SUMMARY
Enhanced surveillance for Hib infection, initially covering Wales and five English regions, began in 1990 and in 1995 was extended to the whole of England and Wales. To determine whether changes in the ascertainment of Haemophilus influenzae may have affected estimates of Hib disease incidence, data from January 1990 to December 2003 were analysed. A total of 8887 and 4020 (45%) cases of H. influenzae and Hib respectively were reported. The proportion of isolates that were serotyped increased over time, and therefore reported incidence may have underestimated the true incidence in the early years of the study. Adjusting for this under-ascertainment, the incidence in children aged <5 years declined from a peak of 28·3/100 000 in 1991 to 0·97/100 000 in 1998 and increased to 3·8/100 000 in 2003. Following the implementation of universal vaccination a dramatic decline in the true incidence of invasive Hib disease occurred. The observation of the subsequent resurgence was real but the highest incidence reached was 85% below the corrected incidence in the pre-vaccine era. Continued high-quality surveillance is needed in order to accurately monitor and detect changes in disease incidence.
INTRODUCTION
Haemophilus influenzae, particularly serotype b (Hib), is a major cause of meningitis, pneumonia, epiglottitis, septicaemia, cellulitis, osteomyelitis and septic arthritis [1, 2]. Around 3 million cases of serious Hib disease are estimated to occur worldwide every year resulting in 700 000 deaths [2]. Over 80% of infections occur in children aged <5 years. In the United Kingdom, like many other industrialized countries, the introduction of the Hib conjugate vaccine into the national childhood immunization programme in 1992 resulted in a dramatic decrease (>90%) in the incidence of Hib, through a combination of direct protection and herd immunity [3–10].
The Health Protection Agency (HPA) estimates the incidence of Hib in the United Kingdom from the number of H. influenzae isolates referred to the HPA Haemophilus Reference Unit (HRU) for serotyping and from hospital laboratory reports of confirmed H. influenzae infections to the HPA Centre for Infections (CFI). In order to assess the need for and impact of the Hib vaccine, enhanced surveillance covering Wales and five English regions was initiated in September 1990. This involved referral of H. influenzae strains to the HRU for confirmation and serotyping. In 1995, following the dramatic decline in the incidence of invasive Hib disease, enhanced surveillance was extended to cover the whole of England and Wales. The HRU now contacts clinicians, public health doctors and hospital laboratories involved with any cases reported to the CFI to obtain clinical information and an isolate where possible. The aim of this analysis was to determine whether changes in the ascertainment of H. influenzae since 1990 may have affected estimates of Hib disease incidence.
MATERIALS AND METHODS
National surveillance of H. influenzae infection is conducted through a combination of routine laboratory reporting and enhanced surveillance, supplemented by clinical reporting schemes. Any case where H. influenzae has been isolated from a normally sterile site is eligible for inclusion. Laboratory reports of confirmed infections from microbiologists in nearly 400 hospital and public health laboratories have been received at the HPA CFI since 1975. To assess the need for and impact of introducing the Hib conjugate vaccine into routine childhood immunization programmes, enhanced surveillance, initially covering Wales and five English regions (East Anglia, Northern, North West, South West and Oxford) representing around 35% of the English population, was initiated in September 1990. In these regions, clinicians, microbiologists and public health doctors were encouraged to report all cases of H. influenzae infections to the HRU, and then to refer a clinical isolate for confirmation and serotyping [11]. The latter was performed using standard slide agglutination and confirmed by molecular techniques [12]. As a consequence of the rapid decline in the incidence of invasive Hib disease following national immunization, the enhanced surveillance was extended nationally to cover all England and Wales in 1995. Since then, reports from any source have been followed by asking the microbiologist to refer the isolate to the HRU.
From October 1992 to September 2000, a national clinical reporting scheme was conducted through the British Paediatric Surveillance Unit (BPSU) [5]. The BPSU functions by asking all paediatricians to report all children with a particular disease on a report card sent to them at the end of each month. The overall response rate, including zero returns, is around 90% each month. The reporting paediatrician is then sent a questionnaire requesting clinical, demographic, and laboratory information for each case. The BPSU surveillance initially requested paediatricians to report all cases of invasive H. influenzae infections (defined by isolation of H. influenzae from a normally sterile site) in children aged <10 years who had received the Hib conjugate vaccine. From November 1995 to September 2000, the definition was expanded to include all children aged <16 years with invasive H. influenzae disease irrespective of vaccination status. The local microbiologist was also contacted and asked to send the clinical isolate to the HRU if this had not already been done.
Eligible cases with isolates referred to the HRU and those reported by laboratories or through enhanced surveillance were entered into a single dataease database, reconciled and de-duplicated regularly. The final database, containing information about all cases reported in England and Wales between January 1990 and December 2003, was transferred into stata 7.0 (Stata Corp., College Station, TX, USA) for statistical analysis. As there were few changes to the laboratory reporting system over the study period, the completeness of laboratory reports was assumed to have remained constant. To compare changes in ascertainment due to enhanced HRU surveillance over time, two periods were chosen. The regional enhanced surveillance started in September 1990 and continued until late 1995, and was therefore fully functional between January 1991 and December 1994. Similarly, the national enhanced surveillance that began in late 1995 was in a transition phase in early 1996, but was fully functional between January 1997 and December 2003.
To estimate the impact of improved serotyping on Hib incidence over the two periods, the proportion of possible Hib cases amongst those without typing results was adjusted using the formula below. This proportion was applied to the untyped cases and the result added to the known Hib infections to estimate the true incidence of Hib infection [13].
Proportions were compared using the χ2 test or Fisher's exact test, while continuous variables were compared using Student's t test with all P values being two-tailed.
RESULTS
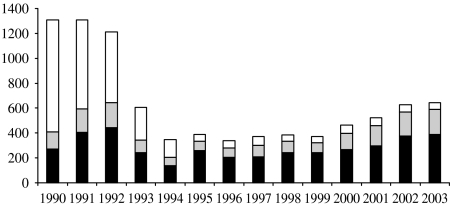
Between January 1990 and December 2003, there were 8887 confirmed cases of H. influenzae. Of these 4020 were due to Hib (45%), 323 due to other capsular strains (3·6%), 2181 due to non-capsular strains (25%) and, in 2363 cases (27%), the serotype was not known. Almost half of the cases (n=3993, 45%) were reported by hospital laboratories and confirmed by the HRU, 35% (n=3107) were reported by hospital laboratories only and 20% (n=1787) by the HRU only (Fig. 1). The H. influenzae serotype was known for almost all isolates (1671/1787 isolates, 93·5%) reported by the HRU only, compared with only 1391 of 3107 isolates (44·8%) reported by hospital laboratories only. During the 14-year study period, the reported number of Hib cases fell from a maximum of 886 cases in 1991 to 36 cases in 1998, followed by a rise in the number of cases to 245 in 2003.
Fig. 1.
Number of H. influenzae cases reported per year by different sources. ■, Surveillance and laboratories; , surveillance only; □, laboratories only.
, surveillance only; □, laboratories only.
Time period between 1991 and 1994
Using 1991–1994 inclusive to represent the time period when the regional enhanced surveillance was in operation, there were 1378 H. influenzae cases (Hib=66%) reported within the surveillance regions and 2091 cases (Hib=58%) from other regions (total 3469 cases, Hib=62%). The proportion of cases reported through hospital laboratories only (i.e. not ascertained by enhanced surveillance) was lower in the surveillance area than in other regions (P<0·0001) (Table) and this difference was present in both children and adults. The proportion of cases in adults that were only reported by laboratories was higher only in the regions outside the surveillance area (P<0·0001).
Table.
Comparison of Hib ascertainment between two time periods: 1991–1994, when enhanced Hib surveillance was performed in five English regions and Wales only, and 1997–2003, when Hib surveillance was in place for the whole of England and Wales
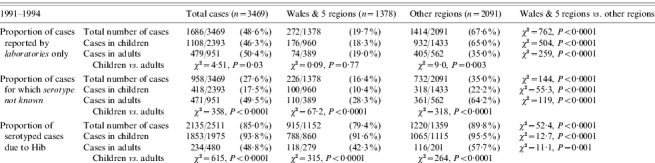
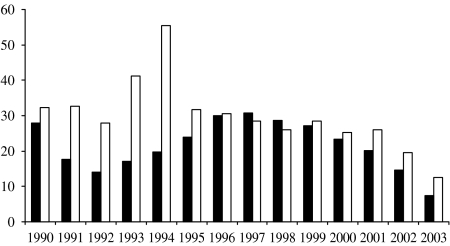
Similarly, the proportion of clinical isolates that were not serotyped was lower within the surveillance regions than elsewhere (Fig. 2). Serotyping was more likely to be performed within the surveillance regions (74% vs. 65%, P<0·0001) and paediatric isolates were more likely to be serotyped compared with adults both within (90% vs. 72%, P<0·0001) and outside (78% vs. 36%, P<0·0001) the surveillance regions (Table). Outside of the surveillance regions, almost two-thirds of adult isolates were not serotyped during this period. There was a higher proportion of cases with a clinical diagnosis available among patients within the surveillance regions than in the rest of the country (91% vs. 72%, χ2=182, P<0·0001).
Fig. 2.
Proportion of clinical isolates that were not serotyped per year in the initial Wales/five regions surveillance (■) and in other regions (□).
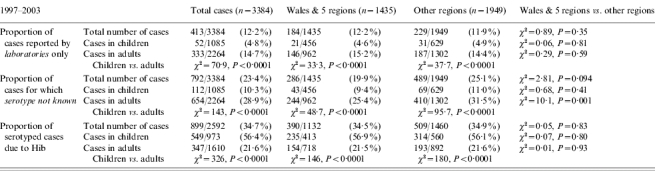
Time period between 1997 and 2003
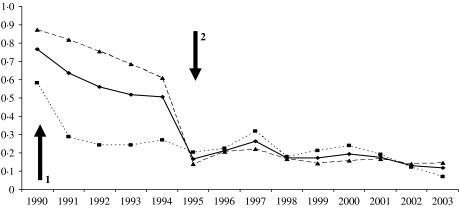
Using 1997–2003 to represent the time period when the national enhanced surveillance was fully operational, there were 1435 H. influenzae cases (Hib=27%) reported within the initial surveillance regions (Wales and five English regions) and 1949 cases (Hib=26%) from other regions (total 3384 cases, Hib 27%). After 1995, there was a dramatic decrease in the number and proportion of cases reported only by hospital laboratories (Table, Fig. 3). By 2003, 88% of cases reported were also confirmed at the HRU and, therefore, in the enhanced surveillance dataset, compared with 51% in 1994. The proportion of cases reported only by hospital laboratories was similar within and outside the original Wales/five-region surveillance areas in both adults (P=0·59) and children (P=0·81) during this time period. Overall, however, the proportion of adult cases only reported by laboratories remained higher than paediatric cases (15% vs. 4·8%, P<0·0001) (Table).
Fig. 3.
Proportion of H. influenzae cases identified by laboratories that were not picked up by enhanced surveillance for all regions (–◆–), Wales/five regions (····■····) and the other regions (- - -▲- - -). Arrow 1=initiation of Wales/five regions enhanced surveillance; arrow 2=extension of enhanced surveillance to all England and Wales.
The overall proportion of clinical isolates serotyped increased every year from 71% in 1997 to 90% in 2003 (χ2 for trend=98, P<0·0001). There was no statistically significant difference in the proportion of isolates serotyped within and outside the original Wales/five-region surveillance areas (P=0·09). However, isolates from adults (75% vs. 69%, P=0·001), but not from children (91% vs. 89%, P=0·41), were still more likely to be serotyped within the Wales/five-region surveillance area than other regions. Overall, isolates from cases in children remained significantly more likely to be serotyped than those from adults (90% vs. 71%, P<0·0001).
Along with improvements in serotyping, there was also an improvement in the collection of clinical, epidemiological and laboratory data, particularly outside the original Wales/five-region surveillance regions. For example, the proportion of cases for whom a diagnosis was available increased from 72% in 1991–1994 to 89% in 1997–2003 (χ2=185, P<0·0001) (Table).
Adjusted incidence of invasive Hib disease
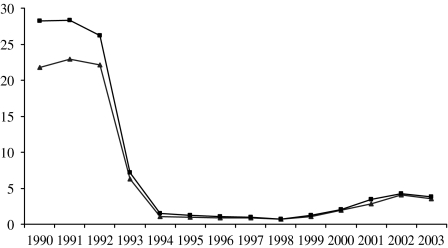
Based on the change in the proportion of isolates serotyped over time, the true number of Hib infections can be estimated by correcting as above. This results in an increase in the estimated number of Hib cases in the early period, but makes little difference in later years (Fig. 4). Using the corrected data, the true incidence of Hib infection in children aged <5 years declined from a peak of 28·3/100 000 in 1991 to 0·97/100 000 in 1998 (Fig. 4). By 2003, the true incidence had increased to 3·8/100 000, but this was still around 85% lower than the pre-vaccine incidence (Fig. 4). A similar trend was observed in adults (data not shown).
Fig. 4.
The incidence of invasive Hib disease in children aged <5 years per 100 000 based on the total number of cases reported (–▲–) and the adjusted number of cases (–■–).
DISCUSSION
The introduction of the Hib conjugate vaccine into the routine childhood immunization programme in 1992 led to a dramatic decline in the incidence of invasive Hib disease by 1995. As a consequence, enhanced surveillance was extended from the five English regions and Wales to cover all regions in both countries. The national BPSU study was also in operation, but as each report was followed by prompt chasing of the isolate, it was impossible to determine which of those isolates referred following a BPSU report would not have been referred as part of normal practice. Overall, however, the combined enhanced surveillance improved collection of important clinical, epidemiological and laboratory information on every case of H. influenzae disease, and resulted in almost all clinical isolates being sent the HRU for confirmation and serotyping. This was particularly important in the era of very low incidence of Hib when most hospitals stopped performing in-house serotyping.
Enhanced surveillance was implemented to ensure that completeness of reporting was maintained before and after the introduction of vaccination. The proportion of cases reported by hospital laboratories that were also picked up by enhanced surveillance increased after 1995 following extension to the whole of England and Wales. We have shown that the contribution of regions outside of the study area to reported cases did not change substantially over the time period of this analysis. This suggests that, by using the reconciled combined national dataset, overall ascertainment of H. influenzae has not changed dramatically over time in England and Wales.
Laboratory reports generally do not contain clinical information and, since the dramatic decline in the incidence of Hib, most H. influenzae isolates were not serotyped within individual hospitals. Extending the enhanced surveillance nationally improved both the collection of clinical information and increased serotyping of H. influenzae isolates throughout the country, so that, by 2003, almost 90% of all clinical isolates were serotyped in both the study regions and the rest of England. In contrast to the conclusions of a study in one region [14] this increase in the completeness of serotyping has markedly improved the ascertainment of Hib infection. This improvement could potentially have contributed to the recent increase in the incidence of Hib [3, 15]. Adjusting for untyped isolates in the post-vaccine era, however, makes very little difference to the incidence of Hib in children aged <5 years. Therefore, our analysis suggests that a true rise in incidence did occur, and cannot be explained by changes in ascertainment. Although the increase was marked, the overall incidence remains 85% lower than the true pre-vaccine incidence, allowing for under-ascertainment in the pre-vaccine era.
Following the detection of this rise an investigation was performed using surveillance data and additional studies. A rapid decline in direct protection, particularly for those vaccinated in infancy, was demonstrated by combining enhanced surveillance data with population coverage information [3]. This decline had been masked by the effects of the initial catch-up campaign. The catch-up campaign had led to high levels of direct protection and high population immunity in the <5 years age group [3, 16]. This campaign also contributed to indirect protection (herd immunity) through a marked reduction in Hib colonization [17]. Surveillance was able to demonstrate the decline in this indirect protection, and to explain the recent resurgence in the incidence of Hib disease in adults [13]. During 2001–2002, because of supply problems, a DTP–Hib combination vaccine containing an acellular pertussis vaccine, known to be less immunogenic than whole-cell pertussis vaccine [18], was used. National data was, therefore, used as a sampling frame for a case-control study that showed poor protection from this combination vaccine [19].
This emphasizes the importance of sustaining high-quality surveillance for many years after apparent disease control. In response to the signals generated from this programme, and to the rapid public health investigation, infant immunization with the less immunogenic combination vaccine was stopped. In 2003, the Department of Health also implemented a booster for all children aged 6 months to 4 years [20]. Surveillance has demonstrated an immediate reduction of disease amongst the target population for this campaign, and a stabilization of the incidence in older individuals. Sustaining long-term protection, however, will probably require the maintenance of high levels of immunity. Therefore, from 4 September 2006, a routine booster of Hib has been recommended for children reaching 1 year of age in the United Kingdom.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Booy R. Getting Hib vaccine to those who need it. Lancet. 1998;351:1446–1447. doi: 10.1016/S0140-6736(98)22020-4. [DOI] [PubMed] [Google Scholar]
- 2.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clinical Microbiology Reviews. 2000;13:302–317. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsay ME et al. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. Journal of Infectious Diseases. 2003;188:481–485. doi: 10.1086/376997. [DOI] [PubMed] [Google Scholar]
- 4.Heath PT et al. Antibody concentration and clinical protection after Hib conjugate vaccination in the United Kingdom. Journal of the American Medical Association. 2000;284:2334–2340. doi: 10.1001/jama.284.18.2334. [DOI] [PubMed] [Google Scholar]
- 5.Heath PT, McVernon J. The UK Hib vaccine experience. Archives of Disease in Childhood. 2002;86:396–399. doi: 10.1136/adc.86.6.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fogarty J, Moloney AC, Newell JB. The epidemiology of Haemophilus influenzae type b disease in the Republic of Ireland. Epidemiology and Infection. 1995;114:451–463. doi: 10.1017/s095026880005216x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herceg A. The decline of Haemophilus influenzae type b disease in Australia. Communicable Diseases Intelligence. 1997;21:173–176. [PubMed] [Google Scholar]
- 8.Garpenholt O et al. The impact of Haemophilus influenzae type b vaccination in Sweden. Scandinavian Journal of Infectious Diseases. 1996;28:165–169. doi: 10.3109/00365549609049069. [DOI] [PubMed] [Google Scholar]
- 9.Hargreaves RM et al. Changing patterns of invasive Haemophilus influenzae disease in England and Wales after introduction of the Hib vaccination programme. British Medical Journal. 1996;312:160–161. doi: 10.1136/bmj.312.7024.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker M, Martin D. Hib disease rate plummets following immunisation programme. New Zealand Public Health Report. 1995;2:13–14. [Google Scholar]
- 11.Heath PT et al. Clinical and immunological risk factors associated with Haemophilus influenzae type b conjugate vaccine failure in childhood. Clinical Infectious Diseases. 2000;31:973–980. doi: 10.1086/318132. [DOI] [PubMed] [Google Scholar]
- 12.Falla TJ et al. PCR for capsular typing of Haemophilus influenzae. Journal of Clinical Microbiology. 1994;32:2382–2386. doi: 10.1128/jcm.32.10.2382-2386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McVernon J et al. Trends in Haemophilus influenzae type b infections in adults in England and Wales: surveillance study. British Medical Journal. 2004;329:655–658. doi: 10.1136/bmj.329.7467.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olowokure B et al. Decrease in effectiveness of routine surveillance of Haemophilus influenzae disease after introduction of conjugate vaccine: comparison of routine reporting with active surveillance system. British Medical Journal. 2000;321:731–732. doi: 10.1136/bmj.321.7263.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trotter CL, Ramsay ME, Slack MP. Rising incidence of Haemophilus influenzae type b disease in England and Wales indicates a need for a second catch-up vaccination campaign. Communicable Disease and Public Health. 2003;6:55–58. [PubMed] [Google Scholar]
- 16.Trotter CL et al. Antibody to Haemophilus influenzae type b after routine and catch-up vaccination. Lancet. 2003;361:1523–1524. doi: 10.1016/s0140-6736(03)13172-8. [DOI] [PubMed] [Google Scholar]
- 17.McVernon J et al. Long-term impact of vaccination on Haemophilus influenzae type b (Hib) carriage in the United Kingdom. Epidemiology and Infection. 2004;132:765–767. doi: 10.1017/s0950268804002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg DP et al. Immunogenicity of a Haemophilus influenzae type b-tetanus toxoid conjugate vaccine when mixed with a diphtheria-tetanus-acellular pertussis-hepatitis B combination vaccine. Pediatric Infectious Disease Journal. 2000;19:1135–1140. doi: 10.1097/00006454-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 19.McVernon J et al. Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with acellular pertussis. Lancet. 2003;361:1521–1523. doi: 10.1016/s0140-6736(03)13171-6. [DOI] [PubMed] [Google Scholar]
- 20.Heath PT, Ramsay ME. Haemophilus influenzae type b vaccine – booster campaign. British Medical Journal. 2003;326:1158–1159. doi: 10.1136/bmj.326.7400.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]