SUMMARY
To enhance the detection of bacterial meningitis in an East Asian surveillance study, we employed cerebrospinal fluid (CSF) bacterial culture, latex agglutination (LA) and polymerase chain reaction–enzyme immunoassay (PCR–EIA) testing for Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae (Sp). The sensitivity and specificity of CSF PCR–EIA testing was compared to LA and culture. A meningitis case was defined by one positive result for any of the three tests. The sensitivity of H. influenzae CSF PCR–EIA, LA, and culture was 100%, 40% and 57·5% respectively; and for Sp CSF PCR–EIA, LA and culture, the sensitivity was 100%, 58·3% and 66·7%, respectively. Hib and Sp specificity was 100% by each method. CSF PCR–EIA was more sensitive than culture or LA for the detection of Hib and Sp meningitis cases increasing their incidence by 74% and 50% compared to culture respectively. CSF PCR–EIA should be included for the detection of bacterial meningitis in surveillance studies.
Introduction
Bacterial meningitis is a life threatening and often neurologically debilitating infectious disease, especially for children. The leading pathogens are Haemophilus influenzae type b (Hib), Streptococcus pneumoniae (Sp) and Neisseria meningitidis, all of which are now vaccine preventable. Worldwide Hib meningitis has the greatest incidence and is estimated to affect annually 400 000 children aged <5 years, 100 000 of which die from the infection [1]. Further, neurological sequelae, including paralysis, cognitive deficits, hearing or vision loss, occur in 10–30% of cases [2]. Sp meningitis is the second most common cause of bacterial meningitis, however, the global disease burden is not well defined. N. meningitidis infections tend to be more regional with the occurrence of both outbreaks and widespread epidemics. Most developed countries now provide routine Hib and Sp conjugate vaccines for children, yet most Asian countries do not. Vaccine cost and lack of appreciation of the disease burden are often given as the reasons for the lack of vaccine use in Asia.
For several reasons the diagnosis of bacterial meningitis in developing countries is difficult to establish. Parents often routinely administer oral antibiotics at the first sign of fever in their infant as antibiotics are readily available from local pharmacies without a prescription. Home antibiotic treatment is prevalent because transportation to a health-care facility for early treatment is often impossible. Medical personnel also routinely administer parental antibiotics at local outpatient clinics for the empirical treatment of febrile infants, without obtaining cerebrospinal fluid (CSF), cultures or other tests. Prior antibiotic treatment will often result in negative CSF culture growth in children with meningitis. In addition, CSF or blood specimens for culture are often not routinely collected because of lack of availability or cost. Further, cultures, if obtained, are often not optimally processed and specimens may be delayed in being cultured in the laboratory. Haemophilus influenzae (Hi) requires specialized media containing X and V factors found in partially lysed blood (chocolate agar) and enriched with supplements. Many Asian countries do not use chocolate agar media prepared in a high-quality standardized manner and may prepare their chocolate agar media suboptimally such that it cannot support the growth of Hi. Some laboratories use a chocolate agar medium prepared from residual human blood, which often contains antibodies to Hi. These and other factors make an accurate assessment of the incidence of bacterial meningitis problematical.
While CSF latex agglutination (LA) antigen testing is reported to improve case detection of bacterial meningitis, it requires a high concentration of bacteria (i.e. ⩾106 c.f.u./ml) for a positive result [3]. Recently, polymerase chain reaction (PCR) testing has been shown to detect as few as 10–100 c.f.u./ml of bacteria in CSF, and therefore may be more sensitive and specific than LA in the diagnosis of bacterial meningitis [4]. Both tests do not require viable bacteria. There is at present, limited capability for PCR testing in most developing countries' laboratories and few population-based surveillance studies have employed PCR or LA technology. In this study we compared the utility of CSF PCR to culture and LA for the diagnosis of Hib and Sp meningitis in a population-based study of children living in Korea, Vietnam and China. Meningococcal meningitis surveillance was not undertaken using polymerase chain reaction–enzyme immunoassay (PCR–EIA) testing because the disease burden could not be accurately assessed in Vietnam and China where routine meningococcal vaccine use is common.
Methods
Study population
A population-based surveillance study of children aged <5 years with signs and symptoms of meningitis was conducted in the Jeonbuk Province of South Korea (n=2176) [5]; Hanoi, Vietnam (n=580) [6]; and the greater area around Nanning, China (n=1192) from September 1999 to December 2002. Children with suspected bacterial meningitis were evaluated by study physicians using standardized clinical and laboratory criteria. We utilized an enhanced active case finding by all physicians in the surveillance area whereby critically ill children that met standardized meningitis signs or symptoms were referred to all tertiary care centres for clinical and laboratory evaluations. Epidemiology and laboratory quality-assurance monitoring activities were periodically performed. Country-specific meningitis incidence rates and further study methods have been published elsewhere [5, 6]. Prior to the collection of clinical data and specimens, the study was explained to the parent/guardian and informed verbal consent was obtained. The study was approved by the Institutional Review Board of the Los Angeles Biomedical Research Institute at Harbor UCLA Medical Center and by an Ethical Research Committee at each of the participating hospitals.
Definitions
Confirmed Hib or Sp meningitis
This was defined as having criteria for suspected bacterial meningitis with either Hib or Sp cultured from CSF or blood.
Probable Hib or Sp meningitis
This was defined as having criteria for suspected bacterial meningitis and either Hib or Sp detected in CSF by Gram stain, LA or PCR.
WHO definition of probable bacterial meningitis
This was defined as having signs and symptoms of meningitis with either a turbid CSF or CSF protein >1·0 g/l or glucose <2·22 mmol/l or WBC >100×106 cells/l (with >80% neutrophils) [7], without an identifiable bacterial pathogen.
Suspected bacterial meningitis
This was defined as a child aged <5 years with signs and symptoms of meningitis [fever, mental status changes (irritability, confusion, or lethargy), headache, bulging anterior fontanelle, forceful vomiting or seizure] and an abnormal CSF (see below).
Abnormal CSF
This included one or more of the following; (1) turbid appearance, (2) elevated CSF white blood cell count (WBC) (>30×106 cells/l for neonates aged ⩽1 month, >10×106 cells/l for infants aged >1 month), (3) CSF neutrophil count >70% of the total WBC in the CSF, (4) elevated CSF protein concentration (>0·75 g/l for infants aged >1 month, or >1·70 g/l for neonates aged ⩽1 month, or (5) CSF glucose concentration <2·22 mmol/l [8].
Specimen collection and processing
Using sterile technique (povidone and then alcohol skin preparation, sterile single-use needle and collection tubes and use of glove and mask), a lumbar puncture was performed and four aliquots totalling 3–5 ml of CSF was collected and transported to the laboratory within 1 h of collection. A Gram stain, cell count, biochemistry and culture were performed on all specimens. A 1 ml aliquot of CSF was placed into a sterile cryovial at the bedside and on arrival in the laboratory was frozen at −70°C and periodically shipped on dry ice to the UCLA Center for Vaccine Research (California, USA) for LA and PCR testing. Smaller study hospitals without deep-freeze facilities froze the LA and PCR aliquot at −20°C for up to 1 week until collection, and it was transported on dry ice by the larger central laboratory staff. Blood was collected and inoculated into Bactec PedsPlus® bottles (Becton, Dickinson & Co., Franklin Lakes, NJ, USA).
Two drops of CSF were streaked on commercial blood and chocolate agar culture media (Becton Dickinson) and residual CSF was also inoculated into 5% Fildes broth [9]. Both agar plates and broth were incubated in 5% CO2 at 37°C for 7 days and checked daily for growth. After 1, 3 and 7 days of incubation, aliquots of broth were sub-cultured on blood and chocolate agar plates. Isolates were identified using standard microbiological criteria [10]. Bactec PedsPlus® bottles were incubated at 37°C for 7 days and in the absence of automated detection systems, the blood was sub-cultured at 1, 3 and 7 days of incubation on chocolate and blood agar media and growth was identified as described above. Bacterial isolates were stored at −70°C in skimmed milk for later species confirmation and serotyping. Hi isolates were serotyped using anti-capsular agglutination (types a–f) (Murex, Remel Inc., Lenexa, KS, USA). All laboratory procedures and reagents were standardized and monitored throughout the study with periodic external proficiency testing performed to ensure sensitivity and accuracy of culture methods and isolate identification [11].
LA antigen testing
LA antigen testing for Hib, Sp and N. meningitidis was performed on all abnormal CSF samples (defined below) and a sample of normal CSF using a commercial LA kit Slidex Meningite® (bioMérieux, Marcy-l'Etoile, France) according to manufacturer's instructions. A total of 1197 out of 3948 (30·3%) CSF specimens collected were tested by LA for the detection of Hib and Sp antigens.
Nucleic acid amplification detection assays
To assess assay sensitivity and specificity, PCR–EIA was performed on DNA of a selection of strains from the American Type Culture Collection (ATCC) and clinical isolates. After log phase growth was obtained, bacteria were diluted ten-fold in sterile normal saline to 0·5 McFarland turbidity. Bacterial DNA was extracted by boiling a dense suspension of an isolate for 10 min with centrifugation at 10 000 g for 5 min. DNA was extracted from boiled bacterial supernate or CSF specimen using the QIAamp® DNA Blood Mini kit (Qiagen, Chatsworth, CA, USA). PCR assay was performed using standard cycling parameters using a digoxigenin-11-dUTP containing nucleotide mixture (PCR DIG Labeling Mix; Roche Diagnostics Inc., Indianapolis, IN, USA) and pathogen specific primer pairs [12]. Non-serotype specific primers of the capsular bexA gene (480-bp segment) were used to amplify Hi DNA [4, 12]. The bexA gene primers detect all capsular types of Hi. As 23 out of 24 (96%) CSF Hi isolates were Hib, we considered a positive Hi PCR–EIA but negative CSF culture to indicate a probable Hib meningitis case. Primers directed to the pneumolysin ply gene (208-bp segment) were used to amplify Sp DNA [13]. The digoxigenin-labelled PCR product was detected using initial hybridization to a biotinylated probe and then binding to streptavidin-coated wells of the PCR ELISA Digoxigenin Detection kit (Roche Diagnostics Inc.) according to manufacturer's instructions. A sample was positive if the mean EIA optical density (OD) value was more than three times the mean OD value for the negative assay control, a concentration where a green colour was first visible. PCR reactions were set up in a class II biosafety cabinet and amplification and product detection was performed in separately ventilated rooms. In addition, aerosol-resistant pipette tips and multiple negative controls were used with each PCR reaction. If one or more of the negative controls had a positive PCR–EIA test result, all samples from the experiment were discarded and the PCR–EIA run was repeated.
Not all CSFs were evaluated by PCR–EIA. We evaluated 1063 (26·9%) CSFs by Hi PCR–EIA and 577 (14·6%) CSFs by Sp PCR–EIA. CSF samples with abnormal cytological or biochemical parameters, or a positive bacterial culture and a sample of CSF with normal indices were tested by PCR–EIA. Whenever possible, abnormal CSF was tested for both Hi and Sp PCR–EIA. However, when there was insufficient CSF volume, prioritization of abnormal CSF testing occurred: Hi PCR–EIA, Sp PCR–EIA and lastly LA.
Statistical methods
The data were analysed using SAS statistical software, version 8.1 (Cary, NC, USA). χ2 and Fisher's exact statistical tests were applied to categorical data when appropriate. Mean CSF parameters were evaluated between groups using the Wilcoxon Signed Rank test.
Results
Laboratory sensitivity and specificity of the PCR–EIA assays
The sensitivity of the Hi and Sp PCR–EIA assay was determined using serial ten-fold dilutions of H. influenzae type b (ATCC 10211) and S. pneumoniae (ATCC 6306) bacterial isolates in normal saline, respectively. The threshold for a positive assay was estimated to be 25 c.f.u./ml for Hib and 185 c.f.u./ml for Sp. The specificity of the PCR–EIA assays was determined using DNA extracts of 10–13 pathogenic bacterial suspensions. No false-positive PCR–EIA results were observed (Table 1). No false-positive Hi or Sp PCR–EIA results were found amongst 19 CSFs that grew other bacterial pathogens; N. meningitidis (n=3), Group B Streptococcus (n=9), E. coli (n=2), K. pneumoniae (n=3), E. faecalis (n=1), S. aureus (n=1). An additional 90 and 54 culture-negative CSFs with normal parameters were tested by Hi and Sp PCR–EIA respectively, and none was PCR–EIA positive.
Table 1.
Polymerase chain reaction–enzyme immunoassay (PCR–EIA) results obtained for suspensions of ATCC and clinical isolates
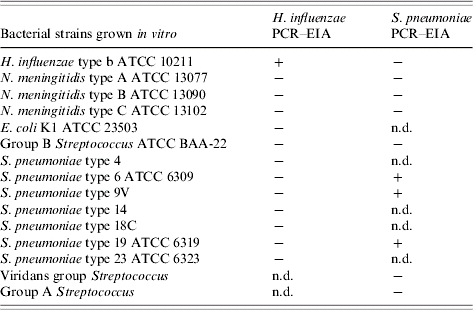
ATCC, American Type Culture Collection.
+, Tested positive; −, tested negative; n.d., not done.
Comparison of culture with LA and PCR–EIA assays
The results of LA and PCR–EIA testing of clinical CSF specimens are presented in Tables 2 and 3. To assess the relative sensitivity of CSF PCR–EIA, LA and culture, we used a positive test in any of the three tests as the comparison standard. Hi PCR–EIA was positive in all 23 CSFs that grew Hib, as well as in an additional 17 culture-negative specimens. LA was positive in 15 of the 23 CSFs that grew Hib, but in only one culture-negative specimen. The sensitivity of Hi CSF PCR–EIA, LA and culture compared to a positive test in any of the three testing methods was 100%, 40% and 57·5% respectively. The specificity of Hi CSF PCR–EIA, LA or culture was 100% for each testing method.
Table 2.
Comparison of CSF Haemophilus influenzae type b polymerase chain reaction–enyzme immunoassay (PCR–EIA) to latex agglutination (LA), culture or a combination of all three tests in specimens from Korea, Vietnam and China
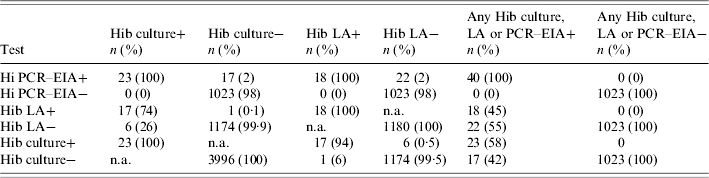
Hib, Haemophilus influenzae type b; n.a., not applicable.
Table 3.
Comparison of CSF Streptococcus pneumoniae polymerase chain reaction–enzyme immunoassay (PCR–EIA) to latex agglutination (LA), culture or a combination of all three tests in specimens from Korea, Vietnam and China
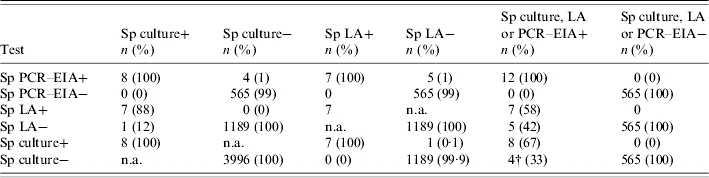
Sp, Streptococcus pneumoniae; n.a., not applicable.
One CSF grew non-typable H. influenzae and was Hi PCR negative (primers directed to only encapsulated H. influenzae) but Sp PCR was repeatedly positive indicating a mixed CSF infection with both non-typable H. influenzae and Sp.
Sp PCR–EIA detected all eight Sp culture-positive specimens and four additional culture-negative specimens. LA detected six of the eight CSF Sp culture-positive and one culture- negative case of Sp meningitis. The sensitivity of Sp CSF PCR–EIA, LA or culture compared to a positive test in any of the three testing methods was 100%, 58·3% and 66·7% respectively. Additionally, the specificity of Sp CSF PCR–EIA, LA or culture was 100% for each testing method. Compared to culture alone, CSF PCR–EIA was more sensitive than LA and increased the observed incidence of meningitis due to Hib and Sp by 74% and 50% respectively.
Clinical characteristics of patients with CSF culture-negative but PCR-positive assays
Twenty-one children ranging from 1·3 to 55 months of age had no bacterial growth from their CSF or blood, but had a positive CSF PCR–EIA (17 Hi PCR and four Sp PCR positive). The clinical characteristics of these children varied. Two-thirds of the cases presented with classic central nervous system (CNS) symptoms of meningitis (headache, irritability, confusion, lethargy or coma) but only one-third of these had abnormal CSF parameters (high WBC, protein and low glucose), consistent with bacterial meningitis. Seven of 21 (33%) children did not have any classical meningitis symptoms on presentation; two had a stiff neck and fever, two had a febrile seizure, one had a seizure without fever and two had fever without any CNS manifestations. The 10-month-old Chinese infant who had a seizure without fever was found to have an infiltrate on chest radiograph. Her CSF revealed a WBC of 900×106 cells/l (68% neutrophils), a CSF protein of 1·13 g/l and CSF LA and PCR–EIA tests were positive for Hib despite negative blood and CSF cultures. Only nine of 21 (43%) culture-negative PCR-positive cases had prior antibiotic use based upon parental interview. Also, only nine of 20 evaluable cases (45%) had abnormal CSF indices compatible with the WHO definition of probable bacterial meningitis.
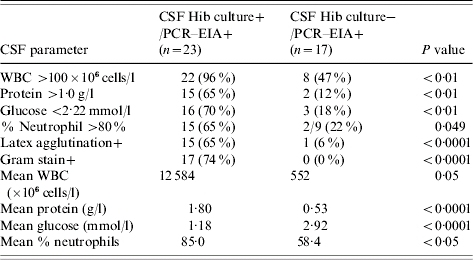
Children with CSF culture-negative Hi PCR–EIA positive results were older (29 vs. 15 months), presented later (3·6 vs. 3·0 days of meningitis symptoms) and were less sick on presentation (less lethargy/coma, septic appearance and stiff neck) than children with positive Hib CSF cultures (Tables 4, 5). There was no difference among children in these groups by sex, prior antibiotic use, mortality or occurrence of seizures or pneumonia. Abnormal neurological findings at hospital discharge were less frequent in CSF Hi PCR–EIA-positive culture-negative cases (6%) compared to CSF Hib culture-positive cases (30%, P=0·107). This correlated with lower indices of inflammation in the CSF. Although over two-thirds of children with culture-positive bacterial meningitis had abnormal CSF indices, only 45% of CSF Hi PCR–EIA-positive culture-negative cases had indices that met the WHO's definition of probable bacterial meningitis. The CSF Gram stain was negative in all CSF culture-negative, Hi PCR–EIA-positive meningitis cases.
Table 4.
Comparison of clinical characteristics of children with Hib culture and polymerase chain reaction–enzyme immunoassay (PCR–EIA)-positive CSF to those with culture-negative but Hib PCR–EIA-positive CSF
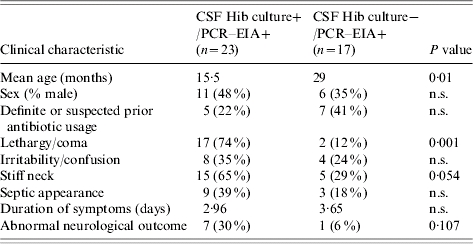
n.s., Not significant.
Table 5.
Comparison of CSF parameters of children with Hib culture and polymerase chain reaction–enzyme immunoassay (PCR–EIA)-positive CSF to those with culture-negative but Hib PCR–EIA-positive CSF
Discussion
We evaluated PCR–EIA and LA testing of CSF in an attempt to improve the detection of Hib and Sp meningitis and to define better the incidence of these diseases in developing countries. The gold standard used for our comparisons was a positive result by any of the three test methods. CSF culture alone was not an adequate standard for comparison because growth of bacteria often does not occur if there is antibiotic in the CSF and if suboptimal culture methods are used, especially when the infection involves a low concentration of fastidious bacteria. We focused our efforts on Hib and Sp meningitis disease burden determination because previous studies showed that they were the most prevalent pathogens in childhood meningitis in these countries. Although we found many abnormal CSFs that were culture negative or were Hib or Sp LA or PCR–EIA negative, we were not able to determine the aetiology of all meningitis cases. It is possible that some cases may be due to meningococci and would only be detected by PCR–EIA, yet we were aware that other enteric bacteria, Group B streptococci and viruses would not be detected due to high rates of prior antibiotic treatment use, unless exhaustive, costly viral culture, PCR and perhaps bacterial 16S rRNA sequence CSF PCR testing were performed.
Previous studies that evaluated CSF PCR in developing countries have shown varied results. A prospective surveillance study in Thailand did not detect any additional cases of Hib meningitis by PCR compared with culture alone, however, only four of 59 abnormal CSFs were positive [14]. A prospective hospital-based study in Vietnam found CSF Hi PCR was positive in all seven positive cultures and in one culture-negative specimen out of 36 abnormal CSFs tested [15]. Another small hospital-based study from India detected Hi PCR-positive results in 14 culture-positive and 23 culture-negative CSF specimens [16]. A larger prospective surveillance study in Burkina Faso involved 1477 children aged <5 years with suspected meningitis found that Hi PCR missed four out of 17 culture-positive cases, but detected an additional 29 positive PCR results among the remaining 1460 culture-negative CSFs [17]. It is unclear why the sensitivity and specificity of Hi PCR in case detection varies between studies. Differences in the sensitivities in PCR primers chosen, methods of specimen processing, DNA extraction and PCR product detection could all account for such discrepancies.
CSF PCR–EIA testing in our study not only detected the culture-positive meningitis cases, but also increased case detection by 74% for Hib meningitis and 50% for Sp meningitis among the culture-negative cases. Since most cases of presumed bacterial meningitis in Asia are culture negative, PCR could significantly enhance bacterial meningitis case detection, especially in areas with presumptive antibiotic use.
To minimize PCR contamination in our laboratory, we instituted several precautions including the use of separate PCR set-up and DNA amplification rooms, use of aerosol-resistant pipette tips and the inclusion of multiple negative controls in each assay. The lack of false-positive CSF PCR–EIA results in culture-negative CSFs with normal indices suggests that PCR contamination was uncommon. Perhaps the reason that bacterial contamination was minimal in this study is because we employed bedside collection of a separate CSF aliquot into a cryovial under sterile conditions by a physician who had received study procedure training. If the specimen had been aliquoted in the laboratory where many plates of bacterial growth are being processed, we may have found PCR contamination to be more of a problem. These data suggest that CSF PCR–EIA testing, in conjunction with CSF culture is a useful adjunct for the diagnosis of childhood bacterial meningitis in surveillance studies. CSF culture will remain important as it provides antibiotic susceptibility and serotype information of isolates for clinicians treating the child, and molecular typing information that will assist public health officers in outbreak control.
Newer PCR methodologies using real-time PCR are available and may be more sensitive than the PCR–EIA method used in this study. However, the latter is easier for field laboratories to use. Real-time PCR instruments contain a luminometer to detect fluorescent signals of PCR products. Such instruments are costly and may not be practical in the field because they rely on uninterrupted electrical supply without power surges. The threshold for a positive PCR result using the PCR ELISA method was a visible colour change. Therefore this PCR method could be performed in remote field sites without the need to use an ELISA plate colorimeter.
The enhanced bacterial detection made possible with the use of PCR–EIA has shown that there is a broad spectrum of clinical presentation of childhood bacterial meningitis. Children with culture-negative, Hi PCR–EIA-positive CSF specimens have a reduced bacterial burden and may present differently from children with culture-positive CSFs. Some of these children had CSF indices indicating inflammation, but we observed 5% without fever, 33% without CNS symptoms, and 9·5% had fever only (no CNS signs or symptoms). Such children may be missed as cases of bacterial meningitis as they fail to show typical signs and symptoms and have less severe CSF inflammation indices. Lumbar punctures may not be performed on such children especially in a busy clinic. These 21 children had a mean CSF WBC of 552×106 cells/l, protein 0·53 g/l, glucose 2·94 mmol/l and all had negative Gram stains. Even if CSF specimens were obtained, in the absence of CSF PCR testing, most clinicians would have diagnosed these children with partially treated, viral or aseptic meningitis or encephalitis. These children developed, in hospital, fewer neurological abnormalities than those with culture-positive meningitis [18, 19].
Studies of bacterial meningitis indicate that prior antibiotic treatment can sterilize CSF and bacterial lipopolysaccharide antigen may not persist in CSF longer than 12–48 h despite ongoing CSF inflammation. It is possible that bacterial DNA may remain detectable in CSF after antibiotic therapy for a finite period only, after which it may not be detected. This may explain the high incidence of probable or suspected bacterial meningitis with abnormal CSF parameters in studies in less developed countries that are culture, antigen and PCR negative [5]. A study involving the quantitative measurement of bacterial DNA using real-time PCR in CSF obtained from serial lumbar punctures from children being treated for bacterial meningitis should be performed to determine the duration of bacterial DNA in CSF during treatment.
Unlike the study from Burkina Faso [17] where >95% of Hib and Sp culture-positive CSFs were also LA positive, our results showed that the LA testing of CSF lacked sensitivity in case detection.
We observed greater prior antibiotic use in culture-negative, PCR–EIA-positive meningitis than culture-confirmed cases. However, more than half of the cases did not report prior antibiotic use. We suspect that the histories obtained from parents were not complete or accurate. In addition, it is possible that they presented early in their illness where the CSF bacterial concentration was below the level of detection by culture and only detectable by PCR–EIA. Our study did not inform us as to whether children with culture-negative, PCR–EIA-positive CSF if left untreated would have eventually presented with fulminate clinical culture-confirmed meningitis. This is because all children in this study with suspected meningitis were admitted to the hospital and given a course of intravenous antibiotics.
Many countries have adopted a WHO rapid Hib disease burden method assessment method [20]. It estimates Hib meningitis incidence using a WHO probable bacterial meningitis definition that defines an abnormal CSF with stringent cut-offs. Our CSF PCR–EIA results show that as many as 26% (10/39) and 23% (3/13) of evaluable Hib and Sp meningitis cases, respectively, would have been missed if this definition alone was used as a case definition. These results compare favourably with those of Asturias et al. [21] that utilized CSF culture and LA testing and found approximately one-quarter of cases of Hib and Sp meningitis had CSF >10 and <100 WBC×106 cells/l. Countries that want to improve bacterial meningitis incidence estimates should consider CSF PCR testing of children with fewer symptoms and abnormal CSF parameters than the WHO definition.
Our results suggest that population-based bacterial meningitis surveillance studies that do not utilize PCR testing of CSF, may not detect a significant proportion of Hib and Sp meningitis cases and therefore underestimate bacterial meningitis incidence by as much as 50–75%. This may be especially true in developing countries with higher rates of prior antibiotic use. As antibiotics are readily available in most developing countries without a prescription, one cannot determine the number of meningitis cases missed by the treatment of a febrile upper respiratory tract infection without CSF obtained. Clinicians in these countries probably miss cases of bacterial meningitis because children present with milder signs and symptoms of meningitis and blood and CSF are not always obtained. For all these reasons, childhood bacterial meningitis incidence rates are likely to be underestimated by small hospital-based, culture-only studies. The use of CSF PCR–EIA testing is an appropriate field technology that improves case detection and increases incidence estimates of Hib and Sp meningitis in surveillance studies of bacterial meningitis.
ACKNOWLEDGEMENTS
We thank all the members of the IVI Invasive Bacterial Disease Surveillance Network in Jeonju, S. Korea, Hanoi, Vietnam and Nanning, China for their tireless efforts in field site development, bacteriological and epidemiological surveillance. In addition, we acknowledge Dr Hai Feng Hwang, Susan Partridge, Jennie Jing, and Dr Joo Yeun Kim, for work in planning the study and development of study operating procedures, data forms, data entry systems, and laboratory database management; and Dr Eunsik Park, and Deok-Ryun Kim, for statistical and database management consultation. We sincerely thank Professor E. Kim Mulholland, Professor Ron Dagan, Dr Jay Wenger, Dr Orin Levine and Professor Zhi Yi Xu for their counsel during development of the IVI multi-country Hib surveillance studies. We are grateful to the medical technologists of the UCLA Center for Vaccine Research for performing the CSF latex agglutination, isolate serotyping and PCR testing. Bill and Melinda Gates Children's Vaccine Program at PATH, GlaxoSmithKline Biologicals, Merck Vaccines, and Wyeth Vaccines supported this study financially.
DECLARATION OF INTEREST
None.
REFERENCES
- 001.Anon.Global Programme for Vaccines and Immunization (GPV). The WHO position paper on Haemophilus influenzae type b conjugate vaccines Weekly Epidemiology Recpord 19987364–68. [PubMed] [Google Scholar]
- 002.Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clinical Microbiology Reviews. 2000;13:302–317. doi: 10.1128/cmr.13.2.302-317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 003.Holloway Y et al. Minimum number of pneumococci required for capsular antigen to be detected by latex agglutination. Journal of Clinical Microbiology. 1992;30:517–519. doi: 10.1128/jcm.30.2.517-519.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 004.Radstrom P et al. Detection of bacterial DNA in cerebrospinal fluid by an assay for simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and streptococci using a seminested PCR strategy. Journal of Clinical Microbiology. 1994;32:2738–2744. doi: 10.1128/jcm.32.11.2738-2744.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 005.Kim JS et al. Incidence of Haemophilus influenzae type b and other invasive diseases in South Korean children. Vaccine. 2004;22:3952–3962. doi: 10.1016/j.vaccine.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 006.Anh DD et al. Haemophilus influenzae type b meningitis among children in Hanoi, Vietnam: epidemiologic patterns and estimates of H. influenzae type b disease durden. American Journal of Tropical Medicine and Hygiene. 2006;74:509–515. [PubMed] [Google Scholar]
- 007.Levine Oet al. Generic protocol for population-based surveillance of Haemophilus influenzae type b. Global Programme for Vaccines and Immunization, WHO/VRD/GEN/95.05, 1996Geneva [Google Scholar]
- 008.Barone MA, McMillan JA. Oski's Pediatrics: Principles and Practices. 3rd edn Vol. 3. New York: Lippincott, Williams & Wilkins; 1999. pp. 2216–2225. , vol. , pp. [Google Scholar]
- 009.Campos J, Murray PR.HaemophilusManual of Clinical Microbiology 6th edn.Washington DC: ASM Press; 1995, pp. 559–560. [Google Scholar]
- 010.Isenberg H, Isenberg H. Essential Procedures for Clinical Microbiology. 1st edn. Washington, DC: ASM Press; 1998. Processing and interpretation of cerebrospinal fluid; pp. 67–126. , pp. [Google Scholar]
- 011.August MH, Huber TW, Sewell KL. Quality Control and Quality Assurance Practices in Clinical Microbiology. 1st edn. Washington, DC: ASM Press; 1990. pp. 1–19. , pp. [Google Scholar]
- 012.van Ketel RJ, de Wever B, van Alphen L. Detection of Haemophilus influenzae in cerebrospinal fluids by polymerase chain reaction DNA amplification. Journal of Medical Microbiology. 1990;33:271–276. doi: 10.1099/00222615-33-4-271. [DOI] [PubMed] [Google Scholar]
- 013.Salo P, Ortqvist A, Leinonen M. Diagnosis of bacteremic pneumococcal pneumonia by amplification of pneumolysin gene fragment in serum. Journal of Infectious Diseases. 1995;171:479–482. doi: 10.1093/infdis/171.2.479. [DOI] [PubMed] [Google Scholar]
- 014.Rerks-Ngarm S et al. Prospective population-based incidence of Haemophilus influenzae type b meningitis in Thailand. Vaccine. 2004;22:975–983. doi: 10.1016/j.vaccine.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 015.Freeman HR et al. The role of polymerase chain reaction in the diagnosis of bacterial meningitis in Vietnam. Annals of Tropical Medicine and Parasitology. 2004;98:65–70. doi: 10.1179/000349804225003046. [DOI] [PubMed] [Google Scholar]
- 016.Singh SC et al. Evaluation of polymerase chain reaction for diagnosing Haemophilus influenzae b meningitis. Annals of Tropical Medicine and Parasitology. 2002;22:347–353. doi: 10.1179/027249302125002010. [DOI] [PubMed] [Google Scholar]
- 017.Parent du Chatelet I et al. Bacterial meningitis in Burkina Faso: surveillance using field-based polymerase chain reaction testing. Clinical Infectious Diseases. 2005;40:17–25. doi: 10.1086/426436. [DOI] [PubMed] [Google Scholar]
- 018.Kaplan SL et al. Association between preadmission oral antibiotic therapy and cerebrospinal fluid findings and sequelae caused by Haemophilus influenzae type b meningitis. Pediatric Infectious Disease. 1986;5:626–632. doi: 10.1097/00006454-198611000-00005. [DOI] [PubMed] [Google Scholar]
- 019.Rothrock SG et al. Pediatric bacterial meningitis: is prior antibiotic therapy associated with an altered clinical presentation? Annals Emergency Medicine. 1992;21:146–152. doi: 10.1016/s0196-0644(05)80149-0. [DOI] [PubMed] [Google Scholar]
- 020.Feikin DR et al. Rapid assessment tool for Haemophilus influenzae type b disease in developing countries. Emerging Infectious Diseases. 2004;10:1270–1276. doi: 10.3201/eid1007.030737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 021.Asturias EJ et al. Meningitis and pneumonia in Guatemalan children: the importance of Haemophilus influenzae type b and Streptococcus pneumoniae. Pan American Journal of Public Health. 2003;14:377–383. doi: 10.1590/s1020-49892003001100002. [DOI] [PubMed] [Google Scholar]


