SUMMARY
The objective of this study is to evaluate how increasing MMR infant vaccination coverage in recent years has modified the epidemiology of rubella in Italy. A cross-sectional population-based seroprevalence study of rubella antibodies was conducted on 3094 sera, in 2004, and results were compared with data obtained by the same method in 1996. The overall proportion of rubella-seropositive individuals was found to be significantly higher in 2004 with respect to 1996 (84·6% vs. 77·4%). However, an increase in seropositivity was observed only in the 1–19 years age groups. Recent increases in childhood MMR vaccination coverage, therefore, have not had an impact on seroprevalence in women of childbearing age, over 5% of whom remain susceptible to rubella. Preconception screening and postpartum vaccination of susceptible women are fundamental if the WHO target of less than one case of congenital rubella syndrome per 100 000 live births is to be attained.
INTRODUCTION
Although rubella is usually a mild exanthematous viral infection in children and young adults, it assumes greater importance in pregnancy because of its frequent transmission to the foetus with disastrous effects [1].
In Italy, rubella immunization has been recommended since 1972, when several attenuated rubella vaccines became available. Initially, a selective vaccination policy targeted at adolescent females was recommended by the Ministry of Health and was adopted by some regions. Starting from 1990, with the introduction of combined measles, mumps and rubella (MMR) vaccine, immunization was recommended for all children within the second year of life, but was offered free of charge only by some regions, mainly located in the North and Centre of Italy. Vaccination coverage therefore remained very low for many years, and wide differences among regions, with lower values in southern Italy, were observed [2]. In fact, according to an Expanded Programme on Immunization (EPI) cluster sampling survey conducted in 1998 among children aged 12–24 months, only ∼56% of children were immunized against MMR, with a range of 25–77% among regions [3].
This vaccination coverage was not sufficient to interrupt virus circulation and it is well known that sub-optimal coverage levels lead to a prolonged inter-epidemic interval and a shift of the disease incidence towards older age groups, including women of childbearing age. Since 1999, therefore, various actions have been undertaken in order to improve MMR vaccination coverage rates in children by 24 months of age [4].
In 1999, MMR vaccination was included in the national vaccination schedule with the recommendation of administering the first dose to children aged between 12 and 15 months. Evaluation of MMR vaccination status was also recommended at ages 5–6 and 11–12 years, when the national schedule calls for administration of booster doses of other vaccines (e.g. diphtheria, and tetanus). At these ages, the MMR catch-up of previously unvaccinated children was therefore to be performed, as well as the administration of the second dose to children who had already received one dose [5].
In 2002, MMR vaccine was included among the vaccinations that each region must provide free of charge to all children [6]. This decision has played an important role in improving MMR vaccination coverage, especially in the South where income levels are substantially lower than in the northern and central parts of Italy. In fact, in the 1998 EPI survey, one of the main reasons reported by parents for not vaccinating their children, in certain regions, was related to the fact that the vaccine was not provided free of charge [2].
A second cluster sampling survey conducted in 2003, by using the same method of the previous survey, revealed a vaccination coverage rate of 77%, in the same age group (regional range 55–90%) [2].
Finally, in 2003, Italy adhered to the WHO European goal of achieving elimination of congenital rubella by 2010 [7], and a National Plan for the elimination of measles and congenital rubella was approved [8]. The National Plan is aimed at eliminating measles and reducing and maintaining the incidence of congenital rubella syndrome (CRS) below 1/100 000 live births per year by 2007, and its operational targets are to reach at least 95% vaccination coverage in children in their second year of life and to perform catch-up vaccinations of older children and adolescents. Additionally, in order to prevent CRS, the National Plan recommends vaccinating susceptible women of childbearing age.
Routine coverage data provided by regions to the Italian Ministry of Health showed that national vaccination coverage rate by 24 months of age reached 85·5% in 2004, and 88·3% in 2005 (http://www.ministerosalute.it/promozione/malattie/documenti/CopVaccPED2005.pdf). Catch-up vaccination of school-age children was performed in 2004–2005, and preliminary data, as of 31 December 2005, show that the national MMR coverage rate in the 6–10 years age group has reached 83% [9].
The objective of this study is to describe how increasing infant vaccination coverage levels obtained in recent years has modified the epidemiology of rubella in the Italian population, by comparing 2004 seroprevalence data with data collected by the same method 8 years previously [10].
METHODS
A national cross-sectional population-based seroprevalence study of rubella antibodies was conducted.
Serosurvey
Assuming an overall rubella antibody prevalence of 70%, a sample size of 2017 sera was required to achieve 95% confidence intervals (CI), with a precision of the estimate of 2%. Serum specimens were obtained by using leftover serum from specimens submitted by the general population to laboratories for diagnostic purposes. One hospital-based reference laboratory was contacted in each of the 20 Italian regions and each laboratory was requested to send about 200 sera stratified by age in equal numbers for males and females. Eighteen out of 20 Italian regions provided serum samples.
A total of 3094 serum samples were collected from January 2003 to October 2004. These included 1332 samples from regions in northern Italy (Piedmont, Lombardy, Autonomous Province of Trento, Autonomous Province of Bolzano, Veneto, Friuli Venezia Giulia, Liguria, and Emilia Romagna), 462 samples from regions in central Italy (Tuscany, Umbria, Marches, and Lazio), and 1300 samples from the southern Italy (Abruzzo, Molise, Apulia, Calabria, Sicily, and Sardinia).
Sera were stratified by age into the following age groups: 0, 1, 2–4, 5–9, 10–14, 15–19, 20–39, ⩾40. For the age group 0, 75 samples were collected, for the age groups between 1 and 19 years ∼100 sera for each 1-year interval was collected, for a total of 1711 sera, while in the age groups 20–39 and ⩾40 years, 751 and 557 sera respectively were collected.
Samples were collected anonymously and only age, sex and date of sampling were recorded. Sera from individuals known to be affected by an immunodepressive condition or by an acute infection or to have recently undergone a blood transfusion were excluded. No other information about health status or symptoms was recorded at the time of blood sampling. All individuals who provided serum samples gave verbal informed consent; consent for minors was provided by parents. Serum samples were stored at −20°C until tested.
Detection of antibodies
Serological testing was performed at the University of Lecce (Laboratory of Hygiene, Department of Biological and Environmental Sciences and Technologies, Faculty of Sciences). The commercial enzyme linked immunosorbent assay (ELISA) (Enzygnost anti-Rubella-virus/IgG, Dade Behring, Germany) was used to detect and quantify human IgG antibodies against rubella virus in serum.
The following criteria apply for the qualitative evaluation: negative (ΔA<0·100, cut-off), positive (ΔA>0·200), equivocal (0·100⩽ΔA⩽0·200). Samples with IgG antibodies activities above the cut-off were evaluated quantitatively with the aid of the α method using the following formula:
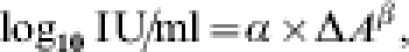 |
where α and β are lot-dependent constants.
Sensitivity and specificity of the method used are, according to the manufacturer, respectively 100% and 98·5%. The results are expressed in IU/ml.
Statistical analysis
Equivocal sera were excluded from the analysis, in order to avoid under- or overestimation of prevalence. Data were summarized as frequencies and positive antibody titres presented as geometric means along with their respective 95% CIs. Differences among percentages of seropositive subjects were assessed by the χ2 test, while differences among geometric titres were assessed by Student's t test of logarithmically transformed values.
Data were also analysed by gender and geographical area, and were then compared with results obtained from a seroprevalence study conducted with the same test method and cut-offs in 1996 [10].
A multivariate analysis was conducted in order to evaluate the role of variables associated with seroprevalence to rubella. Age group, gender and geographical area were included in the logistic regression model.
Analysis by birth cohort and vaccination strategy was also conducted. In detail, the following birth cohorts were considered:
-
●
2004–2003. In 2004, these children were 0–1 years old, being either too young to be vaccinated, or still in the target age for the first MMR dose.
-
●
2002–1998, corresponding to children aged 2–6 years in 2004. From the 1998 birth cohort onwards, the new national vaccination schedule (launched in 1999) was applied.
-
●
1997–1991, corresponding to children aged 7–13 years in 2004. These birth cohorts were all born in a period when the MMR combined vaccine was available. In addition, they were also targeted by the catch-up activity foreseen by the national vaccination schedule.
-
●
1990–1974, corresponding to individuals aged 14–30 years, respectively. These birth cohorts were born before MMR was commercially available, and females were the target of selective rubella immunization.
All statistical analyses were carried out using stata software version 8.2 (Stata Corporation, College Station, TX, USA).
RESULTS
A total of 3094 serum samples were analysed. Overall, after excluding 35 equivocal sera (∼1% of the sample), 84·6% (95% CI 83·2–85·8) of sera were found to be positive for rubella antibodies. Seroprevalence was found to decrease from 51% to 40% between the first and the second year of life, due to loss of maternal antibodies, while a continuous increase was observed after the second year of life. The percentage of subjects positive for rubella antibodies was ∼82% in each of the 2–4, 5–9 and 10–14 years age groups, 85% in the 15–19 years age group and 90% and 95%, respectively, in the 20–39 and ⩾40 years age groups (Table 1). Using the 0 years age group as reference, with multivariate analysis age was significantly associated with seroprevalence from the 2–4 years group onwards. For the 2–4, 5–9, and 10–14 years age groups, the adjusted odds ratios (aOR) were 4·3, 4·1, and 4·1, respectively (P<0·001). In adolescents aged 15–19 years, the aOR was 5·2 (P<0·001), while in adults aged 20–39 years it was 8·0 (P<0·001). The highest aOR was found in the ⩾40 years age group (18·6, P<0·001).
Table 1.
Rubella seroprevalence in Italy, by age group and gender in 2004
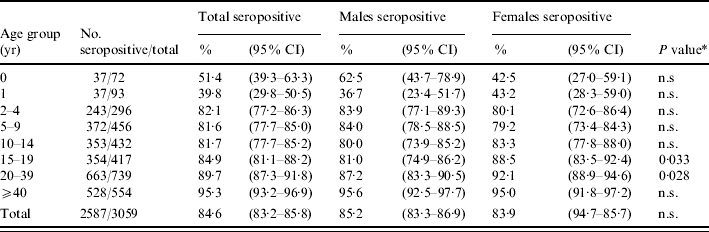
n.s., Non-significant.
Males vs. females.
Analysis of data by age group and gender
In the 15–19 and 20–39 years age groups, seroprevalence was found to be significantly higher among females than among males (P<0·05) (Table 1). The geometric mean titres (GMTs) for rubella reached the highest values at 1 year of age (GMT 123, 95% CI 85·9–175·1) and in the 20–39 years age group (GMT 118, 95% CI 109·9–126·1), and had the lowest values in the 5–9 years (GMT 77, 95% CI 70·0–84·2) and ⩾40 years age groups (GMT 84, 95% CI 76·8–91·4). The trend is similar in males and females, with statistically significant differences observed only in the 5–9 years age group where females showed a higher titre (GMT 91 vs. 65), and in the 20–39 years age group, where males showed a higher titre (GMT 130 vs. 108).
Nevertheless, at multivariate analysis, gender was not significantly associated with seroprevalence.
Analysis of data by geographical area (northern, central and southern Italy) and age group
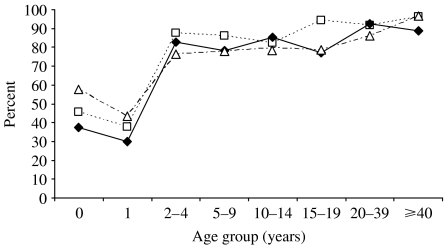
No statistically significant differences in seroprevalence were observed between the three geographical areas, in all age groups up to 14 years of age (Fig. 1). In the 15–19 years age group, the proportion of immune individuals was significantly lower in central and southern Italy compared to northern Italy (P<0·001), while in the 20–39 years age group it was significantly lower in southern Italy with respect to northern and central Italy (P=0·003). In the ⩾40 years age group seroprevalence was lower in central Italy with respect to northern and southern Italy (P=0·010). In addition, when considering only women of childbearing age and more specifically, women in the 20–39 years age group, the proportion of immune individuals was found to be significantly higher in northern Italy (97·1% in the north, vs. 90·2% in the centre and 86·9% in the south, P=0·003) with respect to central and southern Italy. At multivariate analysis, the aOR of being seropositive to rubella was 1·5 in northern Italy compared to southern Italy (P<0·01) while no difference was found between central and southern Italy.
Fig. 1.
Rubella seroprevalence in Italy, by age group and geographical area in 2004. —◆—, Centre; - - -□- - -, North; – - –△– - –, South.
Comparison of seroprevalence data from 1996 and 2004
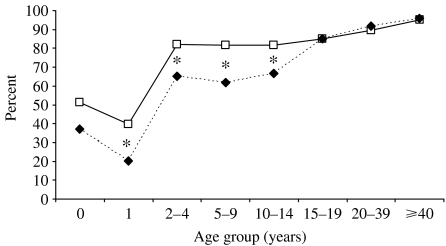
The proportion of seropositive subjects was found to be significantly higher in 2004 only in the age groups from 1 up to 15–19 years (Fig. 2). No changes were detected in the older age groups, and the same trend is evident for both males and females.
Fig. 2.
Comparison between rubella seroprevalence by age group, Italy 1996 (- - -◆- - -) and 2004 (—□—) (* P<0·05).
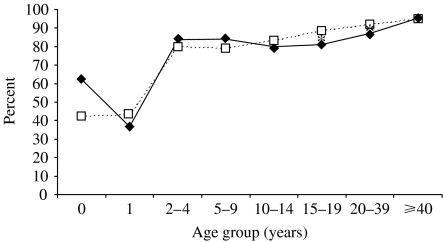
A comparison of data from the two surveys, by age group and gender, shows that in 1996 there were statistically significant differences in seroprevalence between males and females in the 10–19 years age group, while in 2004 the same statistically significant differences are observed in the 15–19 and 20–39 years age groups (Fig. 3). Finally, rubella seroprevalence in females of childbearing age (15–44 years) did not significantly differ in 2004 (91·4%), with respect to 1996 (92·0%).
Fig. 3.
Rubella seroprevalence in Italy, by age group and gender in 2004. —◆—, Male; - - -□- - -, female (* P<0·05).
Analysis by birth cohort also shows that in each cohort in which a comparison was possible, seroprevalence significantly increased from 1996 to 2004 (Table 2). Analysis of data by gender shows that females born in years when selective vaccination was in place had a significantly higher seroprevalence compared to males (79·3% vs. 68·4%, P value<0·0001) in 1996, and despite an increase in seroprevalence rates in these cohorts in both genders, in 2004, the proportion of females immune to rubella was still significantly higher than observed in males (89·1% vs. 84·3%, P=0·03). No significant differences by gender were observed in all the other birth cohorts, in both surveys.
Table 2.
Seroprevalence in 1996 and 2004, by birth cohort and vaccination strategy
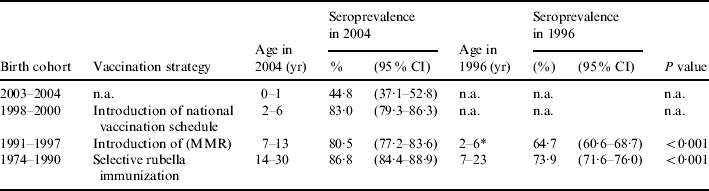
n.a., Not applicable.
Children who were 0–1 year old in 1996 have been excluded from this analysis, since they were too young to be vaccinated.
DISCUSSION
Serum specimens submitted to diagnostic laboratories and used in the present study may not be entirely representative of the Italian population, since they may under-represent the immigrant population, which has minor access to health services, and over-represent people with health problems. However, in spite of these possible biases, the size of the sample is large enough to offer a substantial contribution in better defining the epidemiological picture of rubella infection in Italy.
In this study, the significantly higher proportion of children aged 1–14 years found to be immune to rubella in 2004 with respect to 1996 (82% vs. 62%) is consistent with vaccination coverage estimates and can therefore be related to the increased coverage levels which have taken place in recent years. This is also confirmed by analysis by birth cohort and vaccination strategy, which shows that seroprevalence increased significantly in children born in 1991–1997, who were 7–13 years old in 2004. In addition, a significant seroprevalence increase in the 1984–1990 birth cohorts was also noted, probably due either to catch-up activities, or to natural infection in older unvaccinated individuals.
The significant differences in seroprevalence rates observed in northern, central and southern Italy in 2004 are also attributable to varying vaccination coverage levels achieved in the three areas. In fact, as demonstrated by the two EPI cluster sampling surveys conducted respectively in 1998 and in 2003, MMR coverage rates have always been significantly higher in the northern and central regions with respect to the southern region [2, 3].
Regarding gender differences, it has to be noted that the higher seroprevalence rates observed in females with respect to males in the 10–14 and 15–19 years age groups, in 1996, and attributed to the selective vaccination strategy [11], are now evident in the 15–19 and 20–39 years age groups, reflecting a cohort effect. The role of selective vaccination strategy is highlighted by the birth-cohort analysis, which shows that the proportion of women born in 1973–1982 and immune to rubella is significantly higher than that observed in males.
The proportion of women of child-bearing age susceptible to rubella, estimated at ∼10% in 2004, is still higher than that observed in 1996 in many European countries [11, 12], and comparable to that reported in the late 1990s in Greece and Israel [13, 14].
In order to achieve elimination of congenital rubella the percentage of women of childbearing age susceptible to rubella should be <5%. For this reason, the National Plan includes evaluation of the susceptibility status of women of childbearing age at every opportunity, and their vaccination if necessary, as well as postpartum vaccination of all women found to be susceptible in pregnancy. Nevertheless, these activities have been implemented at the national level only in early 2006, after the conduction of the present study. This may explain why no differences were observed in seroprevalence rates in women in 2004 with respect to 1996. In fact, these percentages remain very similar (11% in the 15–19 years age group and 8% in the 20–39 years age group) in both years, and exceed the level of susceptibility required to obtain a decreased incidence of CRS.
The study has also shown 18% of children aged 2–14 years and 15% of adolescents aged 15–19 years to be seronegative, meaning that the potential for rubella epidemics among this population still exists and the risk of cases occurring in adult women, and of congenital rubella infections, therefore remains high.
The incidence of rubella has decreased in Italy in the last 8 years from 46/100 000 cases in 1996 to 0·8/100 000 in 2004, with the last epidemic occurring in 2002 with an incidence of 10·5/100 000. Incidence data for rubella should be interpreted cautiously; however, incidence is probably underestimated, because of underreporting and because the infection, characterized by a high percentage of asymptomatic cases (up to 50% of acute cases are estimated to be sub-clinical) is often not diagnosed (underdiagnosis). Nevertheless, it is probable that the increase in vaccination coverage levels achieved in children in recent years has reduced the circulation of rubella virus. Cases of rubella in women of childbearing age, however, continue to occur. In fact, even though only 139 cases of rubella were reported to the statutory notification system in 2005, 12 cases occurred in women aged between 15 and 24 years and four in women aged 25–64 years [15].
In Italy, rubella has been a notifiable disease since 1970, while CRS was statutorily notifiable only for a short period, from 1987 to 1991. For the years 1992–2005, no national CRS data are available, but there is evidence that cases did continue to occur [16, 17]. To assess the impact of the National Plan a new surveillance system was set up in January 2005 and CRS and rubella in pregnant women have again become statutorily notifiable [18]. In the same year, two confirmed infections in pregnancy, both of which occurred in immigrant women, and one congenital infection, were detected [15].
The problem of higher susceptibility of minority groups such as the immigrant population has been well documented [19, 20] and, therefore, deserves special attention. In fact, in areas with high vaccination coverage, congenital rubella cases mainly occur in this group. It is, therefore, necessary to conduct further studies to evaluate the susceptibility of immigrant women, who, in addition, have a higher fertility rate than Italian women [21].
CONCLUSION
This study highlights that efforts conducted in recent years to improve MMR vaccination coverage in children, have had an impact on seroprevalence of rubella only in the age groups between 1 and 14 years. In the older age groups, seropositivity is substantially unvaried and mainly a reflection of naturally acquired infection. The strategies provided in the National Plan for measles and congenital rubella elimination [8] have led to good results in children and must be pursued until at least 95% vaccination coverage is achieved and maintained. It must not be forgotten, however, that a large proportion of women of childbearing age are still unprotected from rubella infection and that preconception screening and postpartum vaccination of susceptible women are fundamental if the WHO target, which has been updated in 2005 with the aims of preventing congenital rubella and eliminating rubella [22], is to be attained. In particular, an appropriate immunization strategy should be considered to reach immigrant women who belong to one of the most vulnerable and frequently neglected groups.
APPENDIX. The Serological Study Group
D. Bassetti, S. Boccalini, P. Bonanni, P. Caciagli, A. Gamper, A.M. Campa, W. Caraccio, A. Cavallaro, E. Ciamarra, L. Clerico, L. Cosentino, E. de Simone, A. Favero, A. Focà, L. Genna, A. Giammanco, A. Giancotti, A. Goglio, S. Grandesso, G. Icardi, C. Lolli, D. Marchetti, P. Martelli, L. Mucignat, D. Palladino, C. Passerini, V. Perani, E. Piscione, F. Rizza, C. Rollo, L. Simula, R. Sforza, D. Taglialatela, M. Tronci, D. Villalta.
ACKNOWLEDGEMENTS
This work was partly supported by a grant from GlaxoSmithKline SpA, Verona, Italy.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Plotkin SA, Reef S, Plotkin SA, Orenstein WA.Rubella vaccineVaccines 4th edn.Saunders; 2004707 [Google Scholar]
- 2.Ciofi degli Atti ML et al. Do Changes in policy affect vaccine coverage levels? Results of a national study to evaluate childhood vaccination coverage and reasons for missed vaccination in Italy. Vaccine. 2004;22:4351–4357. doi: 10.1016/j.vaccine.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Salmaso S et al. Epidemiology of measles, mumps and rubella in Italy: estimates by simultaneous EPI cluster surveys of regions. Bulletin of the World Health Organization. 1999;77:843–851. [PMC free article] [PubMed] [Google Scholar]
- 4.Panagiotopoulos T, Antoniadou I, Valassi-Adam E. Increase in congenital rubella occurrence after immunisation in Greece: retrospective survey and systematic review. British Medical Journal. 1999;319:1462–1467. doi: 10.1136/bmj.319.7223.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health. 1999.
- 6.Ministerial Decree 29 November 2001 Official BulletinGazzetta Ufficiale2002
- 7.European Health for All Series. 1999.
- 8.State Regional Conference. 2003. http://governo.it/backoffice/allegati/20894-1712.pdf http://governo.it/backoffice/allegati/20894-1712.pdf
- 9.Ciofi degli Atti ML et al. State of progression of the National Measles and Congenital Rubella Elimination Plan. Bollettino Epidemiologico Nazionale. 2006;19(4) [Google Scholar]
- 10.Gabutti G et al. Epidemiology of measles, mumps and rubella in Italy. Epidemiology and Infection. 2002;129:543–550. doi: 10.1017/s0950268802007677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pebody RG et al. The seroepidemiology of rubella in Western Europe. Epidemiology and Infection. 2000;125:347–357. doi: 10.1017/s0950268899004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amela C, Pachon I, de Ory F. Evaluation of the measles, mumps, and rubella immunisation programme in Spain by using a sero-epidemiological survey. European Journal of Epidemiology. 2003;18:71–79. doi: 10.1023/a:1022567811765. [DOI] [PubMed] [Google Scholar]
- 13.Gioula G et al. Seroepidemiology of rubella in northern Greece. European Journal of Clinical Microbiology and Infectious Diseases. 2004;23:631–633. doi: 10.1007/s10096-004-1172-y. [DOI] [PubMed] [Google Scholar]
- 14.Huerta M et al. Declining seroprevalence of rubella antibodies among young Israeli adults: a 12-year-comparison. Preventive Medicine. 2004;39:1223–1226. doi: 10.1016/j.ypmed.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Ciofi degli Atti ML et al. First cases of rubella infection during pregnancy detected by new reporting system in Italy. Eurosurveillance. 2006;11:E060323.5. doi: 10.2807/esw.11.12.02930-en. [DOI] [PubMed] [Google Scholar]
- 16.Buffolano W et al. Surveillance of congenital rubella: the experience of the Campania Perinatal Infections Registry. Bollettino Epidemiologico Nazionale. 2003;16(5) [Google Scholar]
- 17.Revello MG. One year after the 2002 rubella outbreak. Bollettino Epidemiologico Nazionale. 2003;16(5) [Google Scholar]
- 18.Ministry of Health 2004Official BulletinGazzetta Ufficiale
- 19.Lemos C et al. New features of rubella in Spain: the evidence of an outbreak. Eurosurveillance. 2004;9:9–11. [PubMed] [Google Scholar]
- 20.Tookey P. Rubella in England, Scotland and Wales. Eurosurveillance. 2004;9:21–23. doi: 10.2807/esm.09.04.00464-en. [DOI] [PubMed] [Google Scholar]
- 21.Terra Abrami V. 2005.
- 22.WHO Regional Office for Europe. WHO; 2005. [Google Scholar]