].
With the aim of tracing the heterogeneity of the Spanish clones of Abortusovis serotype, molecular subtyping with PFGE was performed on the 55 isolates collected from different ovine flocks with epidemic abortion from February 1996 to March 2001. This highly discriminatory method is a standard technique for the fingerprinting of Salmonella isolates and allowed us to analyse genetic relatedness and to identify the persistence and dissemination of any particular clonal type(s) of Abortusovis serotype in Spain.
To date, this is the first report where PFGE typing was applied in Abortusovis serotypes, with all the strains being typable. The discriminatory power of PFGE was evaluated using Simpson's diversity index (0·865) that expresses the probability that two unrelated strains will be placed in two different typing groups [18].
PFGE divided the Spanish Abortusovis strains into two clusters. Cluster A, which made up 89·1% of the strains can also be subdivided into two subclusters, A1 and A2. The most frequent PFPs were located within subcluster A1, with >60% similarity of the banding patterns, whereas 76·3% of isolates and 60% of PFPs were grouped. One clone from these clusters, Sabv6, was predominantly found among the Spanish strains studied (38·2%). It was detected for the first time in March 1997 in Santiago Espada (Jaén), and for last time in April 2000 in Monegrillo (Zaragoza).
While Sabv1, Sabv5, and Sabv6 clones were disseminated in five, two, and seven locations respectively, the other PFPs appeared in single places (Table, Fig. 1). However, there was no available information to show if these clones were responsible for outbreaks or if they were endemic strains. Moreover, 20 strains with 16 PFPs emerged in a single episode in 11 areas, indicating that there were probably sporadic infections within the community.
Another event detected corresponded to the co-isolation of two clones in the same flock at the same time as occurred in Jaén, with Sabv 5 and 6, Sabv1 and 3, and Sabv1 and 6 (Fig. 3). This event had also been reported through IS200 fingerprints in two epidemic strains isolated in a single flock from northern Sardinia [10].
However, it is noteworthy that clones Sabv1, 5, 6, 14, 17 and 18, caused repeated infections in a specific area (Table). Hence, clone Sabv1 was identified 15 days and 1 year after the first detection in Ciudad Real and Jaén, respectively, while clone Sabv5 was also isolated 3 months later in Zamora. Similarly, clone Sabv6 was detected 4 months later in Huesca and Zaragoza, and 1 year later in Jaén (Fig. 3). Finally, in Bilbao, Sabv14, Sabv17 and Sabv18 were recovered 5 years, 11 months, and 8 months later, respectively, after first detection.
Factors which could possibly lead to the spread and increased prevalence of infection due to a specific clone of this organism corresponded to: a lack of food, which can contribute to growth of Salmonella in the rumen, changes in the digestive flora, intestinal and hepatic parasites, and individual factors associated with breed and genetic susceptibility. Other factors include animals meeting by seasonal migration or markets and the nomadic lifestyle of ovine flocks, insufficient health management and transport and a high proportion of pregnant ewes [1, 11].
Among the identified genotypes of the available Abortusovis serotype strains, it has been observed that closely related clones, with one band difference (90–96% similarity), emerged in the same geographic area: Sabv1 and 2, 1 year later in Badajoz; Sabv 1 and 3, nearly 1 year in Jaén; Sabv5 and 6, 5 months later in Jaén; and Sabv14 in Bilbao 1 year later; while in nearby places, e.g. Teruel and Zaragoza Sabv7 and 8 appeared. These data may suggest a common ancestor or a clonal evolution among strains yielded in a given area. An Italian survey of the presence of IS200 in Abortusovis strains from Sardinia also found that strains from nearby geographic regions showed high degrees of relatedness in their hybridization patterns, suggesting that four clones derive from a single chromosomal clonal line [10].
An epidemiological survey performed with 39 Iranian Abortusovis strains using IS200 fingerprinting, identified four genotypes in strains collected in three provinces. Whereas 23 out of 34 strains from one province showed the same IS200 profile, as did three out of a total of three strains in a nearby province. This main genotype remained in that area from 1970 to the present day [11]. Furthermore, it was detected that the other three IS200 profiles of strains isolated in the west of Iran shared the four bands of the prevalent profile, plus an additional band. As stated by Nikbakht et al. [11] all these observations, suggest an ancestral clone which circulated throughout Iran for 30 years, and from which the other genotypes could be derived.
In the prevention of abortion caused by Abortusovis serotype, different antimicrobials such as streptomycin, tetracycline, chloramphenicol, furazolidone and more recently fluoroquinolones, have been used, although, results can be disappointing because some of these can induce endotoxemia [1]. Approximately 58·2% of our Abortusovis strains were susceptible to 16 out of the 18 antimicrobials tested. Resistance to streptomycin and spectinomycin were found in all strains, possibly as a consequence of overuse of these aminoglycosides in routine ovine practice in Spain. As a serotype, Abortusovis appears to be quite sensitive to β-lactams, quinolones, tetracycline, chloramphenicol, polimixin B, and to other tested aminoglycosides. Conversely, as cited for other adapted serotypes (Typhimurium, Enteritidis, Hadar, Typhi), high levels of antimicrobial resistance in veterinary Salmonella strains as a consequence of antibiotic presence in feeds [22] has not been reported in Abortusovis [1, 23]. In contrast, resistance in Abortusovis strains to sulphamethoxazole was high (41·8%), while fewer isolates were trimethoprim–sulphamethoxazole resistant (7·3%), which is explained by the use of individual sulphonamides (or in association with tetracycline) as the most common antimicrobials used in the prophylactic treatment of numerous ovine pathologies (metritis, mastitis, diarrhoea, pneumonia, foot diseases or coccidiosis). However, antibiotic surveillance would be desirable as a consequence of affecting new antimicrobials, because ampicillin and tetracycline resistance has been previously reported in 10·3% and 3·4% of Italian Abortusovis strains recovered from 1997 to 2003 [24].
We did not detect a special gathering of clusters reflecting sulphamethoxazole and trimethoprim–sulphamethoxazole resistance, although, differences in susceptibilities were observed within clones Sabv1, 6, 14, and 18, either inside or outside the community.
In summary, fingerprinting of genomic DNA by XbaI–PFGE was particularly useful for defining clonal lines of Abortusovis strains and their spread in close geographic areas. It provided a good level of intra-serotype discrimination with zoonotic relevance which helps to detect the emergence of new strains and could be recommended as a typing method as has previously been used for other Salmonella spp. serotypes. During the 1996–2001 period, 20 clones of Abortusovis serotypes were identified in 11 Spanish provinces, and some events have been detected in this study: (i) most of the clones appeared in single places, although clones Sabv1, 5, and 6 were disseminated in five, two, and seven locations, respectively; (ii) 16 clones emerged in a single episode in specific areas, but some clones (Sabv1, 5, 6, 14, 17 and 18) caused repeated infections; (iii) several clones are co-detected in the same flock at the same time (Sabv5 and 6, Sabv1 and 3, Sabv1 and 6); (iv) clones Sabv6, 1, 14 and 5 were the predominant cause of the 38·2%, 12·7%, 9·1%, and 7·3% of studied Abortusovis episodes, respectively.
ACKNOWLEDGEMENTS
We thank all the Spanish laboratories which sent Abortusovis strains to LNRSSE. This work was supported by a grant from the Instituto de Salud Carlos III (02/0008) and Junta de Andalucía (Investigation Group AGR-149).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Pardon P et al. Ovine Salmonellosis by Salmonella abortusovis [in French] Annales de Recherches Véterinaires. 1988;19:221–235. [PubMed] [Google Scholar]
- 2.Jack EJ. Salmonella abortion in sheep. Veterinary Annual. 1968;12:57–63. [Google Scholar]
- 3.Uzzau S et al. Host-adapted serotypes of Salmonella enterica. Epidemiology and Infection. 2000;125:229–255. doi: 10.1017/s0950268899004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute for International Cooperation in Animal Biologics Salmonella abortusovishttp://www.vm.iastate.edu/services/institutes/iicab/iicab.htm
- 5.Jack EJ. Salmonella abortusovis: an atypical Salmonella. Veterinary Annual. 1971;82:558–561. [Google Scholar]
- 6.Tainturier D et al. Miscarriage in small ruminants [in French] Le Point Véterinaire. 1997;28:41–49. [Google Scholar]
- 7.Astorga R et al. Pathology of small ruminants in pictures. Perinatal and mastitis syndromes [in Spanish] Ciencias Veterinarias. 2000;1:31–38. [Google Scholar]
- 8.Nastasi A et al. Epidemiological evaluation by rRNA-DNA hybridation of strains of Salmonella enterica subsp. enterica serovar Abortusovis isolated in southern Italy in the years 1981–1989. Bollettino dell Istituto Sieroterapico Milanese (Milano) 1991;70:475–481. [PubMed] [Google Scholar]
- 9.Colombo MM et al. Phenotypic features and molecular characterization of plasmids in S. abortusovis. Journal of General Microbiology. 1992;138:725–731. [Google Scholar]
- 10.Schiaffino A et al. Strain typing with IS200 fingerprints in Salmonella abortusovis. Applied and Environmental Microbiology. 1996;62:2375–2380. doi: 10.1128/aem.62.7.2375-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikbakht GH et al. Fingerprinting of Salmonella enterica subsp. enterica serotype Abortusovis in Irán. Epidemiology and Infection. 2002;128:333–336. doi: 10.1017/s0950268801006732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters TM et al. The Salm-gene project: a European collaboration for DNA fingerprinting for food-related salmonellosis. Eurosurveillance. 2003;8:46–50. doi: 10.2807/esm.08.02.00401-en. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. Wayne, PA: CLSI; 2005. [Google Scholar]
- 14.Holmes DS, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Analytical Biochemistry. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 15.Beuzón CR et al. Identification of Salmonella abortusovis by PCR amplification of a serovar-specific IS200 element. Applied and Environmental Microbiology. 1997;63:2802. doi: 10.1128/aem.63.5.2082-2085.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenover FC et al. Interpreting chromosomal DNA restriction patterns produced by pulse-field gel electrophoresis: criteria for bacterial strain typing. Journal of Clinical Microbiology. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nei M, Li WH. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proceedings of the National Academy of Sciences USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter PR, Gaston MA. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. Journal of Clinical Microbiology. 1988;26:2465–2466. doi: 10.1128/jcm.26.11.2465-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumler AJ et al. Evolution of host adaptation in Salmonella enterica. Infection and Immunity. 1998;66:4579–4587. doi: 10.1128/iai.66.10.4579-4587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uzzau S et al. Salmonella enterica serovar-host specificity does not correlate with the magnitude of intestinal invasion in sheep. Infection and Immunity. 2001;69:3092–3099. doi: 10.1128/IAI.69.5.3092-3099.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sojka WJ et al. The incidence of Salmonella infection in sheep in England and Wales, 1975 to 1981. British Veterinary Journal. 1983;139:386–392. doi: 10.1016/s0007-1935(17)30383-4. [DOI] [PubMed] [Google Scholar]
- 22.Marwick C. Animal feed antibiotic use raises drug resistance fear. Journal of the American Medical Association. 1999;282:120–122. [PubMed] [Google Scholar]
- 23.Malorny B, Schroeter A, Helmut R. Incidence of quinolone resistance over the period 1986 to 1998 in veterinary Salmonella isolates from Germany. Journal of Clinical Microbiology. 1999;43:2278–2282. doi: 10.1128/aac.43.9.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Curtis P, Filippini G, Magistrali C. Salmonella Abortusovis: an up-to-date problem [in Spanish] http://www.pg.izs.it/webzine.html Webzine Sanità Pubblica Veterinaria. 23 [Google Scholar]