SUMMARY
On 3 February 2004, the Vermont Department of Health received reports of acute gastroenteritis in persons who had recently visited a swimming facility. A retrospective cohort study was conducted among persons attending the facility between 30 January and 2 February. Fifty-three of 189 (28%) persons interviewed developed vomiting or diarrhoea within 72 h after visiting the facility. Five specimens tested positive for norovirus and three specimen sequences were identical. Entering the smaller of the two pools at the facility was significantly associated with illness (RR 5·67, 95% CI 1·5–22·0, P=0·012). The investigation identified several maintenance system failures: chlorine equipment failure, poorly trained operators, inadequate maintenance checks, failure to alert management, and insufficient record keeping. This study demonstrates the vulnerability of recreational water to norovirus contamination, even in the absence of any obvious vomiting or faecal accident. Our findings also suggest that norovirus is not as resistant to chlorine as previously reported in experimental studies. Appropriate regulations and enforcement, with adequate staff training, are necessary to ensure recreational water safety.
INTRODUCTION
Noroviruses cause about 23 million cases of acute gastroenteritis in the United States each year [1]. Illness is often characterized by abrupt onset of vomiting or diarrhoea and consequent dehydration may be severe enough to require hospitalization [2]. The acute phase of illness lasts 12–72 h and illness resolves without specific treatment. Since a wide diversity of norovirus strains exist and immunity to infection appears to wane over time, norovirus illness affects persons of all ages [3].
Noroviruses are transmitted by many routes, including contaminated food, person-to-person contact, contaminated environmental surfaces, and airborne droplets of vomitus [3]. Contaminated drinking or recreational water has also been reported as a source of infection [4–7]. Outbreaks of norovirus illness associated with swimming pools are infrequently reported [7], even though there are at least 360 million visits to recreational water venues each year in the United States [8]. Guidelines on pool water treatment aim to prevent transmission of bacterial and parasitic agents that have caused several pool-associated outbreaks such as E. coli O157 and Cryptosporidium [7]. Unlike many bacterial agents, laboratory confirmation of norovirus infection is not routinely available. Moreover, norovirus has a very low infectious dose, is a common cause of diarrhoeal illness [1, 2], and is reportedly resistant to levels of chlorination used in swimming pools [9]. For these reasons swimming-pool transmission of norovirus may be common but substantially underreported. We investigated a swimming pool-associated outbreak of norovirus disease in order to elucidate the source of infection, the risk factors for transmission, and to recommend strategies for control and prevention.
METHODS
On 3 February 2004, the Vermont Department of Health (VDH) was notified of several persons who developed acute gastroenteritis after visiting a private indoor swimming facility over the previous weekend (31 January to 1 February 2004). Reported symptoms were acute onset vomiting, diarrhoea, and nausea and generally occurred within 12–36 h of attending the facility and lasted up to 2 days. Stool specimens collected from four persons who first reported illness tested positive for norovirus at the VDH Laboratory.
Epidemiological investigation
A retrospective cohort study was conducted of all persons who visited the swimming facility between the evening of Friday, 30 January and noon Monday, 2 February 2004. This included members, as well as non-members who had reserved the swimming facility for private group events. These events included: three classes of mother–baby swimming lessons; two groups of girls aged 6–12 years from a girls' organization; two birthday parties for children aged 5–10 years; and a preschool swimming class. The facility provided names of participants for the mother–baby swimming lessons and the preschool swimming class, but could only provide one contact name for each of the other non-member groups. Through these persons we obtained a complete roster of contact information of those individuals who attended. For two groups of the girls' organization, we were permitted only to select randomly the names of half the families on each roster.
A questionnaire was developed to gather information on times of attendance at the facility, occurrence of illness, and on possible exposures such as the specific pool that was used, locker room use, food and beverage consumption, whether the head was submerged in water, and whether water entered the swimmer's mouth. Questionnaires were administered by telephone between 12 and 22 February 2004. Visitors were also asked whether they had witnessed anyone vomiting or a faecal accident at the swimming facility, and to describe the chlorine smell, temperature, and appearance of each pool they used. Lastly, we also gathered information on illness in all household contacts. Verbal consent was obtained from all adult interviewees, and child assent with parental verbal consent for all interviewees younger than 18 years of age.
Primary cases were defined as persons who attended the swimming facility between Friday, 30 January and Monday, 2 February 2004, and who experienced vomiting or diarrhoea (⩾3 episodes in a 24-h period) within 72 h of their visit. Secondary cases were defined as household contacts of primary cases, and either with no exposure to the swimming facility during the study period and with illness onset ⩾24 h after the primary case, or with exposure to the swimming facility but with illness onset >72 h after the pool visit.
Statistical analyses
Risk ratios (RR) and 95% confidence intervals (CI) were calculated to evaluate associations between risk factors and illness. All analyses were conducted using stata 8.0 [10].
Environmental investigation
A comprehensive inspection and evaluation of the pools and pool maintenance systems was conducted by the VDH sanitarian on 3 February 2004 after the report of the initial cases. During this visit, several water samples were tested for temperature, chlorine, and pH from both pools and the hot tub. Pool filtration and chlorination systems were inspected to ensure their adequacy for servicing the pools, that they were operating at the appropriate settings, and that there were no signs of mechanical failure or maintenance needs. Equipment maintenance logs were examined and standard pool operating procedures were reviewed with the pool maintenance supervisor. All staff who had worked between 30 January and 2 February 2004 were interviewed about standard pool operating procedures, pool policies, knowledge of pool maintenance and equipment, and prior certification or training. Specifically, staff members were asked to recall the readings of chlorine and pH taken from each of the pools, and the quantity, time, and type of chemical solution they added to each pool over the weekend.
Laboratory methods
An additional six stool specimens were collected and tested for bacterial agents by routine culture and for norovirus by reverse transcription–polymerase chain reaction (RT–PCR) with degenerate primers targeted to a unique 172-bp region of the polymerase gene (region B), using previously described methods [11]. Amplified products from the four initial and additional positive stools were confirmed by nucleotide sequencing of samples in their entirety in both directions and detected sequences were analysed by use of the GCG suite of programs [12].
RESULTS
The swimming facility contained two indoor pools: the small activity pool (13 900 gallons) and a lap pool (111 000 gallons); a hot tub (1000 gallons); men's and women's locker rooms; and a birthday party room where food was served at private events.
Epidemiological findings
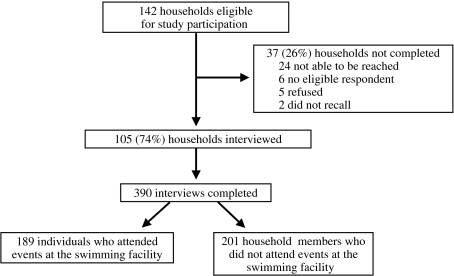
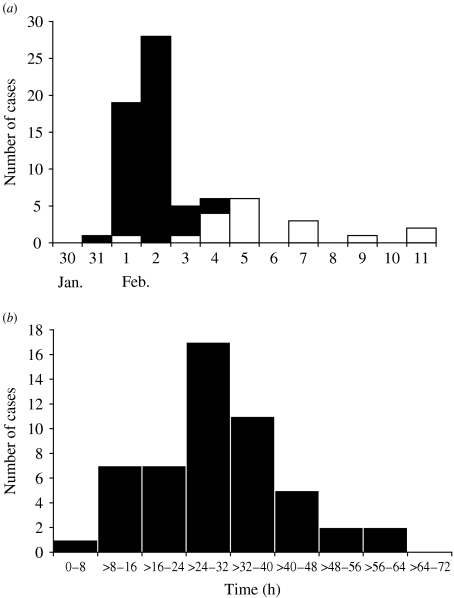
Of 142 households of registered members or private event attendees who attended the swimming facility during the study period interviews of at least one household member were completed for 105 (74%) (Fig. 1). The 105 completed household interviews included information on a total of 390 individuals. This consisted of 189 persons who had attended events at the swimming facility from 30 January to 2 February, and 201 of their household members who did not attend the swimming facility. The median age of swimming pool attendees was 13 years (range 5 months to 73 years), and most (68·3%) were female. Fifty-three of 189 (28%) attendees interviewed met the definition of a primary case. The median age of primary cases was 7 years (range 5 months to 61 years); 31 (58%) were female. In addition, 16 of 74 (21·6%) household contacts of a primary case fitted the definition of a secondary case. Most primary cases became ill on 1 or 2 February, and most secondary cases developed illness on 4 or 5 February (Fig. 2a). The median incubation time from exposure to the swimming facility to onset of symptoms for primary cases was 30 h (range 8–62 h) (Fig. 2b). Of the 53 primary cases, most had vomiting (89%), and almost half (49%) reported diarrhoea. Other common symptoms included nausea (77%), stomach cramps (68%), chills (58%), and a low-grade fever (53%). Seven cases (13%) visited a physician.
Fig. 1.
Flow chart of study participants.
Fig. 2.
(a) Cases of illness associated with attendance at a private swimming facility by date of onset, Vermont, 30 January to 11 February 2004. ■, Primary cases (n=53); □, secondary cases (n=16). (b) Incubation period between first exposure to the activity pool and onset of symptoms for primary cases of illness (n=53).
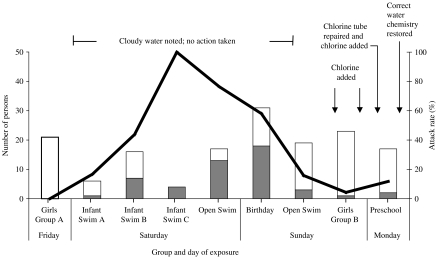
There were 52 primary cases in the 160 interviewees (attack rate 33%) who either swam or accompanied children that swam in the smaller activity pool, and these cases had no exposure to the larger lap pool. Only one of the 21 (4·8%) persons whose activities were restricted to the lap pool and none of the seven persons who only utilized other parts of the facility (e.g. hot tub, locker rooms) subsequently developed gastrointestinal illness. Since this strongly implicated either the activity pool or associated exposure as the source of infection, further analyses of risk factors were restricted to a cohort of 157 persons who were at the activity pool and for whom complete data were available. The attack rates in persons using the activity pool was 0% in the groups using the pool on 30 January and 100% in the participants of the mother–baby swim class mid-morning on 31 January. Parents and staff noted that the pool water had been turbid on the Saturday. The attack rate dropped precipitously in the second girls' group who used the pool after chlorine had been added to the pool on the afternoon of 1 February. Illnesses were still reported following exposures on 2 February after repair of the chlorinator tube. The pool chemistry was not returned to normal until the evening of 2 February, after which time no more illnesses were reported to the health club or VDH (Fig. 3).
Fig. 3.
Number of well (□) and ill (■) persons and attack rates ( ) of acute gastroenteritis among persons attending events at the programme pool, by exposure group and day; Vermont, 30 January to 2 February 2004. Six employees (3 ill, 3 well; 50% attack rate) not depicted because they had multiple exposures to the activity pool over the period of interest.
) of acute gastroenteritis among persons attending events at the programme pool, by exposure group and day; Vermont, 30 January to 2 February 2004. Six employees (3 ill, 3 well; 50% attack rate) not depicted because they had multiple exposures to the activity pool over the period of interest.
Only 2 of 30 (7%) persons who were at the activity pool but did not swim became ill, compared to 48 of 127 (38%) who went into the pool (RR 5·67, 95% CI 1·5–22·0, P=0·012, Table). Of persons who went into this pool, getting water in the mouth was significantly associated with an increased risk of illness (RR 2·49, 95% CI 1·3–4·7, P=0·004), but neither swimming (versus wading; RR 0·82, 95% CI 0·4–1·6, P=0·58) nor using the slide (RR 0·89, 95% CI 0·6–1·4, P=0·59) were independently associated with illness. Since getting water in the mouth was most commonly reported in children aged <10 years (61·5% of those that got water in their mouths), and these children swam mostly on Saturday when contamination was probably highest, this finding could be the result of confounding with time of swimming. When the analysis was restricted to the three groups (n=26) who used the activity pool on Saturday morning, the point estimate for relative risk still exceeded 1 (RR 4·29, 95% CI 0·7–29·4, P=0·12), although it did not reach statistical significance because of small sample size. Only 18 of the 53 (34%) primary cases ate food or drank in the private party room while visiting the swimming facility, and these activities were restricted to the birthday party group who attended events on Sunday morning and also had high rates of illness. Use of the locker rooms or showers was not significantly associated with risk of gastrointestinal illness (RR 2·0, 95% CI 0·3–11·7, P=0·44 and RR 0·8, 95% CI 0·5–1·2, P=0·25, respectively). There were no reported incidents of vomiting or faecal accidents at the swimming facility during the implicated weekend or the previous week. No persons reported having gastrointestinal illness in the 2 weeks prior to visiting the pool. All infants and toddlers wore waterproof nappies while swimming in the pool, and all of them were changed before and after pool use at designated changing facilities in the locker rooms.
Table.
Univariate analysis of risk factors among participants attending events at the activity pool at a private swimming facility (n=157)*, Vermont, 30 January 2006 to 2 February 2006
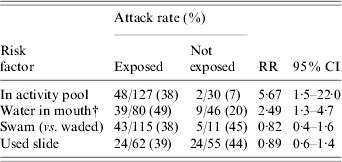
RR, Risk ratio; CI, confidence interval.
In total, 160 persons were at the activity pool, however, information on pool exposures was missing for three persons. The analysis was restricted to the 157 persons with complete data.
Pool behaviors were assessed among the 127 persons who went into the activity pool.
Environmental findings
Separate automated chlorine feeders and filtration systems supplied each of the two pools and the hot tub. At the time of the inspections after the outbreak had been reported, no equipment failures or irregularities were identified, and chlorine, pH, and temperatures were well maintained. However, several deficiencies in pool operation and maintenance were identified, including lack of standardized training or national certification among aquatic staff, poor record-keeping for maintenance or monitoring of pool chemistry, and lack of knowledge of standard operating procedures for reporting and response to pool maintenance lapses.
In light of incomplete record-keeping, descriptive information from interviews with participants and aquatic staff was used to reconstruct events occurring at the pool facility, specific to the activity pool, over the period from 30 January to 2 February 2004 (Fig. 3). While there were no abnormalities in pool water appearance noted by persons who visited the club on Friday, 30 January, several staff and persons who were there on Saturday reported a marked cloudiness of the water. This appearance persisted through Sunday morning, at which point the staff alerted general maintenance personnel who sampled the water chemically. The maintenance manager on duty recalled that readings of chlorine and pH taken at that time were below acceptable standards for disinfection (1–2 ppm chlorine and pH 7·4–7·6). The pool was hyperchlorinated to 3·5 ppm at mid-day Sunday and again that evening with several cups of 65% chlorine granules. Early Monday morning, on 2 February, the pool operator, who did not work the previous weekend, discovered a kink in the chlorinator tube supplying the activity pool, and replaced it at that time. A water sample was collected, and the pool analysis demonstrated an imbalance of free chlorine (0·5 ppm; normal range 1–2 ppm) and pH (6·8; normal range 7·4–7·6), and an elevated level of organic matter in the pool (total dissolvable solids=300). The swimming facility received guidelines from a private pool company on how to restore chemical balance in the pool, and added the appropriate compounds at closing on Monday, 2 February 2004.
Laboratory findings
Overall, stool specimens were collected from 10 affected persons, and five (four initially collected plus one additional) of these specimens tested positive for norovirus by RT–PCR. The nucleotide sequences of RT–PCR products amplified from three positive stool specimens were found to be identical, and permitted classification of the virus into NoV genogroup II.
DISCUSSION
We report an outbreak of norovirus illness with a high attack rate associated with swimming pool contamination and a mechanical failure of automated pool water disinfection equipment. Although no faecal or vomiting episode was reported at the swimming facility, the lack of cases in those exposed on Friday and the rapid rise in attack rates of those swimming on Saturday morning strongly suggest that the virus was introduced into the activity pool during one of the infant swim classes on Saturday morning. Although all infants used waterproof nappies, this outbreak underscores that they may not adequately contain liquid stool. Lack of chlorination due to a kinked chlorinating tube was compounded with procedural failures: water quality was observably poor by Saturday morning, and almost two full swim days elapsed before any action was taken. Although the outbreak may have begun to decline prior to any intervention, attack rates continued to drop after addition of chlorine and repair of the chlorination tube on Monday. The delay in response was due to a lack of staff training on appropriate water testing and record-keeping, poor communication, and a lack of a clear response plan. Assessment of risk factors suggested that getting water contaminated with norovirus in one's mouth may be associated with an increased risk of illness. Similar practices, described as immersion of the swimmer's head under water, has been previously described as a risk factor for norovirus infection [13]. These findings highlight the need to educate swimmers about the potential risks involved with ingesting public pool water.
Our study also provides an insight into the resistance of norovirus to chlorine in water. Norovirus is reputed to be resistant to chlorine up to 10 ppm. This is in large part based on a study in which volunteers were fed stool filtrates from norovirus-infected persons along with varying concentrations of chlorine [9]. However, although illness still occurred in 5 out of 8 persons when fed a mixture of virus that was inactivated with 3·75 ppm chlorine for 30 min, the virus was suspended in veal infusion broth, a proteinaceous, high-chlorine demand solution which rapidly depleted free chlorine to levels insufficient for complete virus inactivation before ingestion. Our study suggests that norovirus is more susceptible to chlorine, since the outbreak ended abruptly after the pool was chlorinated to 3·5 ppm, suggesting concentrations at or below this level may inactivate norovirus. More recent studies on inactivation of feline calicivirus, a norovirus surrogate, suggest that 1–2 ppm free chlorine take 15–30 min for feline calicivirus inactivation [14].
Since norovirus is the most common known cause of acute gastroenteritis and has a low infectious dose, swimming pool contamination with this virus may be more common than previously appreciated. Norovirus outbreaks associated with swimming pools however are not commonly reported. Although this may in part be due to under-ascertainment of these events, pool chlorine concentrations currently accepted as standard may be sufficient to prevent norovirus transmission. Indeed, outbreaks of gastroenteritis from swimming pools are more commonly associated with chlorine-resistant organisms, such as Cryptosporidium and Giardia. Waterborne outbreaks of norovirus more commonly reported are those associated with recreational water such as fountains, lakes and rivers where chlorination was absent [7]. Similar to our findings, the only previously reported pool outbreak of norovirus in the United States was associated with a failure of chlorination [15]. Outbreaks associated with drinking water have also been reported, again often caused by inadequate chlorination or a relatively massive contamination from a septic tank [4]. Interestingly, in several published outbreak reports, no clear faecal or vomiting accident could be identified [7] – we similarly could not identify an event. This may suggest that a contamination event may be relatively insignificant. It is also possible that the virus is transmitted by asymptomatic infected persons, estimated in volunteer studies to be responsible for 30% of infections [16].
There were some limitations to our study. First, the initial contact with health authorities occurred several days after the peak of illness transmission and after the pool water had been repeatedly hyperchlorinated, impeding the ability to gather representative water samples for testing for norovirus and other pathogens. Even if samples had been obtained before hyperchlorination, techniques for detection of norovirus in water are not very sensitive [17]. Detection of norovirus in the water would have been useful, but was not necessary to implicate the pool as the source of infection. The delay and the time required to gather names of persons using the pool from the swim facility may have resulted in poor recall of activities among interviewees. Only a few persons who had experienced illness had suspected the pool as the source. They were among those who initially reported to the health department, so it is unlikely that there was differential recall by illness status that would have affected our results by producing a false association. A faecal or vomiting accident may have occurred but we were unable to document it either because that household was not interviewed or because the interviewee failed to report it. Documentation of such an event would have helped us understand how the virus could have been introduced into the pool. The temporal association between level of pool contamination and illness and distinctly different demographic characteristics among participants by day of exposure limited the ability to evaluate risk factors such as age or conduct multivariate analyses including demographic factors. Food and beverages are common vehicles of norovirus transmission; however, the birthday party on Sunday morning, which experienced high rates of illness, was the only group that reported eating or drinking at the facility. Lack of other groups for comparison and the timing of attendance of the birthday party group with elevated pool contamination limited the ability to validly interpret the contribution of eating or drinking to illness. Only 10 biological specimens were tested for presence of norovirus, of which three were available for sequencing and suggested a single contamination event. The testing of more stools may have detected more than one norovirus strain in the water and provided further insight into transmission, and possible evidence for multiple sources of contamination.
This investigation of an outbreak at a swimming facility highlights the susceptibility of recreational water sources to contamination with norovirus, causing gastroenteritis if disinfection is inadequate. All aquatics staff should be well trained in water testing and pool operators should remain on site or be available for consultation during weekends when pool usage is usually highest. In addition, all aquatics facilities should develop standard operating procedures and emergency response plans detailing how water quality complaints are to be handled, the appropriate response, and clear lines of communication to personnel certified in public pool operations.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Mead PS et al. Food-related illness and death in the United States. Emerging Infectious Diseases. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widdowson MA et al. Norovirus and foodborne disease, United States, 1991–2000. Emerging Infectious Diseases. 2005;11:95–102. doi: 10.3201/eid1101.040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green KY, Chanock RM, Kapikian AZ, Fields BN, Knipe DM, Howley PM. Fields Virology. 4th edn Philadelphia: Lippincott Williams & Wilkins; 2001. Human Caliciviruses; pp. 841–874. , vol 1. , pp. [Google Scholar]
- 4.Blackburn BG et al. Surveillance for waterborne-disease outbreaks associated with drinking water – United States, 2001–2002. MMWR Surveillance Summary. 2004;53:23–45. [PubMed] [Google Scholar]
- 5.Kukkula M et al. Outbreak of viral gastroenteritis due to drinking water contaminated by norwalk-like viruses. Journal of Infectious Diseases. 1999;180:1771–1776. doi: 10.1086/315145. [DOI] [PubMed] [Google Scholar]
- 6.Maunula L et al. Wading pool water contaminated with both noroviruses and astroviruses as the source of a gastroenteritis outbreak. Epidemiology and Infection. 2004;132:737–743. doi: 10.1017/s0950268804002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoder JS et al. Surveillance for waterborne-disease outbreaks associated with recreational water – United States, 2001–2002. MMWR Surveillance Summary. 2004;53:1–22. [PubMed] [Google Scholar]
- 8.US Bureau of the Census. Statistical abstract of the United States. 115th edn. Washington, DC: US Bureau of the Census; 1995. [Google Scholar]
- 9.Keswick BH et al. Inactivation of Norwalk virus in drinking water by chlorine. Applied and Environmental Microbiology. 1985;50:261–264. doi: 10.1128/aem.50.2.261-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.StataCorp TX: StataCorp LP; 2004. . Stata Version 8.0. College Station, [Google Scholar]
- 11.Anderson AD et al. A waterborne outbreak of Norwalk-like virus among snowmobilers, Wyoming, 2001. Journal of Infectious Diseases. 2003;187:303–306. doi: 10.1086/346239. [DOI] [PubMed] [Google Scholar]
- 12.Accelrys Inc Accelrys Inc.; San Diego, CA: . Wisconsin Package Version 10.3, [Google Scholar]
- 13.Koopman JS et al. Norwalk virus enteric illness acquired by swimming exposure. American Journal of Epidemiology. 1982;115:173–177. doi: 10.1093/oxfordjournals.aje.a113288. [DOI] [PubMed] [Google Scholar]
- 14.Jeanette A et al. Chlroine inactivation of adenovirus type 40 and feline calicivirus. Applied and Environmental Microbiology. 2003;69:3979–3985. doi: 10.1128/AEM.69.7.3979-3985.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kappus KD et al. An outbreak of norwalk gastroenteritis associated with swimming in a pool and secondary person-to-person transmission. American Journal of Epidemiology. 1982;116:834–839. doi: 10.1093/oxfordjournals.aje.a113474. [DOI] [PubMed] [Google Scholar]
- 16.Okhuysen PC et al. Viral shedding and fecal IgA response after Norwalk virus infection. Journal of Infectious Diseases. 1995;171:566–569. doi: 10.1093/infdis/171.3.566. [DOI] [PubMed] [Google Scholar]
- 17.Karim MR, Pontius FW, LeChevallier MW. Detection of noroviruses in water – summary of an international workshop. Journal of Infectious Diseases. 2004;189:21–28. doi: 10.1086/380132. [DOI] [PubMed] [Google Scholar]