SUMMARY
This review examines the current situation of bovine tuberculosis (bTB) in southern African savannah systems, and uses theory on multi-species host–pathogen systems to suggest possible options for future research and management. In southern Africa, the buffalo (Syncerus caffer) and the Kafue lechwe [Marsh antelope] (Kobus leche) have been found to be maintenance hosts for this disease, but the importance of other host species is becoming apparent. The role of other host species in the maintenance and spread of the disease varies, depending on the spatial distribution and resource utilization patterns of the species, disease susceptibility, transmission modes and the ecology of both host(s) and vector(s). Future research needs to identify the pathogenicity of bTB in each of the host species, and the mechanisms and rates of inter- and intra-specific transmission among different species, in order to develop multi-host models to understand the development and spread of the disease.
BACKGROUND
Bovine tuberculosis (bTB) poses a serious threat to free-ranging wildlife and domestic animals, as well as having significant zoonotic potential. Control and eradication programmes for this disease have focused mainly on tuberculosis (TB) in domestic cattle because they are the traditional hosts and have economic importance. Bovine TB also poses a threat to trade in animals and their products [1]. The importance of TB in wild animals specifically has been acknowledged recently [2]. Once infected, many wild animals have shown the potential to act as reservoirs of infection for both domestic cattle and other valuable wildlife species [2]. The brushtail possum (Trichosurus vulpecula), European badger (Meles meles), bison (Bison bison), African buffalo (Syncerus caffer), Kafue lechwe (Kobus leche) and white-tailed deer (Odocoileus virginianus) can all act as maintenance hosts for bTB, allowing the persistence of the infection in wildlife and enabling the horizontal transmission of the pathogen between species [2]. In addition, some species act as ‘spillover’ hosts or ‘dead-end’ hosts [3]. Spillover or dead-end hosts have only a limited possibility of maintaining the disease in the population in the absence of a persistent alternate source of infection [2]. For example, lions (Panthera leo), leopards (Panthera pardus), cheetahs (Acinonyx jubatus) and other carnivore species do not appear to be able to maintain infection in the absence of an infected maintenance host in the system.
In Africa, bTB is present in cattle in the majority of countries, although there are strong regional differences in the number of outbreaks, cases and deaths (Tables 1 and 2). Only seven nations in Africa apply disease control measures and consider bTB as a notifiable disease [6]. Although measures to control bTB in domestic stock are becoming established, the infection has relatively recently infected certain populations of native wild bovids, most notably the African buffalo. This species is considered the main reservoir throughout Africa [7] and is thought to be responsible for infection of other sympatric wildlife and the possible re-infection of cattle. This poses a serious obstacle for control and eradication of bTB [8]. However, the more recent detection of other potential maintenance hosts indicates that bTB in Africa exists as a multi-host pathogen within a multi-species system. In addition, because the number of interacting large mammal species in certain savannah regions of Africa is perhaps higher than any other geographic area of similar size [9], bTB has spread rapidly through these ecosystems.
Table 1.
Occurrence of bovine TB in cattle and/or other wildlife in southern African countries between 1998 and 2004 [4]
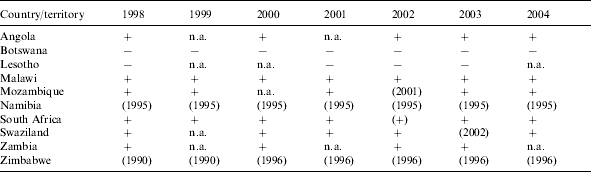
+, Infection reported or known to be present; −, infection not reported and date of last outbreak unknown; n.a., no information available; (year), date of the last reported occurrence of disease; (+), disease limited to specific zones.
Table 2.
Bovine TB and control statistics for southern African countries in 2004 [5]
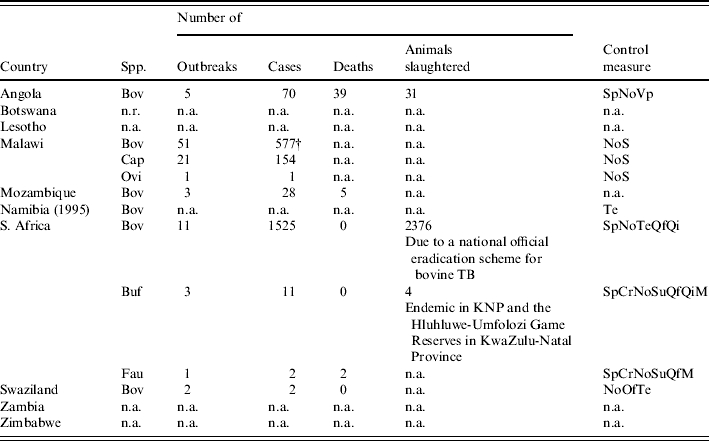
Cases, animals infected by the disease (sick animals and animals that have died from the disease that have been detected in clinical outbreaks of the disease, in abattoirs or during active surveillance); Deaths, animals that have died naturally from the disease (not including those culled); Animals slaughtered, animals killed for disease control purposes; No, notifiable disease; Cr, control of wildlife reservoirs; M, monitoring; Qf, precautions at border; Qi, movement control inside the country; Sp, modified stamping out; Su, surveillance; Te, screening; Vp, vaccination prohibited; Bov, cattle; Buf, buffaloes; Cap, goats; Fau, wild animals; Ovi, sheep; n.a., no information available; n.r., disease not reported; (year), date of the last reported occurrence of the disease; † meat inspection recorded cases.
Although bTB occurs in many African countries, this paper will focus on countries within southern Africa, where the majority of studies on bTB in wildlife has been carried out. The theory behind multi-species host–pathogen systems will be discussed followed by the origin and epidemiology of bTB in southern Africa, and a review of the wild mammals affected by the disease in southern Africa. The current theoretical knowledge of the role of multi-host interactions in maintaining infectious disease will then be used to help understand the problem and provide possible options for future management.
MULTI-SPECIES HOST–PATHOGEN SYSTEMS
Most research on host–pathogen interactions has focused on single-host, single-pathogen interactions [10]. However, many pathogens and parasites, including bTB, may infect multiple species [11]. The dynamics of multi-species host–pathogen systems has become a focus of recent research [12–15]. In single-host systems the density of a host population needs to exceed a threshold for the disease to invade and persist in the population [16]. The threshold population densities (Nt) for invasion and persistence differ for each pathogen and host species. Similarly, many pathogens can infect and spread through a population but are unable to persist [17]. In multi-host systems, the threshold density theory is replaced by a threshold community configuration, which can be described by an isocline with the axes corresponding to the densities of each host. The shape of the threshold isocline depends on the ratio of inter- to intra-species transmission rates [15]. The rate of inter-species transmission is dependent on the interaction rate between the host species. Closely interacting species may provide a single resource for the pathogen and therefore will operate effectively as a single population in relation to the threshold [18]. If host species only interact weakly, the existence of an alternative host may provide an additional, but possibly occasional, resource and pathogen establishment will only be slightly enhanced. However, this could still be important for the maintenance of a pathogen if this interaction occurs at specific times of year, for example where animals congregate at a specific limiting resource such as a water hole or supplementary food supply.
Pathogen host specificity is another important consideration; generalist pathogens tend to pose a greater threat than those that are more specialized [19]. Local environmental conditions affect the development of many pathogens in both time and space, so the availability of infective stages of some pathogens can vary seasonally and geographically [11]. The transmission rate between species is largely determined by the spatial distribution [15], timing of contact [11] and resource utilization patterns of the hosts. However, determining such rates is difficult and further complicated by the existence of multiple hosts [15]. Another important determinant is the basic reproductive rate (R0) of the pathogen, defined as the average number of successful secondary infections produced when one infected individual is introduced into a susceptible population. R0 must be ⩾1 for the pathogen to persist indefinitely, although diseases may persist and represent a continued threat for significant periods when R0 <1 during the extinction phase, especially for chronic infections. Mycobacterium bovis, the causative agent of bTB, is one such pathogen, with most infected individuals remaining infectious until they die, possibly representing a continued source of infection for a number of years.
ORIGIN AND EPIDEMIOLOGY OF bTB IN SOUTHERN AFRICA
According to Myers & Steele [20], bTB existed in the Mediterranean even before classical times. From northern Italy it spread to western Europe and Great Britain, and it was from Holland and Great Britain that cattle first carried the disease to many parts of the world that had been colonized by those countries [21]. It is therefore not surprising that most of the ex-British and ex-Dutch colonies are infected with bTB today, since it was introduced in the 19th century with the livestock of colonial settlers. In South Africa, bTB was first diagnosed in cattle in 1880, and it was first reported in wildlife (greater kudu, Tragelaphus strepsiceros) in the eastern Cape Province in 1928 [22]. In the Kruger National Park (KNP), a single case of Mycobacteriosis was identified in an impala (Aepyceros melampus) in 1967 [23], but it was not until 1990 that the bacterium was first isolated in buffalo within KNP [24]. Strong circumstantial evidence suggests that bTB entered KNP in the south during the late 1950s or early 1960s. Buffalo herds contracted the disease by grazing with infected cattle in the Komatipoort/Malelane region before returning, infected, to the park [25]. There were two cattle farms in the immediate vicinity at that time, which eventually had to be slaughtered out to control TB [26], and there were also reports of cattle deaths from buffalo-associated theileriosis, confirming that buffalo and cattle had shared rangeland.
TB has played an important historical role in the health and culture of developed nations, and consequently much is known about the epidemiology and control of bTB in cattle in these countries. However, information on bTB in relation to humans and animals within developing countries is often difficult to obtain [27]. In most developed countries, the establishment of control programmes and the widespread pasteurization of milk appear to have substantially reduced the disease caused by M. bovis in both cattle and humans [6]. It is not possible, however, to evaluate the relative contribution of M. bovis to the current TB epidemic in humans in many developing countries, where current human TB diagnostics do not differentiate between M. bovis and M. tuberculosis infection. TB remains the greatest cause of human deaths and economic loss in many developing countries including those in Africa [8]. This is largely due to the lack of funds [28], trained professionals, and previous under-estimation of both the economic and zoonotic consequences of the disease by governments and donor agencies in these countries [8]. Globally, there were more than 8·8 million new cases of TB in 2002 and these were heavily concentrated in the developing world [29]. TB is a major opportunistic infection in people infected with human immunodeficiency virus (HIV) [30] and the increasing occurrence of HIV in developing countries is resulting in a human epidemic of TB [6]. The economic losses to the agricultural sector as a result of bTB are substantial. Milk yields are reduced and infected carcasses intended for sale and consumption may be condemned, which has serious implications for exporting cattle or their products.
Understanding the epidemiology of the infection within and between species is crucial to the control of the disease in both domestic and wild animals. The transmission from infected domestic animals to susceptible wildlife (and vice versa) is highest when they share pasture or territory [31]. However, although many species may become infected, most do not have maintenance host potential [3]. The wide range of wildlife species becoming infected via inter-specific routes gives a new dimension to the determinants of disease, although these ‘inter-specific epidemiological links’ [32] are poorly understood.
The two main transmission pathways are the respiratory and alimentary routes. In respiratory (also known as aerosol or droplet) transmission, mycobacteria from open pulmonary lesions are aerosolized in the respiratory tract, resulting in the classical mode of transmission; this is the main cause of disease spread within human and cattle populations [6, 33, 34]. Certain traditional societies in Africa have close contact with their cattle, often sharing living space with them, providing an ideal situation for people to inhale bTB and for infected people to infect their livestock [35].
Alimentary transmission may occur in one of two ways. Firstly, transmission may occur through the excretion of mycobacteria in sputum, draining sinuses, faeces or urine of an infected individual, and the subsequent consumption of contaminated material by other animals. For example non-aggressive herbivorous species may share range or even habitat niches, so alimentary transmission through consumption of secondary contaminated material (vegetation) may occur. Grazing animals in Africa often congregate at water points, salt supplementary points, or at night for protection from predators, thus facilitating the spread of the disease [36]. The second alimentary transmission route may account for the spread of disease during aggressive inter-specific encounters and prey to predator transmission. This involves the consumption of primary infected material (i.e. lesions, tissues, blood and internal organs) by a susceptible individual. Transmission by this route is becoming cause for concern in the conservation of Africa's high profile carnivores that live in areas with infected prey species. The consumption of animal products, such as unpasteurized milk [37] poses a serious threat to rural pastoralists and small-scale farmers, as well as consumers in urban areas of Africa [35].
A third, but less known, mode of transmission is via the percutaneous route. This has been documented in kudu, where contaminated thorns may scratch or abrade the delicate ear or facial skin of this species. It has also been documented in large predators, where fight wounds contaminated by M. bovis result in chronic granulomatous infections of the skin, subcutis or muscles [24].
Irrespective of the precise route of infection, it may take years for clinical signs to develop [3] and the spread of M. bovis within the animal is considered to be a relatively slow process in ruminants and large carnivores, with most infected animals being asymptomatic until disseminated lesions develop during the advanced stages [27, 34]. The clinical signs exhibited across most species include emaciation, coughing and associated respiratory problems, swollen lymph nodes, draining sinuses and lameness (carnivores). The clinical signs are generally related to the route of infection and target organs involved, and these frequently differ between species (Table 3).
Table 3.
Clinical signs, most common sites of gross lesions, route of transmission, epidemiological status, and estimated length of infectious period prior to death of free-ranging African wildlife [2]; (R. G. Bengis, unpublished observations)
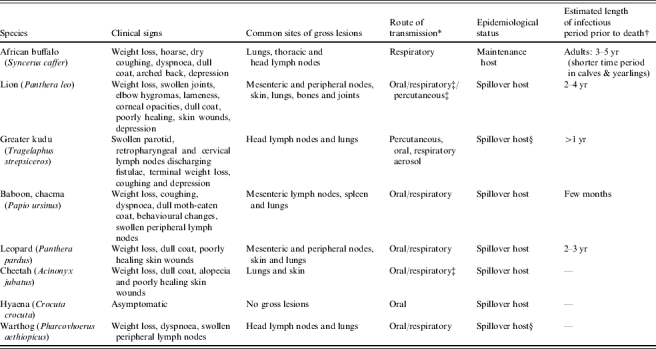
Route of transmission is frequently linked to sites of gross lesions but secondary haematogenous or lymphogenous spread, and infection of abdominal organs via coughing and swallowing infectious material, also occur;
Limited data available;
Possible alternative route of transmission;
In high densities may be maintenance hosts of M. bovis.
The severity of bTB in any individual is dependent on the infectious dose (number of organisms and number of exposures), the route of infection, and the immune robustness of the individual. Bovine TB only becomes visible during the ‘active’ or ‘clinical’ stage of the disease, when characteristic lesions develop and progress, ultimately leading to death. Many animals are subclinically infected and may remain asymptomatic until such time that they experience a repeat bTB infection, suffer from poor nutrition or advancing age, or become super-infected due to other disease agents [38]. Most notable of the other disease agents are the immunodeficiency viruses, which may affect primates (simian immunodeficiency virus, SIV), cats (feline immunodeficiency virus, FIV) or humans (HIV), and which have the potential to exacerbate mycobacterial infections [39].
Wild mammals affected by bTB in Southern Africa
Most mammalian species are susceptible to TB, and the number of wild African mammal species in which bTB has been reported is increasing [3]. Three main factors appear to have contributed to this increase. First, an increase in domestic livestock numbers and expanding interface with wildlife has increased bTB infection pressure. Infected wildlife maintenance hosts then become an additional source of infection. Second, public awareness of bTB and its potential economic and environmental impacts has resulted in heightened passive surveillance and increased amounts of specific research. Lastly, active surveillance and monitoring in conjunction with improved ante-mortem diagnostics have provided the means to assess the prevalence and incidence of TB in wildlife, both directly (observation) and indirectly (e.g. blood sampling). Although many species are known to be infected, only a few high profile species have received much formal attention. The following provides a brief synopsis of the historical and general ecological aspects of four most important African species or groups susceptible to bTB.
African buffalo
African buffalo are highly gregarious animals and are distributed throughout savannah regions in herds numbering hundreds. In KNP there are approximately 100 herds distributed across 22 000 km2, with an average herd size of 270 individuals [40]. This once healthy buffalo population was probably subclinically infected for a long time before the TB prevalence reached levels where the disease became clinically detectable. Drought stress in the early 1990s may also have played a role [3]. The pattern of lesion development that occurs in buffalo suggests that bTB spreads mainly through aerosol transmission, making this highly gregarious and susceptible species an ideal maintenance host in the southern African ecosystem [24]. Within 3–6 months of infection, most buffalo develop lesions in the lymph nodes of the head, tonsils, lung or thoracic lymph nodes. The infection may then spread by local expansion, or via the blood or lymph vessels to more distal sites (R. G. Bengis, unpublished observations). Necropsies show the lung lesions to be poorly encapsulated, indicating a weak immune response, which in turn suggests that buffalo may be recent evolutionary hosts with naive immunity. Further progression of lesions frequently results in caseous necrosis, which may be followed by cavitation and liquefaction, at which stage the host becomes super-infective [24]. Adult buffalo may remain infected for 3–5 years before mortality; and although calves and yearlings are less likely to become infected, the disease appears to progress much faster in these age classes (R. G. Bengis, unpublished observations). The potential impact of this slow progressive disease on buffalo population biology is unknown, but recent studies in the Hluhluwe/Umfolozi Park have demonstrated that bTB may affect population growth, resilience and fecundity (A. E. Jolles et al., unpublished observations).
Following the first recorded case in KNP in 1990 [41], follow-up surveys have demonstrated a gradient of infection from south to north. This latitudinal gradient was not unexpected in view of the likely southern entry of the disease to the park, followed by the progressive northward spread. In 1998, a survey involving the sampling of most buffalo herds in KNP revealed an average bTB prevalence of 38·2% in the southern region of the park, 16·0% in the central region and 1·5% in the northern region [42]. Rodwell et al. [43, 44] found that, between 1991 and 1998, bTB had increased in prevalence and spread northwards. The high prevalence seen in some buffalo herds is due almost entirely to intra-specific transmission (buffalo in the immediate social group of those infected receive high and possibly multiple exposures to the disease, although this may vary depending on the severity of lesions in the individual). The prevailing environmental conditions, especially rainfall [38], may also play a role, because that in turn frequently affects the behaviour of the buffalo. There is no sexual bias in disease vulnerability but it is suggested that there is an age-related increase in prevalence [38, 44]. Caron et al. [38] found significant regional variation in the age structure of buffalo populations, suggesting that a decrease in body condition of adults and subsequent reduction in milk production caused a decrease in calf survival. Modelling has predicted that prevalence could rise to as high as 90% over the next 25 years [42], with associated consequences for predatory species. However, not all infected individuals will be in the same stage of disease due to the involvement of several other factors. It is likely that the pathogenesis of the disease is directly related to the animal's genetic resistance, condition and nutritional status [44, 45]. These in turn may be linked to seasonal fluctuations of environmental variables such as rainfall, grazing, temperature and ultraviolet exposure [40, 46]. Poor body condition increases the risk of developing TB, and it has been demonstrated that bTB may operate in synergy with both parasitism and resource limitation to reduce body condition significantly [38].
Greater kudu
The greater kudu is a gregarious herbivore, which forms herds of up to 12 individuals consisting of yearlings, calves and adult females. Males are generally solitary and visit females during the autumn/winter rut. Thirteen cases of bTB have been confirmed in kudu in KNP since the first diagnosis was made in 1996 in an animal from an adjacent commercial game ranch [47]. However, staff and tourists have reported at least 30 additional kudu with characteristic head swellings, symptomatic of bTB [24]. Bovine TB in kudu in KNP generally presents with severe abscessation of the head lymph nodes, with draining sinus tracts. The draining tracts in the parotid area of the head allow infectious exudate to contaminate leaves and thorns on the vegetation [47]. During dry spells, the animals compete for browse, on palatable thorn trees (Acacia spp. and Xiziphus spp.). During this process, contaminated thorns scarify the skin and contaminated browse is ingested, passing on the infection to other kudu feeding in a similar manner. Furthermore, as a result of ingesting thorny material, kudu frequently have micro-scarification of pharyngeal and oesophageal mucous membranes, which may act as the port of entry through which bTB infects associated tissues such as the tonsils, retropharyngeal, mandibular and cervical lymph nodes. Infection frequently spreads to the lungs and other distal sites, including the abdominal organs, and infected animals then develop emaciation, coughing and blindness. The characteristic swollen head and neck lymph nodes (and associated draining tracts) make kudu one of the few species in which it is possible to diagnose TB at a distance. Kudu with advanced TB and draining sinus tracts are super-shedders of infection, and have the potential to act as a maintenance host species. Their infected organs are also highly infectious and may be responsible for inter-specific transmission, especially to their predators [24]. Prevalence is likely to be maintained at low levels because of low population densities, perhaps rising during the dry season with greater contact and feeding transmission. The common ‘buffalo strain’ of M. bovis has been isolated from some kudu. However, a different genotype has also been found in a group of kudu in KNP, which indicates that, as well as being susceptible to the dominant strain of infection, kudu may also be able to maintain a separate infection cycle, which may have implications for their potential role as maintenance hosts [48]. It is unknown how long the infected kudu can survive before the disease becomes fatal; however, in KNP, one male kudu showing typical clinical swellings of the head lymph nodes died 11 months later (R. G. Bengis, unpublished observations).
Chacma baboon
All primates are susceptible to TB and although both human and bTB occur in captive individuals, either form is rarely found in free-ranging primates [24]. However, in 1998, a single troop of free-ranging chacma baboons (Papio ursinus) in KNP became infected from feeding on carcasses scavenged either from the wild or from a post-mortem facility. The troop denned at night in a deserted pump house on an old river bridge, and in this confined space, aerosol and oral transmission (faeces and urine) were facilitated, resulting in 50% prevalence of infection. This high local prevalence in the troop probably resulted from multiple exposures. The most frequent clinical signs seen in the baboons were emaciation, coughing and dyspnoea. Some baboons were noted to suffer what appeared to be depression. The most consistent necropsy findings were severe miliary lesions of the lungs and spleen, indicating that the disease rapidly enters the blood stream and then spreads to distal sites [49]. The kidneys, liver, vertebrae, mesenteric and peripheral lymph nodes also suffered extensive lesions [49]. TB isolates were identical to the common African buffalo isolate in KNP. In contrast to the more commonly affected species, bTB in baboons appears to produce a fulminating infection and is usually fatal within a few months (R. G. Bengis, unpublished observations). Following the closure of their den and the death of many of the most severely infected animals, a large proportion of the troop was captured and tested in a follow-up operation. Positive animals were culled and negative animals were released. When the baboons were reassessed 26 weeks later, no further cases were detected and no spillover to other troops had occurred [49] suggesting that the disease is unable to be maintained in this species in the absence of an outside source.
Carnivores
The first reported free-ranging carnivore infections occurred in 1995 when lions, and then cheetah were diagnosed with bTB. Following this, leopards were diagnosed in 1998 and a further 50 cases of lion infection have since been confirmed. Most of the confirmed cases have occurred in the southern and central region of KNP, corresponding positively with the region of high prevalence of bTB in buffalo [24]. The KNP has a lion population of approximately 1700 of which Keet et al. [32] estimated that approximately 500 live in areas where there is high TB prevalence in buffalo. Restriction fragment length polymorphism analysis has confirmed that the M. bovis strain in lions is the same as that isolated from buffalo [32]. This provides further evidence of prey to predator transmission. Predators selectively prey on weaker individuals and scavenge on dead animals, which increases the likelihood that they will come into contact with extremely infectious material [38]. Most carnivores become infected with bTB from eating infected prey animals [24, 32]. Most infectious material is present in infected organs such as lungs and lymph nodes, and the ingestion of muscle tissue alone poses minimum risk to the consumer because M. bovis does not readily multiply in meat [27]. Transmission of infection from prey to predator may also occur via the respiratory route during terminal bite asphyxiation of infected prey [3]. In the absence of infectious prey species, the low population density of lions (and other carnivore species), coupled with relatively low rates of horizontal transmission, suggests that they are likely to be dead-end hosts.
The risk of lions becoming infected by bTB may be increased because of simultaneous infection with FIV, potentially making them more susceptible to bTB. In lions, the common clinical signs of bTB include emaciation, staring hair-coat with poorly healing skin lesions, swollen joints and limbs, lameness and blindness. Macroscopically and microscopically, there are lesions throughout the lymphatic system, especially the mesenteric, peripheral and head lymph nodes. The lesions are generally well developed but do not show signs of caseation or calcification and their macro-appearance are unlike those of classical TB lesions of primates or ruminants [24]. However, the general symptoms are largely similar to those exhibited by humans who historically contracted TB through ingestion of unpasteurized contaminated milk [37]. Lions are social cats, and when compromised by infection, they retain the support of other members of the pride; therefore infected individuals have a better opportunity of surviving for longer than solitary species without this support system. The time from infection to death has been estimated to be between 2 and 5 years (R. G. Bengis, unpublished observations), but is probably related to initial infectious dose, as well as number of exposures.
Leopards rarely catch buffalo and any successful catches are usually young individuals that are less likely to be infected with bTB. Leopards probably become infected by scavenging carcasses of dead buffalo, or catching infected kudu or warthogs. Leopards are solitary, and do not have a social support system like lions. Therefore, once infected, the disease is likely to be fatal to leopards within a shorter period of time.
Management of bTB in Southern Africa
South Africa has an established bTB eradication scheme in place for cattle, but the large, free-ranging wildlife source, represented mainly by buffalo, may pose problems in the future. The South African National Parks and the Directorate of Animal Health of the Department of Agriculture have been evaluating possible containment, control and eradication strategies since the early 1990s. An electrified perimeter fence has been erected to protect farms adjacent to KNP. Initial plans to separate northern buffalo herds from the infected southern herds by means of a buffalo-free zona sanitaire or a double fence have been shelved because infection has now been detected in a significant number of northern herds. In addition, the detection of TB infection in kudu and warthog has confounded the whole disease control option, and also clouded any ideas or intentions of embarking on a buffalo-centred intervention.
In the Hluhuwe/Umfolozi Park a capture test and slaughter policy is being carried out. Buffalo are mass-captured in corrals; the test-positive animals are killed and the test-negative animals are released. Because the animals are held in a confined area for 72 h before the intradermal tuberculin test can be read, it was feared that this may result in a high rate of transmission between the infected and non-infected individuals, thereby reducing the effectiveness of this control technique [2]. However, a more recent evaluation of this project showed that this technique was indeed succeeding in reducing TB prevalence in those buffalo herds, and so this project will continue in the future. To date, a similar project has not been implemented in KNP because of the size of the individual buffalo herds and their home ranges, and certain habitat and terrain challenges. In KNP an additional initiative is proving successful. A breeding programme has been established to produce disease-free buffalo calves, which are then removed from the park and used to set up a breeding herd of KNP genotype buffalo that can be used for re-stocking conservation areas [50].
In the long term, it would appear that vaccines offer the most widely acceptable solution for safeguarding both domestic and wild animals. Research into the development of a vaccine has previously focused on controlling infection in man and domestic animals, but more recent work is addressing the need for the protection of wildlife species, especially those endangered or valuable [2]. Bacillus Calmette-Guerin (BCG) is currently the only available vaccine that can be used for wildlife but its effectiveness is very variable, ranging from 0% to 70% protection in humans [2, 51]. The Wildlife Tuberculosis Study Group (WTBSG) is currently undertaking research to examine the effectiveness of this vaccine in buffalo. Once an effective vaccine has been identified, the next challenge will be to develop an effective and practical delivery system. For example, non-living vaccines may be delivered to free-ranging wildlife through the use of oral baits, but these are often not as effective as live vaccines given directly into the duodenum [50] and may be inappropriate for species that are never artificially fed, for example the African buffalo. Other methods of administration suggested are aerosol vaccines distributed by helicopter, or self-replicating recombinant vaccines containing important mycobacterial antigens [2].
To help in the development and eventual implementation of the vaccine option, reliable infection models in buffalo have been developed and validated by the WTBSG for future post-vaccination challenge studies. Field research is also underway to study TB transmission rates in buffalo herds, TB-related mortality rates, buffalo herd fission–fusion events, buffalo movements and exchanges between herds. The results of these field studies are being used to develop mathematical models that may be more appropriate for this multi-host system. Some preliminary results from these models predict that the buffalo population growth will start to decline when herds have a sustained TB prevalence of 40% or more.
Understanding the dynamics of multi-host–pathogen systems is an extremely important aspect of the conservation of susceptible species. The establishment and prevalence of diseases is largely influenced by host-community structure. Resource utilization patterns and spatial distribution of hosts partly influence the transmission rate between species, but pathogen life cycles, and host and vector ecology are also important [15]. Multi-species host–disease models, as for example proposed by Holt et al. [15], can provide general insights into how community structure affects the establishment and maintenance of an infection. Similar models could be used to investigate the role of different host species, within a multi-species host complex, in maintaining real infections, and also the effects of management strategies, directed at specific hosts, on the persistence of these infections. However, even basic formulations of such models for real disease–host scenarios are heavily reliant on good-quality data on parameters such as disease-induced mortality, the infectious period in different host species and intra- and inter-specific disease transmission rates. At present, such data are either based on very small sample sizes or are not available, and even general data on inter-specific interactions (and therefore potential transmission opportunities) are sparse or anecdotal.
The existence of bTB in free-ranging mammals in southern Africa poses significant threats to conservation and tourism. Bovine TB is virulent in lions and other top carnivores, and these species exist at relatively low densities in most of the infected area. If these were the only potential host species, the infection probably would not be able to persist. However, gregarious large herbivores such as the buffalo and the kudu occur at higher densities, and the pathogen may persist in these host species, and be maintained in the absence of any additional disease source. Consequently, if inter-specific transmission occurs between buffalo or kudu and large carnivores, or if the infection passes from these species to carnivores indirectly via another host species that also acts as prey for large carnivores, the impacts of the infection on populations of large carnivores will be exacerbated. Strategies for the management of the disease in some parks already focus on eradication of the infection from buffalo herds by the test and slaughter technique. Conceptual multi-species host–disease models suggest that proactive control to reduce populations of hosts such as buffalo and kudu may help by reducing the overall level of infection (via reductions in the number of susceptible animals) in the host community. The general principles emerging from these models may, therefore, have significant implications for the development and assessment of management options for bTB in national parks and other areas throughout sub-Saharan Africa, and efforts should be made to obtain the necessary data to develop these conceptual models into practical models to underpin management.
CONCLUSION
The emergence of bTB in free-ranging African wildlife populations may be the most threatening disease event in certain African ecosystems, since the rinderpest epidemic of 1898–1902 [24]. Although the increased reports of bTB may have several underlying components, an increase in both the number of species infected and the prevalence within those species, as well as spatial spread is certain. Research is being carried out in three main areas: improving understanding of the epidemiology and interaction of TB in wildlife and cattle, developing better diagnostic tests, and developing vaccines [27].
The increased awareness of bTB and its potential environmental and socio-economic implications has led to significant improvements in diagnostics, prevention and control. However, the eradication of bTB from wildlife is highly unlikely to occur in the near future and thus the threat of re-infection of domestic livestock from a wildlife source will remain. Numerous options exist to contain, control and eradicate bTB, but many are considered unacceptable on economic, moral and environmental grounds. Although culling wildlife is considered morally unacceptable by some it remains a viable option in certain conservation areas. Potential management initiatives must address whether there are multiple wildlife maintenance hosts that could negate single-species eradication schemes, and what potential impact of bTB or its control will have on wildlife [24]. Theoretical multi-species host–disease models suggest that serious consideration should be given to more radical options for management, such as proactive reduction in populations of principal host species like buffalo, rather than the removal of infected animals alone. The pathogenicity of bTB in each of the different host species, and the inter- and intra-specific transmission rate among different species need to be identified. This information can be used to develop more specific models to understand the spatio-temporal patterns of TB in African wildlife and help to guide management decisions. Until an effective vaccine is developed to ensure the disease-free and long-term viability of both domestic and wild animals, bTB will continue to play a major environmental and socio-economic role in the African ecosystems.
ACKNOWLEDGEMENTS
We are grateful to Mike Gray for assistance with the initial research. We also thank Tony Leiman, Carl-Erik Schultz, Charles Perrings and Anita Michel for useful comments and discussion at various stages of this research.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Clifton-Hadley R, Wilesmith J, Griffin F, de Lisle G.An epidemiological outlook on bovine tuberculosis in the developed worldTuberculosis in Wildlife and Domestic Animals 1995 [Google Scholar]
- 2.de Lisle GW et al. Tuberculosis in free-ranging wildlife: detection, diagnosis and management. Revue Scientifique et Technique de l'Office International des Epizooties. 2002;21:317–334. doi: 10.20506/rst.21.2.1339. [DOI] [PubMed] [Google Scholar]
- 3.Keet DF. 2000. http://btb.animaldisease.org http://btb.animaldisease.org
- 4.Office International des Epizooties. 2005. http://www.oie.int/hs2/sit_mald_freq_pl.asp?c_cont=1&c_mald=35 http://www.oie.int/hs2/sit_mald_freq_pl.asp?c_cont=1&c_mald=35
- 5.Office International des Epizooties. 2005. http://www.oie.int/hs2/sit_mald_cont.asp?c_mald=35&c_cont=1 http://www.oie.int/hs2/sit_mald_cont.asp?c_mald=35&c_cont=1
- 6.Cosivi O et al. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerging Infectious Diseases. 1998;4:59–70. doi: 10.3201/eid0401.980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michel AL. Implications of tuberculosis in African wildlife and livestock. Domestic Animal/Wildlife Interface: Issue for Disease Control, Conservation, Sustainable Food Production, and Emerging Diseases. 2002;969:251–255. doi: 10.1111/j.1749-6632.2002.tb04387.x. [DOI] [PubMed] [Google Scholar]
- 8.Cosivi O et al. Epidemiology of Mycobacterium bovis infection in animals and humans, with particular reference to Africa. Revue Scientifique et Technique de l'Office International des Epizooties. 1995;14:733–746. doi: 10.20506/rst.14.3.875. [DOI] [PubMed] [Google Scholar]
- 9.UNEP. 2002. http://africa.unep.net/Biodiversity_Degrad/index.asp http://africa.unep.net/Biodiversity_Degrad/index.asp
- 10.Anderson RM, May RM. Infectious Diseases of Humans. Oxford: Oxford University Press; 1991. [Google Scholar]
- 11.Morgan ER et al. Ruminating on complexity: macroparasites of wildlife and livestock. Trends in Ecology and Evolution. 2004;19:181–187. doi: 10.1016/j.tree.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Holt RD, Pickering J. Infectious-disease and species coexistence – a model of Lotka-Volterra form. American Naturalist. 1985;126:196–211. [Google Scholar]
- 13.Hochberg ME, Holt RD. The coexistence of competing parasites 1. The role of cross-species infection. American Naturalist. 1990;136:517–541. [Google Scholar]
- 14.Bowers RG, Begon M. A host-host pathogen model with free-living infective stages, applicable to microbial pest-control. Journal of Theoretical Biology. 1991;148:305–329. doi: 10.1016/s0022-5193(05)80240-1. [DOI] [PubMed] [Google Scholar]
- 15.Holt RD et al. Parasite establishment in host communities. Ecology Letters. 2003;6:837–842. [Google Scholar]
- 16.Anderson RM, May RM.Population ecology of infectious disease agentsTheoretical Ecology Sinauer Associates; Sunderland, MA: 1981318–355. [Google Scholar]
- 17.Begon M et al. Rodents, cowpox virus and islands: densities, numbers and thresholds. Journal of Animal Ecology. 2003;72:343–355. [Google Scholar]
- 18.Bowers RG, Turner J. Community structure and the interplay between interspecific infection and competition. Journal of Theoretical Biology. 1997;187:95–109. doi: 10.1006/jtbi.1997.0418. [DOI] [PubMed] [Google Scholar]
- 19.McCallum H, Dobson A. Detecting disease and parasite threats to endangered species and ecosystems. Trends in Ecology and Evolution. 1995;10:190–194. doi: 10.1016/s0169-5347(00)89050-3. [DOI] [PubMed] [Google Scholar]
- 20.Myers JA, Steele JH. Bovine Tuberculosis Control in Man and Animals. St Louis, MO: Warren H. Green Inc.; 1969. [Google Scholar]
- 21.Webb GB. Clio Medica: tuberculosis. New York: 1936. [Google Scholar]
- 22.Paine R, Martinaglia G. Tuberculosis in wild buck living under natural conditions. Journal of Comparative Pathology and Therapeutics. 1929;XL11(1) [Google Scholar]
- 23.de Vos V, McCully RM, Van Niekerk CAWJ. Mycobacteriosis in the Kruger National Park. Koedoe. 1977;20:1–9. [Google Scholar]
- 24.Bengis RG, Keet DF. 2000. http://btb.animaldisease.org http://btb.animaldisease.org
- 25.South African National Parks. http://www.parks-sa.co.za/frames.asp?mainurl=sitemap/sitemap.html. 2000. http://www.parks-sa.co.za/frames.asp?mainurl=sitemap/sitemap.html
- 26.Annual Reports of the State Veterinarian. 1958.
- 27.Krebs J Bovine Tuberculosis in Cattle and Badgers. London: Ministry of Agriculture, Fisheries and Food (MAFF) Publications; 1997. p. 191. [Google Scholar]
- 28.World Health Organization. Zoonotic tuberculosis (Mycobacterium bovis): Memorandum from a WHO meeting (with the participation of FAO) Bulletin of the World Health Organization. 1994;72:851–857. [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. 2004.
- 30.Raviglione MC, Snider DE, Kochi A. Global epidemiology of tuberculosis. Journal of the American Medical Association. 1995;273:220–226. [PubMed] [Google Scholar]
- 31.O'Reilly LM, Daborn CJ. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tubercle and Lung Disease. 1995;76:1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 32.Keet DF, Kriek NP, Michel A.Proceedings of the 3rd International ConferenceCambridge, UK: 2000 [Google Scholar]
- 33.Thoen CO, Bloom BR.Tuberculosis in wild and domestic mammalsTuberculosis, Pathogenesis, Protection and Control Washington: ASM Press; 1994 [Google Scholar]
- 34.Menzies FD, Neill SD. Cattle-to-cattle transmission of bovine tuberculosis. Veterinary Journal. 2000;160:92–106. doi: 10.1053/tvjl.2000.0482. [DOI] [PubMed] [Google Scholar]
- 35.Daborn C, Griffin F, de Lisle G.TB in humans and domestic animals in the developing worldTuberculosis in Wildlife and Domestic Animals 1995 [Google Scholar]
- 36.Ayele WY et al. Bovine tuberculosis: an old disease but a new threat to Africa. International Journal of Tuberculosis and Lung Disease. 2004;8:924–937. [PubMed] [Google Scholar]
- 37.Schenken JR, Burns EL, Anderson WAD.Gastrointestinal tractPathology 4th ednSt. Louis: C. V. Mosby Co.1961 [Google Scholar]
- 38.Caron A, Cross PC, Du Toit JT. Ecological implications of bovine tuberculosis in African buffalo herds. Ecological Applications. 2003;13:1338–1345. [Google Scholar]
- 39.World Health Organization. 2002.
- 40.Tanner M, Michel AL. Investigation of the viability of M. bovis under different environmental conditions in the Kruger National Park. Onderstepoort Journal of Veterinary Research. 1999;66:185–190. [PubMed] [Google Scholar]
- 41.Bengis RG et al. An outbreak of bovine tuberculosis in a free-living African buffalo (Syncerus caffer-Sparrman) population in the Kruger National Park – a preliminary report. Onderstepoort Journal of Veterinary Research. 1996;63:15–18. [PubMed] [Google Scholar]
- 42.Rodwell TC.Syncerus cafferPh.D. ThesisUniversity of California; Davis: 1999 [Google Scholar]
- 43.Rodwell TC et al. Prevalence of bovine tuberculosis in African buffalo at Kruger National Park. Journal of Wildlife Diseases. 2000;37:258–264. doi: 10.7589/0090-3558-37.2.258. [DOI] [PubMed] [Google Scholar]
- 44.Rodwell TC, Whyte IJ, Boyce WM. Evaluation of population effects of bovine tuberculosis in free-ranging African buffalo (Syncerus caffer) Journal of Mammalogy. 2001;82:231–238. [Google Scholar]
- 45.Morris RS, Pfeiffer DU, Jackson R. The epidemiology of Mycobacterium bovis infections. Veterinary Microbiology. 1994;40:153–177. doi: 10.1016/0378-1135(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 46.Keet DF et al. Advanced tuberculosis in an African buffalo (Syncernus caffer) Journal of the South African Veterinary Association. 1994;65:79–83. [PubMed] [Google Scholar]
- 47.Bengis RG et al. Tuberculosis, caused by Mycobacterium bovis, in a kudu (Tragelaphus strepsiceros) from a commercial game farm in the Malelane area of the Mpumalanga Province, South Africa. Onderstepoort Journal of Veterinary Research. 2001;68:239–241. [PubMed] [Google Scholar]
- 48.Michel AL, Maré L, Salmon MD, Morley PS, Ruch-Gallie R.Mycobacterium bovisProceedings of the 9th Symposium of the International Society for Veterinary Epidemiology and EconomicsBreckenridge, Colorado: 20001205–1207. [Google Scholar]
- 49.Keet DF et al. The rise and fall of tuberculosis in a free-ranging chacma baboon troop in the Kruger National Park. Onderstepoort Journal of Veterinary Research. 2000;67:115–122. [PubMed] [Google Scholar]
- 50.Bengis RG, Grobler DG.Proceedings of the North America Veterinary ConferenceOrlando, FL: 20001032–1033. [Google Scholar]
- 51.Buddle BM et al. Intraduodenal vaccination of brushtail possums with bacilli Calmette-Guerin enhances immune responses and protection against Mycobacterium bovis infection. International Journal of Tuberculosis Lung Disease. 1991;1:377–383. [PubMed] [Google Scholar]


