SUMMARY
Mycobacterium avium subspecies paratuberculosis is the causative agent of Johne's disease, a chronic enteritis in ruminants including cattle, sheep, goats, and farmed deer. Recently, this bacterium has received an increasingly wide interest because of a rapidly growing body of scientific evidence which suggests that human infection with this microorganism may be causing some, and possibly all, cases of Crohn's disease. Recent studies have shown that a high percentage of people with Crohn's disease are infected with M. avium subsp. paratuberculosis; whether the association of this bacterium and Crohn's disease is causal or coincidental is not known. Crohn's disease is a gastrointestinal disease in humans with similar histopathological findings to those observed in the paucibacillary form of Johne's disease in cattle. The search for risk factors in Crohn's disease has been frustrating. However, epidemiologists have gathered enough information that points to an association between M. avium subsp. paratuberculosis and Crohn's disease. This paper reviews epidemiological models of disease causation, the major philosophical doctrines about causation, the established epidemiological criteria for causation, and the currently known epidemiological evidence of M. avium subsp. paratuberculosis as a possible cause of Crohn's disease.
INTRODUCTION
Mycobacterium avium subspecies paratuberculosis is a pathogenic bacteria in the genus Mycobacteria. It is often abbreviated as M. paratuberculosis, M. avium subsp. paratuberculosis or MAP. MAP causes paratuberculosis or Johne's disease, a chronic granulomatous gastroenteritis in ruminants [1]. Johne's disease occurs worldwide and is primarily a disease of domesticated ruminants, including cattle (both beef and dairy), sheep, goats, and farmed deer [2,3]. The host range for Johne's disease has been reported to include wild ruminant species, such as deer [4–7], as well as non-ruminants, such as wild rabbits [8,9], their predators, including foxes and stoats [10], and primates, such as mandrills and macaques [11,12]. The disease is characterized by profuse and intractable diarrhoea, severe weight loss and diagnostic changes in the lining of the small intestine [13,14].
Crohn's disease is a chronic inflammatory disease of the intestines in humans [15]. The disease primarily causes ulcerations of the small and large intestines, although it can affect the digestive system anywhere from the mouth to the anus. Common symptoms of Crohn's disease include severe bouts of watery or bloody diarrhoea, cramping, abdominal pain, fever, weight loss, and bloating [15]. Morphological changes in Crohn's disease include chronic inflammation involving all layers of the intestinal wall (transmural involvement), thickening of involved segments, with narrowing of lumen, linear ulceration of the mucosa, submucosa oedema with elevation of the surviving mucosa, producing a characteristic cobblestone appearance. Crohn's disease in humans has long been suspected of having a mycobacterial cause [1, 16–18]. This proposition was first advanced by Dalziel [19]. According to Clarke [20], the histopathology of Johne's disease ranges from the more common pluribacillary or lepromatous form to the less common paucibacillary or paucimicrobial tuberculoid form like leprosy in humans. Due to the histopathological features of Crohn's disease closely resembling those found in animals with the paucibacillary form of Johne's disease, it has been suggested that the two diseases shared the same aetiology [13,14,21,22]. The objectives of this paper were: (i) to review the epidemiological evidence involving the potential association of MAP with Crohn's disease in humans, and (ii) to determine if causation of Crohn's disease can be inferred based upon the evidence reviewed.
Epidemiology of Johne's disease
Mycobacterium avium subsp. paratuberculosis (MAP) is a member of the M. avium complex [23]. M. avium strains are widely distributed in the environment as well as in birds, animals, and humans [24–26]. M. avium strains do not usually cause disease unless the host is debilitated or immunocompromised. By contrast MAP is a specific pathogen with the ability to cause chronic inflammation of the intestine (Johne's disease) in many species [27–30]. MAP is a well recognized cause of disease and economic loss in dairy herds, and most control programmes have been designed for the dairy industry [31–33]. It is estimated that nearly 40% of United States dairy herds are infected with MAP and that losses to the dairy industry may exceed $1·5 billion per year [34,35]. MAP is most commonly transmitted via the faecal–oral route [36,37]. However, it can also be transmitted in the semen of bulls, in milk (or colostrum), and in utero across the placenta to the newborn calf [2]. Moreover, it has been suggested that MAP can exist within the tissues of animals for years without causing clinical disease [38]. Subclinically or clinically infected animals shed MAP in faeces and milk, enabling dissemination to susceptible calves, the environment, and in retail milk [39]. MAP in milk may survive pasteurization [39]. In the United Kingdom, the United States, and the Czech Republic, MAP has been cultured from 1·6% to 2·8% of units of retail pasteurized cow's milk [39–42], and it has been suggested that live organisms might be transmitted to humans by this route.
Epidemiology of Crohn's disease
Crohn's disease occurs throughout the world, with a prevalence of 161–319 cases/100 000 people in Canada [43]. It is most prevalent in Europe and North America [44]. The disease affects between 400 000 and 600 000 people in North America alone [45]. Prevelance estimates for Northern Europe have ranged from 27–48/100 000 [43]. The incidence of Crohn's disease in North America has been estimated at 6/100 000 per year, and is thought to be similar in Europe, but lower in Asia and Africa [46,47]. The incidence of Crohn's disease in industrialized parts of the world has been reported to be increasing [48–51]. The disorder occurs most frequently among people of European origin, is 3–8 times more common among Jews than among non-Jews [52]. However, this excess risk is not evident in the Jewish population of Israel [53]. Although the disorder can begin at any age, its onset most often occurs between 15 and 30 years of age [54–57].
Satsangi et al. [58] reported that parents, siblings or children of people with Crohn's disease were 3–20 times more likely to develop the disease than the general population. Twin studies show a concordance of greater than 55% for Crohn's disease [59–61]. Mutations in a gene called NOD2/CARD15 are associated with Crohn's disease [62–64], and with susceptibility to certain phenotypes of disease location and activity [65]. The NOD2/CARD15 susceptibility does not apply to Chinese [66], Japanese [67], Korean [68], Tunisian [69] or Turkish [70] patients with Crohn's disease. A susceptibility locus for Crohn's disease has been mapped to chromosome 16 [71]. Three independent studies reported that mutations within the NOD2/CARD15 gene were strongly linked to Crohn's disease in Europeans [62,71,72]. However, Greenstein [21] reported that the presence of a gene that is associated with an increased susceptibility to Crohn's disease does not preclude the possibility that the disease may be caused by an infectious agent. Another study [67], suggested the possibility of genetically identifiable subpopulations having different tendencies to develop Crohn's disease when exposed to the same infectious agent. Recent studies have identified an association between inflammatory bowel disease (IBD) and mutations in yet another gene termed NRAMP1 (also known as SLC11A1) [73]. This gene has been reported to be associated with both Crohn's disease and ulcerative colitis.
Epidemiological models for causation
Epidemiology is the scientific inquiry into the causation of disease; it is the search for the risk factors that cause the effect or the disease [74]. In this search, various models or theories for causation have been developed over the years in an attempt to explain the interaction of risk factors and their effect on disease; Models are purposely simplified representations of that interaction [75]. The various models of causation include: epidemiological triad/triangle [76,77], web of causation [78], wheel of causation [79] and Rothman's causal pie [75].
Epidemiological triangle/triad
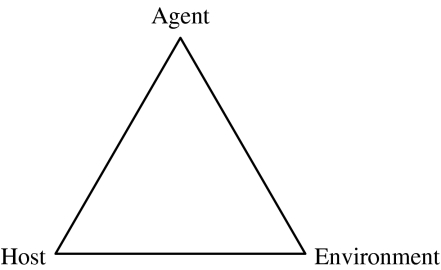
This model makes the agent a component of causation along with the host and environment (Fig. 1). The model implies that all components are equally important in disease causation and that a change in any one of them would change the frequency of disease. The model applies to both infectious or non-infectious diseases. For instance, in Johnne's disease the agent would be the bacterium, MAP; host factors include non-immune, weakened resistance, poor nutrition, age, gender; and environmental factors include animal stocking density, poor environmental conditions (such as temperature, humidity, wind velocity, precipitation, poor housing as in crowded conditions, poor ventilation, and bad sanitation).
Fig. 1.
Epidemiological triangle/triad [77].
Wheel of causation
The wheel model places genetic factors in the core of the wheel and varies the size of the host and environmental components depending on their influence in the particular disease process [78]. Surrounding the host is the total environment divided into the biological, physical, and social environments (Fig. 2). These divisions, of course, are not true divisions – there are considerable interactions among the environment types. Although it is a general model, the wheel of causation does illustrate the multiple aetiological factors of human infectious diseases [79]. According to Jantchou et al. [80], many environmental factors for IBD have been investigated, including infectious agents, diet, drugs, stress and social status. Among these factors, MAP, oral contraceptives and antibiotics could play a role in Crohn's disease [80–84]. Sicilia et al. [81] reported that the pathogenesis of IBD probably involves an interaction between genetic and environmental factors: cigarette smoking, appendectomy and oral contraceptives are the factors most frequently linked to its aetiology.
Fig. 2.
Wheel of causation [79].
Web of causation
This model refers to the ‘web’ of interconnected factors which lead to disease. The web of causation merely reflects the fact that there is a complex mixture or a ‘web’ of factors that can cause disease [78]. Many aetiological factors for Crohn's disease have been suggested, including autoimmune, genetic, dietary components plus various infectious agents including MAP [82–84].
Causal pie model
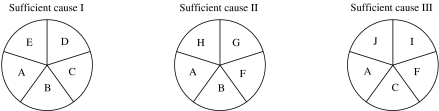
The main causal model used by epidemiologists today is Rothman's ‘pies’ [75]. The idea is that a sufficient causal complex (a pie) is represented by the combination of several component causes (slices of the pie) (Fig. 3). A set of component causes occurring together may complete the ‘pie’, creating a sufficient cause and thus initiating the disease process. Rothman & Greenland [75] define both ‘necessary’ cause and ‘sufficient’ cause. A ‘sufficient’ cause is one that always results in disease [75], while a ‘necessary’ cause is one that must be present but might not be the cause of a disease to develop. In other words, a necessary cause is a component cause that is a member of every sufficient cause [85–91].
Fig. 3.
Causal pie model [92]. This illustration shows a disease that has three sufficient causal complexes, each having five component causes. A is a necessary cause since it appears as a member of each sufficient cause. B, C, and F are not necessary since they fail to appear in all three sufficient causes.
Rothman [93] defines ‘a cause of a disease event as an event, condition, or characteristic that preceded the disease event and without which the disease event either would not have occurred at all or would not have occurred until a later time’. If disease does not develop without the factor being present, then the causative factor is termed ‘necessary’. If the disease always results from the factor, then the causative factor is termed ‘sufficient’. In reference to Rothman's causal pie model, the possibility exists that MAP is a ‘necessary’ but not a ‘sufficient’ cause of Crohn's disease. As a necessary cause, MAP is required to be present to trigger the inflammatory reaction seen in Crohn's disease. However, not being a sufficient cause means that MAP cannot cause Crohn's disease alone but acts in concert with immune dysfunction and genetic susceptibility in order for Crohn's disease to occur [82,84]. Therefore, not every one with the presence of MAP in the intestine would suffer from Crohn's disease. Moreover, the failure to detect MAP in some cases of Crohn's disease may not necessarily indicate that MAP is absent. Low specificity and sensitivity of the test, among other factors, may explain the inability to detect MAP in some patients [94]. It has been reported that the sensitivity of nucleic-acid based tests is influenced by the bacterial burden, as exemplified by the compromised sensitivity of PCR-based assays for sputum smear-negative tuberculosis [95–97]. By extension, assays that reliably detect abundant MAP organisms in livestock with Johne's disease may not provide sufficient sensitivity to study human Crohn's disease [94].
The major philosophical doctrines about causation
The two major philosophical doctrines that have influenced modern science include inductivism and refutationism.
Inductivism
This doctrine holds that science proceeds from observation to theory, beginning with observations derived from experiments, and extrapolating from these to general laws [76, 98–102]. Bacon's vision of the ‘true induction’ comprises three interrelated stages: (i): Observation and Experiment (ii) Classification and Concept Formation and (iii) Eliminative Induction and Causal Inference [102]. The traditional view of science is that induction – the formation of a hypothesis based on observation – is cardinal to the scientific method. However Hume [103], the deductivist and others [104] argued that a hypothesis that is derived by induction is flawed because it can be refuted by the first observation that proves an exception. Deduction refers to reasoning that proceeds from the general to the particular and relies on general theory to infer particular conclusions [76]. A century after Hume, Mill [105] proposed a canon of five methods to infer causes from their effects incorporating some of the ideas that had been proposed earlier by Bacon [106]. The canons of Mill [107] have evolved into inferential criteria that are in use today.
Refutationism or falsificationism
This theory is a rival account of the processes involved in scientific research to inductivism. While inductivism holds that science proceeds from observation to theory, beginning with observations derived from experiments, and extrapolating from these to general laws, falsificationism suggests that science proceeds in the opposite direction, beginning with scientific theories or ‘conjectures’, and then conducting experiments and eliminating those theories that are falsified by results [108–111]. Karl Popper, one of the most influential philosophers of science of the twentieth century [112], followed Hume in rejecting induction, claiming that it is always possible to produce a theory to fit any set of observations [113]. According to Karl Popper ‘Our belief in a hypothesis can have no stronger basis than our repeated unsuccessful critical attempts to refute it’ [114]. Popper and other scientists believed that causation is established through a process of conjecture and refutation, and that science advances only by disproofs [113,115,116]. Popper insisted strictly on deduction, allowing the sole capability of science to be the falsification of prior hypotheses (the so-called hypothetico-deductive method), rejecting any place for verification [108].
Relationship between association and causation
Association is an identifiable relationship between an exposure and disease. Association implies that exposure might cause disease [75,78]. Epidemiologists infer causation based upon the association and several other factors [80–82]. Causation implies that there is a true mechanism that leads from exposure to disease [77, 80, 82, 117–120]. However, the presence of an association does not necessarily mean that the relationship is causal [74,77,82].
Deriving causal inferences
The variation among the viewpoints of epidemiologists with regard to causality is rooted in the variation among philosophical viewpoints. However, Hill's criteria provide interpretive guidelines for evaluating epidemiological evidence. Hill established the following classic operational causal criteria: strength of association, consistency, specificity, temporality, biological plausibility, dose–response effect, coherence, experimental evidence, and analogy [121–124]. According to Hill [121], not all of these guidelines will be applicable in all situations and that there may be times when we wish to conclude that a putative cause–effect relationship is real even when some of the criteria are not met. According to Rothman [125], only the criterion of temporality is a sine qua non for causality. If the putative cause did not precede the effect, that indeed is indisputable evidence that the observed association is not causal. Other than that one condition, there is no necessary or sufficient criterion for determining whether an observed association is causal [125]. This conclusion is in accordance with the view of Hume, Popper, and others that causal inferences cannot attain the certainty of logical deductions [103,110,111]. There is no explicit consensus about what constitutes sufficient evidence to establish causation from association.
Established epidemiological criteria for causation
The established epidemiological criteria for causation are meant to be guidelines in assisting judgement as to whether an association is causal or not. Criteria of causation refer to a set of criteria used to assess the strength of a relation between a cause and an effect, and provide a way of reaching jugdements on the likelihood of an association being causal. The most widely cited list of causal criteria, originally posed as a list of standards, is attributed to Hill [121], who adapted them from the U.S. Surgeon General's 1964 report on smoking and health [126]. Most of these lists stem from the canons of inference described by Mill [107] and the rules given by Hume [103]. The widely adopted criteria that have been refined by several scientists [77,78,109,127,128] include: (i) strength of association; (ii) consistency of effect; (iii) specificity of effect; (iv) temporality; (v) biological gradient or dose response; (vi) biological plausibility.
Strength of association
Strength of association refers to the extent to which a supposed cause and effect are related and should not be confused with statistical significance [109]. The most common measure of strength of association is relative risk or rate ratio [109]. Other measures of association in epidemiology include the odds ratio, a correlation coefficient and attributable risk [109]. According to Chamberlin et al. [129], technical advances have allowed the identification and/or isolation of MAP from a significantly higher proportion of Crohn's disease tissues than from controls. These methodologies include: (i) improved culture techniques; (ii) development of MAP-specific polymerase chain reaction assays; (iii) development of a novel in situ hybridization method; (iv) efficacy of macrolide and anti-mycobacterial drug therapies; and (v) discovery of Crohn's disease-specific seroreactivity against two specific MAP recombinant antigens [129]. Several studies [130,131] reported that 50% of Crohn's disease patients and 22% of ulcerative colitis patients were MAP positive and MAP was not cultured from the non-IBD patients. Some researchers suggest that all of IBD may be due to MAP [130,131]. Chiodini et al. [132], described the isolation of a slow-growing, mycobactin-dependent Mycobacteria species from the intestinal mucosa of Crohn's disease patients but not from control tissue.
Consistency of effect
This epidemiological criteria refers to the fact that an association is found in many studies despite different circumstances, research designs, or time-periods [78]. Relationships that are demonstrated in multiple studies are more likely to be causal than those that are not. Several studies conducted at different times by different research methods have reported on the isolation of MAP from patients with Crohn's disease [129, 133–138]. MAP has been found in Crohn's disease patients by genetic probes (including both DNA, and RNA) [94]. The insertion element IS900, found at 14 to 18 copies per genome has been shown to be genomically specific for MAP [138] and, has been widely used as a target for PCR [129, 131, 139–146].
Specificity of effect
Specificity describes the precision with which a factor will predict the occurrence of a specific disease; it adds plausibility to the causal claim but, if absent, does not detract from it [111]. Routine culture of MAP from Crohn's disease patients' tissues is difficult because when present MAP is commonly in spheroplast form (cell wall deficient), which does not thrive in standard culture conditions [129,143]. It has also proved difficult to detect MAP in Crohn's disease tissues by other methods: the mycobacterial cell wall Ziehl–Neelsen (ZN) staining techniques, first described in 1882 [147,148] have not shown MAP in humans because MAP exists in the cell-wall-deficient form [21]; serology studies have been beset by problems of non-specificity because of antigen cross reactivity [149], although more recent studies have reported a specific high immune reactivity to recombinant MAP antigens in Crohn's patients [150,151]. These difficulties reflect the fact that MAP microorganisms when present in Crohn's disease are few in number, relative to bovine cases of MAP infection (Johne's disease) [152]. An assay for MAP in Crohn's disease must be able to specifically detect small numbers of organsisms with tissue, near or below the threshold of microscopic detection [94]. Molecular methods have been used to determine the prevalence of MAP in cases of Crohn's disease [153]. An important limitation of studies looking for novel pathogens is that information about the sensitivity and specificity of assays applied is generally lacking [94]. In separate studies, it has been shown that IS900 element is genomically specific for MAP [139] and that IS900 sequences from a heterogenous collection of MAP are invariant [154]. According to Sechi et al. [138], MAP has been identified by in situ hybridization to the MAP-specific IS900 gene in tissue specimens of Crohn's disease. However, despite these favourable considerations, the IS900-based in situ probe was prone to non-specific hybridization, compromising the utility of IS900-based in situ hybridization and indirect in situ PCR [155]. Jeyanathan et al. [94], reported that the alternative means of increasing specificity and sensitivity involves the use of rRNA-specific oligonucleotide probe in situ hybridization. Probes targeting rRNA provided excellent specificity resulting in forms that were morphologically consistent with ZN-positive organisms on adjacent sections. Ryan et al. [146], reported the detection of MAP DNA in 40% of Crohn's cases where microdissected granulomas were examined. However, only half of the granuloma-positive cases had corresponding whole tissue sections that were positive for MAP. The greater detection rate of MAP in laser capture microdissection (LCM) isolated granulomas compared with whole tissue sections may have been attributable to better targeting of MAP DNA in granulomas – PCR may suffer loss of sensitivity because of the potential dilutional effect of the large quantities of non-target DNA found in whole tissue sections. Failure to detect MAP in some studies may have been attributable to inefficient amplification of long sequences (>250 bp) [156].
Temporality
Temporality refers to the necessity that the cause precedes the effect in time [128]. Causation is not possible without the cause occurring before the effect [93]. Data exist that indicate that temporal sequence criteria have been fulfilled for the association between Crohn's disease and MAP [157,158]. In a study by Van Kruiningen et al. [157], a goat was infected with MAP organism taken from a human patient with Crohn's disease and showed progression to Johne's disease. A 1991 report found that 24-day-old specific pathogen-free Leghorn-Cochin chicks could be infected by multiple exposure routes using the same MAP strain (‘Linda’) [158].
Dose–response relationship
A dose–response effect is present when the effect increases with the dose or level of exposure. In a study conducted by Schwartz et al. [159], the intestinal mucosal layer from patients with IBD had high numbers of bacteria compared with people without Crohn's disease, however, there was no correlation between the numbers of bacteria present and either the degree of inflammation or the use of anti-inflammatory agents or sulfasalazine compounds [159]. This study suggests that a demonstration of dose–response criterion may not be applicable to a relationship between MAP and Crohn's disease. The pivotal event that convinced a totally sceptical gastroenterological community to accept that Helico bacter pylori was the aetiological factor in peptic ulcers was the cure rate that was achieved when the putative H. pylori infection was treated with appropriate antibiotics [21]. Similarly, Greenstein [21] suggested that the failure to cure IBD with anti-MAP antibiotics is the main impediment to convincing a sceptical gastroenterological community that MAP is zoonotic. Possible reasons that could account for this inability to cure patients with Crohn's disease include, the use of the wrong antibiotics and lack of satisfactory performed studies that are prospective, randomized, double blinded and placebo controlled, that have been performed using acknowledged satisfactory anti-MAP antibiotics [21]. Recently, Greenstein et al. [160], demonstrated that methotrexate and 6-mercaptopurine inhibit MAP growth in vitro. However, the dosages of methotrexate and 6-mercaptopurine in clinical use have not been titrated according to standard antibiotic conventions [160].
Biological plausibility
A hypothesized effect is biologically plausible if it makes sense in the context of current biological knowledge [75]. By the 1930s, Johne's disease was found to be caused by an odd bacteria named Mycobacteria paratuberculosis. This organism is from the same family of bacteria which cause tuberculosis and leprosy. Current concepts regarding the cause of Crohn's disease emphasize a dysfunction of the immune system resulting in a prolonged and intense process of inflammation [161–165]. The damage to the bowel appears to be due to this inflammatory process [163–165]. MAP is thought to produce disease by over-stimulating the immune system. The bacterium lives inside the cells of the host, where it divides only once about every 2–12 h. By way of contrast, other bacteria in the gut such as Escherichia coli, Salmonella spp., Shigella spp., divide about once every 20 min. There are no toxins or poisons produced by MAP. Disease happens when the immune system recognizes the ‘foreign’ proteins of the bacteria, even inside a living cell and mounts a furious attack [161–163]. The immune ‘attack’ focuses on the infected cells in the mucosal layer of the digestive system and results in massive inflammation, as well as ulcers, diarrhoea and weight loss [159, 161–163].
CONCLUSION
This paper has attempted to highlight current scientific evidence in regard to fulfilling the epidemiological criteria for a causal association between MAP and Crohn's disease. We were able to demonstrate that data exist that show that the MAP Crohn's disease phenomenon has fulfilled at least four (strength of association, consistency of effect, temporality and biological plausibility) of the six epidemiological causal criteria outlined by Hill.
In summary, the current epidemiological evidence strongly supports the conjecture that Crohn's disease is caused by MAP especially for those who believe in the theory of inductivism. Several studies that demonstrated scientific evidence, including temporality, necessary to infer a causal association between MAP and Crohn's disease were highlighted. For the followers of Popper who believe in falsification/deductivism, whether enough observations or experiments have been conducted to falsify the MAP/Crohn's disease phenomenon is a matter of personal judgement. Moreover, there are people who believe that studies can falsify a theory only to a certain degree.
ACKNOWLEDGEMENTS
The authors thank the Department of Chemistry, Biochemistry, and Molecular Biology, and the Department of Veterinary and Microbiological Sciences of North Dakota State University, for their support.
DECLARATION OF INTEREST
None.
REFERENCES
- Harris JE, Lammerding AM. Crohn's disease and Mycobacterium avium subsp. paratuberculosis: current issues. Journal of Food Protection. 2001;64:2103–2110. doi: 10.4315/0362-028x-64.12.2103. [DOI] [PubMed] [Google Scholar]
- Tiwari A et al. Johne's disease in Canada [Review Article] Canadian Veterinary Journal. 2006;47:874–882. [PMC free article] [PubMed] [Google Scholar]
- Kennedy DJ, Benedictus G. Control of Mycobacterium avium subsp. paratuberculosis infection in agricultural species. Review of Science and Technology. 2001;20:151–179. doi: 10.20506/rst.20.1.1274. [DOI] [PubMed] [Google Scholar]
- Chiodini RJ, Van Kruiningen HJ. Eastern white-tailed deer as a reservoir of ruminant paratuberculosis. Journal of the American Veterinary Medical Association. 1983;182:168–169. [PubMed] [Google Scholar]
- Buergelt CD, Ginn PE. The histopathologic diagnosis of subclinical Johne's disease in North American bison (Bison bison) Veterinary Microbiology. 2000;77:325–331. doi: 10.1016/s0378-1135(00)00317-5. [DOI] [PubMed] [Google Scholar]
- Cook WE et al. Radiometric culture of Mycobacterium avium subsp. paratuberculosis from the faeces of tule elk. Journal of Wildlife Diseases. 1997;33:635–637. doi: 10.7589/0090-3558-33.3.635. [DOI] [PubMed] [Google Scholar]
- Manning EJB et al. Testing for Mycobacterium avium subsp. paratuberculosis infection in asymptomatic free-ranging tule elk from an infected herd. Journal of Wildlife Diseases. 2003;39:323–328. doi: 10.7589/0090-3558-39.2.323. [DOI] [PubMed] [Google Scholar]
- Greig A et al. Paratuberculosis in wild rabbits (Oryctolagus cuniculus) Veterinary Record. 1997;140:141–143. doi: 10.1136/vr.140.6.141. [DOI] [PubMed] [Google Scholar]
- Beared PM et al. Natural paratuberculosis infection in rabbits in Scotland. Journal of Comparative Pathology. 2001;124:290–299. doi: 10.1053/jcpa.2001.0466. [DOI] [PubMed] [Google Scholar]
- Beard PM et al. Paratuberculosis infection of nonruminant wildlife in Scotland. Journal of Clinical Microbiology. 2001;39:1517–1521. doi: 10.1128/JCM.39.4.1517-1521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure HM et al. Mycobacterium paratuberculosis infection in a colony of stumptail macaques (Macaca arctoides) Journal of Infectious Diseases. 1987;155:1011–1019. doi: 10.1093/infdis/155.5.1011. [DOI] [PubMed] [Google Scholar]
- Zwick LS et al. Paratuberculosis in a mandrill (Papio sphinx) Journal of Veterinary Diagnostic Investigation. 2000;14:326–328. doi: 10.1177/104063870201400409. [DOI] [PubMed] [Google Scholar]
- Grant IR, Holland CV. Zoonotic potential of Mycobacterium paratuberculosisModern Perspectives on Zoonoses Dublin: Royal Irish Academy; 1997, pp. 75–83. [Google Scholar]
- Collins MT et al. Results of multiple diagnostic tests for Mycobacterium avium subsp. paratuberculosis in patients with inflammatory bowel disease and in controls. Journal of Clinical Microbiology. 2000;38:4373–4381. doi: 10.1128/jcm.38.12.4373-4381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauer SB. Inflammatory bowel disease. New England Journal of Medicine. 1996;334:841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- El Zaatari FA, Osato MS, Graham DY. Aetiology of Crohn's disease: the role of Mycobacterium avium paratuberculosis. Trends in Molecular Medicine. 2001;7:247–252. doi: 10.1016/s1471-4914(01)01983-9. [DOI] [PubMed] [Google Scholar]
- Hulten K et al. Identification of cell wall deficient forms of M. avium subsp. paratuberculosis in paraffin embedded tissues from animals with Johne's disease by in situ hybridization. Journal of Microbiological Methods. 2000;42:185–195. doi: 10.1016/s0167-7012(00)00185-8. [DOI] [PubMed] [Google Scholar]
- Quirke P. Antagonist. Mycobacterium avium subspecies paratuberculosis is a cause of Crohn's disease. Gut. 2001;49:757–760. doi: 10.1136/gut.49.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel TK. Chronic intestinal enteritis. British Medical Journal. 1913;ii:1068–1070. [Google Scholar]
- Clarke CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. Journal of Comparative Pathology. 1997;116:217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- Greenstein RJ. Is Crohn's disease caused by a mycobacterium? Comparison with leprosy, tuberculosis, and Johne's disease. Lancet Infectious Diseases. 2003;3:507–514. doi: 10.1016/s1473-3099(03)00724-2. [DOI] [PubMed] [Google Scholar]
- Moss MT et al. Polymerase chain reaction detection of Mycobacterium paratuberculosis and Mycobacterium avium subsp. silvaticum in long term cultures from Crohn's disease and control tissues. Gut. 1992;33:1209–1213. doi: 10.1136/gut.33.9.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel MF, Krichevsky M, Levyfrebault VV. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. International Journal of Systematic Bacteriology. 1990;40:254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- Hermon-Taylor J. Protagonist. Mycobacterium avium subspecies paratuberculosis is a cause of Crohn's disease. Gut. 2001;49:755–756. doi: 10.1136/gut.49.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primm TP, Lucero CA, Falkinham JO. Health impacts of environmental mycobacteria. Clinical Microbiology Reviews. 2004;17:98–106. doi: 10.1128/CMR.17.1.98-106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinski E, Rynearson TK. Mycobacteria in soil and their relationship to disease associated strains. American Review of Respiratory Disease. 1968;97:1032–1037. doi: 10.1164/arrd.1968.97.6P1.1032. [DOI] [PubMed] [Google Scholar]
- Chiodini RJ, Van Kruiningen HJ, Merkal RS. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Veterinarian. 1984;74:218–262. [PubMed] [Google Scholar]
- Cocito C et al. Paratuberculosis. Clinical Microbiology Reviews. 1994;7:328–345. doi: 10.1128/cmr.7.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris ND, Barletta RG. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clinical Microbiology Reviews. 2001;14:489–512. doi: 10.1128/CMR.14.3.489-512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning EJ, Collins MT. Mycobacterium avium subspecies paratuberculosis: pathogen, pathogenicity and diagnosis. Review of Science and Technology. 2001;20:133–150. doi: 10.20506/rst.20.1.1275. [DOI] [PubMed] [Google Scholar]
- Goodger WJ et al. Epidemiological study of on-farm management practices associated with prevalence of Mycobacterium paratuberculosis infections in dairy cattle. Journal of the American Veterinary Medical Association. 1996;208:1877–1881. [PubMed] [Google Scholar]
- Johnson-Iferulundu Y, Kanneene JB. Distribution and environmental risk factors for paratuberculosis in dairy cattle herds in Michigan. Journal of the American Veterinary Medical Association. 1999;60:589–596. [PubMed] [Google Scholar]
- Ott SL, Wells SJ, Wagner BA. Herd-level economic losses associated with Johne's disease on US dairy operations. Preventive Veterinary Medicine. 1999;40:179–192. doi: 10.1016/s0167-5877(99)00037-9. [DOI] [PubMed] [Google Scholar]
- Jones RL, Milner AR, Wood PR. Johne's Disease: Current Trends in Research, Diagnosis and Management. Commonwealth Science and Industrial Research Organization; Melbourne, Victoria, Australia: 1989. Review of the exonomic impact of Johne's Disease in the United States; pp. 46–50. , pp. [Google Scholar]
- Stabel JR. Johne's disease: a hidden trait. Journal of Dairy Science. 1998;81:283–288. doi: 10.3168/jds.S0022-0302(98)75577-8. [DOI] [PubMed] [Google Scholar]
- Fort Collins, Colorado: Centers for Epidemiology and Animal Health, United States Department of Agriculture; 1999. p. 4. United States Department of Agriculture. Info sheet – veterinary services: what do I need to know about Johne's disease in beef cattle? , pp. [Google Scholar]
- Reed C et al. Environmental risk factors for infection with Mycobacterium avium complex. American Journal of Epidemiology. 2006;164:32–40. doi: 10.1093/aje/kwj159. [DOI] [PubMed] [Google Scholar]
- Çetinkaya B, Egan K, Morgan KL. An abbattoir-based study of the prevalence of subclinical Johne's disease in adult cattle in south west England. Epidemiology and Infection. 1996;16:373–379. doi: 10.1017/s0950268800052705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar D et al. IS900 PCR to detect Mycobacterium paratuberculosis in retail supplies of whole pasteurized cowsmilk in England and Wales. Applied and Environmental Microbiology. 1996;62:3446–3452. doi: 10.1128/aem.62.9.3446-3452.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant IR, Ball HJ, Rowe MT. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy processing establishments in the United Kingdom. Applied and Environmental Microbiology. 2002;68:2428–2435. doi: 10.1128/AEM.68.5.2428-2435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayele WY et al. Mycobacterium avium subspecies paratuberculosis cultured from locally and commercially pasteurized cow's milk in the Czech Republic. Applied and Environmental Microbiology. 2005;71:1210–1214. doi: 10.1128/AEM.71.3.1210-1214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson JL et al. Detection of viable Mycobacterium avium subsp. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. Journal of food protection. 2005;68:966–972. doi: 10.4315/0362-028x-68.5.966. [DOI] [PubMed] [Google Scholar]
- Bernstein CN et al. The epidemiology of inflammatory bowel disease in Canada: A population-based study. American Journal of Gastroenterology. 2006;101:1559–1568. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 2000. p. 23. Scientific Committee on Animal Health and Animal Welfare. Possible links between Crohn's disease and paratuberculosis. SANCO/B3/R16/2000 European Commission Directorate-General Health & Consumer Protection Directorate B – Scientific Health Opinions Unit B3. Adopted 21 March . , pp.
- Loftus EV, Schoenfeld P, Sandborn WJ. The epidemiology and natural history of Crohn's disease in population-based patient cohorts from North America: a systematic review. Alimentary Pharmacology and Therapeutics. 2002;16:51–60. doi: 10.1046/j.1365-2036.2002.01140.x. [DOI] [PubMed] [Google Scholar]
- Hiatt RA, Kaufman L. Epidemiology of inflammatory bowel disease in a defined northern California population. Western Journal of Medicine. 1988;149:541–546. [PMC free article] [PubMed] [Google Scholar]
- Moum B et al. Incidence of Crohn's disease in four counties in southeastern Norway, 1990–1993 Scandinavian Journal of Gastroenterology. 1996;31:355–361. doi: 10.3109/00365529609006410. . A prospective population-based study. The Inflammatory Bowel South-Eastern Norway (IBSEN) Study Group of Gastroenterologists. [DOI] [PubMed] [Google Scholar]
- Calkins M, Mendeloff AI. Epidemiology of inflammatory bowel disease. Epidemiological Reviews. 1986;8:60–91. doi: 10.1093/oxfordjournals.epirev.a036296. [DOI] [PubMed] [Google Scholar]
- Loftus EV, Jr. et al. Crohn's Disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- Hermon-Taylor J, Rachmilewitz D Inflammatory Bowel Diseases. London: Kluwer Academic Publishers; 1994. pp. 51–57. , pp. [Google Scholar]
- Armitage E et al. Increasing incidence of both juvenile-onset Crohn's disease and ulcerative colitis in Scotland. European Journal of Gastroenterology and Hepatology. 2001;13:1439–1447. doi: 10.1097/00042737-200112000-00007. [DOI] [PubMed] [Google Scholar]
- Podolsky DK. Inflammatory bowel disease. New England Journal of Medicine. 2002;346:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- Niv Y, Abuksis G, Fraser GM. Epidemiology of Crohn's disease in Israel: a survey of Israeli kibbutz settlements. American Journal of Gastroenterology. 1999;94:2961–2965. doi: 10.1111/j.1572-0241.1999.01371.x. [DOI] [PubMed] [Google Scholar]
- Loftus EV., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Card T, Hubbard R, Logan RFA. Mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology. 2003;125:1583–1590. doi: 10.1053/j.gastro.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Shivananda SJ et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996;39:690–697. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus A et al. Incidence of Crohn's disease in Stockholm county 1955–1989. Gut. 1997;41:480–486. doi: 10.1136/gut.41.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satsangi J, Jewell DP, Bell JI. The genetics of inflammatory bowel disease. Gut. 1997;40:572–574. doi: 10.1136/gut.40.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysk C et al. Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988;29:990–996. doi: 10.1136/gut.29.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orholm M et al. Concordance of inflammatory bowel disease among Danish twins. Results of a nationwide study. Scandinavian Journal of Gastroenterology. 2000;35:1075–1081. doi: 10.1080/003655200451207. [DOI] [PubMed] [Google Scholar]
- Thompson NP et al. Genetics versus environment in inflammatory bowel disease: results of a British twin study. British Medical Journal. 1996;312:95–96. doi: 10.1136/bmj.312.7023.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y et al. Frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Fielding JF. The relative risk of inflammatory bowel disease among parents and siblings of Crohn's disease patients. Journal of Clinical Gastroenterology. 1986;8:655–657. doi: 10.1097/00004836-198612000-00013. [DOI] [PubMed] [Google Scholar]
- van Heel DA et al. Inflammatory bowel disease: Progress toward a gene. Canadian Journal of Gastroenterology. 2000;14:207–218. doi: 10.1155/2000/361309. [DOI] [PubMed] [Google Scholar]
- Cuthbert AP et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002;122:867–874. doi: 10.1053/gast.2002.32415. [DOI] [PubMed] [Google Scholar]
- Leong RW et al. NOD2/CARD15 gene polymorphisms and Crohn's disease in the Chinese population. Alimentary Pharmacology and Therapeutics. 2003;15:1465–1470. doi: 10.1046/j.1365-2036.2003.01607.x. [DOI] [PubMed] [Google Scholar]
- Inoue N et al. Lack of common NOD2 variants in Japanese patients with Crohn's disease. Gastroenterology. 2002;123:86–91. doi: 10.1053/gast.2002.34155. [DOI] [PubMed] [Google Scholar]
- Lee GH et al. Frequency analysis of NOD2 gene mutations in Korea patients with Crohn's disease. The Korean Journal of Gastroenterology. 2005;45:162–168. [PubMed] [Google Scholar]
- Zouten-Mekki L et al. Card15/NOD2 in a Tunisian population with Crohn's disease. Digestive Diseases Sciences. 2005;50:130–135. doi: 10.1007/s10620-005-1290-0. [DOI] [PubMed] [Google Scholar]
- Uyar FA et al. Distribution of common CARD15 variants in patients with sporadic Crohn's disease: Cases from Turkey, 2006. Digestive Disease Science. 2006;51:706–710. doi: 10.1007/s10620-006-3195-y. [DOI] [PubMed] [Google Scholar]
- Hugot JP et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Hampe J et al. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet. 2001;16:1925–1928. doi: 10.1016/S0140-6736(00)05063-7. [DOI] [PubMed] [Google Scholar]
- Kojima Y et al. Inflammatory bowel disease is associated with a novel promoter polymorphism of natural resistance-associated macrophage protein 1 (NRAMP1) gene. Tissue Antigens. 2001;58:379–384. doi: 10.1034/j.1399-0039.2001.580606.x. [DOI] [PubMed] [Google Scholar]
- Parascandola M, Weed DL. Causation in epidemiology. Journal of Epidemiology and Community Health. 2001;55:905–912. doi: 10.1136/jech.55.12.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Causation and causal inference in epidemiology. American Journal of Public Health. 2005;95:S144–S150. doi: 10.2105/AJPH.2004.059204. [DOI] [PubMed] [Google Scholar]
- Last JM. A Dictionary of Epidemiology. 4th edn. New York: Oxford University Press; 2001. p. 196. , pp. [Google Scholar]
- Torrence ME. Understanding Epidemiology. St Louis; MO: Mosby Inc.: 1997. pp. 133–151. , pp. [Google Scholar]
- Krieger N. Epidemiology and the web of causation: has anyone seen the spider. Social Science and Medicine. 1994;39:887–903. doi: 10.1016/0277-9536(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Mausner JS, Bahn AK. Epidemiology: An Introductory Text. Philadelphia, PA: W. B. Saunders; 1986. [Google Scholar]
- Jantchou P, Monnet E, Carbonnel F. Environmental risk factors in Crohn's Disease and ulcerative colitis (excluding tobacco and appendicectomy) Gastroenterologie Clinique et Biologique. 2006;30:859–867. doi: 10.1016/s0399-8320(06)73333-4. [DOI] [PubMed] [Google Scholar]
- Sicilia B et al. Environmental risk factors and Crohn's Disease: a population based, case-control study in Spain. Digestive and Liver Disease. 2001;33:762. doi: 10.1016/s1590-8658(01)80693-9. [DOI] [PubMed] [Google Scholar]
- Chiodini RJ, Rossiter CA. Paratuberculosis: A potential zoonosis. Veterinary Clinics of North America. 1996;12:457–467. doi: 10.1016/s0749-0720(15)30417-5. [DOI] [PubMed] [Google Scholar]
- Dumonceau JM et al. No Mycobacterium paratuberculosis found in Crohn's Disease using polymerase chain reaction. Digestive Diseases and Sciences. 1996;41:421–426. doi: 10.1007/BF02093838. [DOI] [PubMed] [Google Scholar]
- El Zaatari FA et al. Nucleotide sequence analysis and seroreactivities of the 65K heat shock protein form Mycobacterium paratuberculosis. Clinical and Diagnostic Laboratory Immunology. 1995;2:657–664. doi: 10.1128/cdli.2.6.657-664.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmreck TC. An Introduction to Epidemiology, 3rd edn. Sudbury, MA: Jones & Bartlett Publishers; 2002. [Google Scholar]
- Greenland S. Evolution of Epidemiological Ideas: Annotated Readings on Concepts and Methods. Epidemiological Resources Inc.; Chestnut Hill, MA, USA: 1987. [Google Scholar]
- Gordis L.EpidemiologyPhiladelphia: Saunders; 2000 [Google Scholar]
- Elwood M. Causal Relationships in Medicine: A Practical System for Critical Appraisal. New York: Oxford University Press; 1988. [Google Scholar]
- Lilienfeld DE, Stolley PD. Foundations of Epidemiology. 3rd edn. New York: Oxford University Press; 1994. [Google Scholar]
- Last JMA.Dictionary of Epidemiology2nd ednNew York: Oxford University Press; 1988 [Google Scholar]
- Kleinbaum DG, Kupper LL, Morgenstern HL. Epidemiological Research: Principles and Quantitative Methods. Belmont, CA: Lifetime Learning Publications; 1982. [Google Scholar]
- Rothman KJ, Greenland S. Modern Epidemiology. 2nd edn. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- Rothman KJ, Schottenfield D, Fraumeni JF. Cancer Epidemiology and Prevention. Philadelphia: Saunders; 1982. Causation and Causal Inference. [Google Scholar]
- Jeyenathan M et al. Evaluation of in situ methods used to detect Mycobacterium avium subsp. paratuberculosis in samples from patients with Crohn's disease. Journal of Clinical Microbiology. 2006;44:2942–2950. doi: 10.1128/JCM.00585-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes BA. Critical Assessment of gene amplification approaches on the diagnosis of tuberculosis. Immunology Investigations. 1997;26:105–116. doi: 10.3109/08820139709048919. [DOI] [PubMed] [Google Scholar]
- Ieven M, Goossens H. Relevance of nucleic acid amplification techniques for diagnosis of respiratory tract infections in the clinical laboratory. Clinical Microbiology Reviews. 1997;10:242–256. doi: 10.1128/cmr.10.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordhoek GT et al. Multicenter quality control study for detection of Mycobacterium tuberculosis in clinical samples by nucleic amplification methods. Clinical Microbiology and Infection. 2004;10:295–301. doi: 10.1111/j.1198-743X.2004.00825.x. [DOI] [PubMed] [Google Scholar]
- Weed DL, Gorelic LS. The practice of causal inference in cancer epidemiology. Cancer Epidemiology, Biomarkers and Prevention. 1996;5:303–311. [PubMed] [Google Scholar]
- Susser M. What is a cause and how do we know one? A grammar for pragmatic epidemiology. American Journal of Epidemiology. 1991;133:635–648. doi: 10.1093/oxfordjournals.aje.a115939. [DOI] [PubMed] [Google Scholar]
- Rychetnik L et al. A glossary for evidence based public health. Journal of Epidemiology and Community Health. 2004;58:538–545. doi: 10.1136/jech.2003.011585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C. An Introduction to the Study of Experimental Medicine (Greene HC, translator) New York: Henry Schuman Inc.; 1949. [Google Scholar]
- Bacon F. Novum organum. Chicago: Open Court; 1994. [Google Scholar]
- Hume D, Nidditch PH. A Treatise of Human Nature. New York: Oxford University Press; 1978. Of the inference from the impression to the idea. [Google Scholar]
- Hendel CWJ. Hume Selections. New York: Charles Scribners; 1955. pp. 22–28. , pp. [Google Scholar]
- Mill JS. Philosophy of Scientific Method. New York: Hafner; 1950. [Google Scholar]
- Bacon F, Jones R. Essays, Advancements of Learning, New Atlantis, and Other Pieces. New York: Odyssey Press; 1937. pp. 266–363. , pp. [Google Scholar]
- Mill JS. A System of Logic, Ratiocinative and Inductive. 5th edn. London: Parker, Son and Bowin; 1862. , pp. [Google Scholar]
- Rothman KJ. Causal Inference. Chestnut Hill, MA: Epidemiology Resources; 1988. [Google Scholar]
- Pearce N, Crawford-Brown D. Critical discussion in epidemiology: problems with the Popperian approach. Journal of Clinical Epidemiology. 1989;42:177–184. doi: 10.1016/0895-4356(89)90053-x. [DOI] [PubMed] [Google Scholar]
- Kuhn TS. The Structure of Scientific Revolutions. 2nd edn. Chicago: University of Chicago Press; 1970. [Google Scholar]
- Susser M, Rothman KJ. Causal Inference. Chestnut Hill, MA: Epidemiology Resources; 1988. pp. 33–57. , pp. [Google Scholar]
- Susser M. The logic of Sir Karl Popper and the practice of epidemiology. American Journal of Epidemiology. 1986;124:711–718. doi: 10.1093/oxfordjournals.aje.a114446. [DOI] [PubMed] [Google Scholar]
- Buck C. Popper's philosophy for epidemiologists. International Journal of Epidemiology. 1975;4:159–168. doi: 10.1093/ije/4.3.159. [DOI] [PubMed] [Google Scholar]
- Popper KR. The Logic of Scientific Discovery. 3rd edn. London: Hutchinson; 1972. [Google Scholar]
- Page GP et al. Are we there yet?: deciding when one has demonstrated specific genetic causation in complex diseases and quantitative traits. American Journal of Human Genetics. 2003;73:711–719. doi: 10.1086/378900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt JR. Strong inference. Science. 1964;164:347. doi: 10.1126/science.146.3642.347. [DOI] [PubMed] [Google Scholar]
- Vineis P, Kriebel D. Causal models in epidemiology: past inheritance and genetic future. Environmental Health. 2006;5:21. doi: 10.1186/1476-069X-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis L. Epidemiology. 3rd edn. Philadelphia: Saunders; 2004. [Google Scholar]
- Rothman KJ.EpidemiologyAn Introduction 1st ednNew York: Oxford University Press; 2002 [Google Scholar]
- Kundi M. Causality and the interpretation of epidemiological evidence. Environmental Health Perspectives. 2006;114:969–974. doi: 10.1289/ehp.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AB. The environment and disease: association or causation. Proceedings of the Royal Society of Medicine. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AB. Principles of Medical Statistics. 9th edn. New York: Oxford University Press; 1971. pp. 288–296. , pp. [Google Scholar]
- Höfler M. The Bradford Hill considerations on causality: a counterfactual perspective. Emerging Themes in Epidemiology. 2005;2:11. doi: 10.1186/1742-7622-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CV, Goodman KJ. The Messed Lessons of Sir Austin Bradford Hill. Toronto: Congress of Epidemiology; 2001. p. 765. , pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. Modern Epidemiology. Boston: Little Brown and Co.; 1986. [Google Scholar]
- Washington, DC: Government Printing Office; 1964. United States Department of Health, Education and Welfare. Smoking and Health: Report of the advisory committee to the surgeon general of the public health service, public health service publication 1103. [Google Scholar]
- Susser M. Rules of inference in epidemiology. Regulatory Toxicology and Pharmacology. 1986;6:116–128. doi: 10.1016/0273-2300(86)90029-2. [DOI] [PubMed] [Google Scholar]
- Fletcher RH, Fletcher SW, Wagner EH. Clinical Epidemiology: the Essentials. 3rd edn. Baltimore: Williams and Wilkins; 1996. [Google Scholar]
- Chamberlin W et al. Review article: Mycobacterium avium subsp. paratuberculosis as one cause of Crohn's disease. Alimentary Pharmacology and Therapeutics. 2001;15:337–346. doi: 10.1046/j.1365-2036.2001.00933.x. [DOI] [PubMed] [Google Scholar]
- Naser SA et al. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn's disease. Lancet. 2004;364:1039–1044. doi: 10.1016/S0140-6736(04)17058-X. [DOI] [PubMed] [Google Scholar]
- Mishina D et al. On the aetiology of Crohn disease. Proceedings of the National Academy of Sciences USA. 1996;93:9816–9820. doi: 10.1073/pnas.93.18.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini RJ et al. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. Journal of Clinical Microbiology. 1984;20:966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermon-Taylor J et al. Causation of Crohn's disease by Mycobacterium avium subsp. paratuberculosis. Canadian Journal of Gastroenterology. 2000;14:521–539. doi: 10.1155/2000/798305. [DOI] [PubMed] [Google Scholar]
- Hulten K et al. Detection of Mycobacterium avium subsp. paratuberculosis in Crohn's diseased tissues by in situ hybridization. American Journal of Gastroenterology. 2001;96:1529–1535. doi: 10.1111/j.1572-0241.2001.03751.x. [DOI] [PubMed] [Google Scholar]
- Ikonomopoulos JA et al. Sensitive differential detection of genetically related mycobacterial pathogens in archival material. American Journal of Clinical Pathology. 2000;114:940–950. doi: 10.1309/7ABR-E7MJ-18V9-CM4M. [DOI] [PubMed] [Google Scholar]
- McFadden JJ et al. Crohn's disease-isolated mycobacteria are identical to Mycobacterium paratuberculosis, as determined by DNA probes that distinguish between mycobacterial species. Journal of Clinical Microbiology. 1987;25:796–801. doi: 10.1128/jcm.25.5.796-801.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechi LA et al. Detection and Isolation of Mycobacterium avium subsp. paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn's disease in Sardinia. American Journal of Gastroenterology. 2005;100:1529–1536. doi: 10.1111/j.1572-0241.2005.41415.x. [DOI] [PubMed] [Google Scholar]
- Sechi LA et al. Identification of Mycobacterium avium subsp. paratuberculosis in biopsy specimens from patients with Crohn's disease identified by in situ hybridization. Journal of Clinical Microbiology. 2001;39:4514–4517. doi: 10.1128/JCM.39.12.4514-4517.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turenne CY et al. Sequencing of hsp65 distinguishes among subsets of the Mycobacterium avium complex. Journal of Clinical Microbiology. 2006;44:433–440. doi: 10.1128/JCM.44.2.433-440.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autschbach F et al. High prevalence of Mycobacterium avium subsp. paratuberculosis 1S900 DNA in gut tissues from individuals with Crohn's disease. Gut. 2005;54:944–949. doi: 10.1136/gut.2004.045526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull TJ et al. Characterization of IS900 loci in Mycobacterium avium subsp. paratuberculosis and development of muliplex PCR typing. Microbiology. 2000;146:2185–2197. doi: 10.1099/00221287-146-9-2185. [DOI] [PubMed] [Google Scholar]
- Bull TJ et al. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. Journal of Clinical Microbiology. 2003;41:2915–2923. doi: 10.1128/JCM.41.7.2915-2923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini RJ et al. Spheroplastic phase of mycobacteria isolated from patients with Crohn's disease. Journal of Clinical Microbiology. 1986;24:357–363. doi: 10.1128/jcm.24.3.357-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermon-Taylor J et al. Molecular biology of Crohn's disease mycobacteria. Baillieres Clinical Gastroenterology. 1990;4:23–42. doi: 10.1016/0950-3528(90)90037-h. [DOI] [PubMed] [Google Scholar]
- Moss MT et al. Specific detection of Mycobacterium paratuberculosis by DNA hybridization with a fragment of the insertion element IS900. Gut. 1991;32:395–398. doi: 10.1136/gut.32.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P et al. Mycobacterium paratuberculosis detected by nested PCR in intestinal granulomas isolated by LCM in cases of Crohn's disease. Methods in Molecular Biology. 2002;193:205–211. doi: 10.1385/1-59259-283-X:205. [DOI] [PubMed] [Google Scholar]
- Ziehl F. Zur Farbung des Tuberkelbacillus. Deutsche Medizinische Wochenschrift. 1882;8:451. [Google Scholar]
- Neelsen FCA. Ein casuistischer Bietrag zur Lehre von der Tuberkulos. Zentralblatt für die Medizinischen Wissenschaften. 1883;21:497–501. [Google Scholar]
- Fiocchi C. Inflammatory bowel disease: aetiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Naser S, Shafran I, El-Zaatari F. Mycobacterium avium subsp. paratuberculosis in Crohn's disease is serologically positive [Letter] Clinical and Diagnostic Laboratory Immunology. 1999;6:282. doi: 10.1128/cdli.6.2.282-282.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zaatari FA et al. Characterization of Mycobacterium paratuberculosis p36 antigen and its seroreactivities in Crohn's disease. Current Microbiology. 1999;39:115–119. doi: 10.1007/s002849900430. [DOI] [PubMed] [Google Scholar]
- Sanderson JD et al. Mycobacterium paratuberculosis DNA in Crohn's disease tissue. Gut. 1992;33:890–896. doi: 10.1136/gut.33.7.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald TT.The Hypothesised Link Between Dairy products, Mycobacterium paratuberculosis and Crohn's DiseaseLondon The Dairy Council (UK)2001 [Google Scholar]
- Semret M et al. Differentiating host-associated variants of Mycobacterium avium by PCR for detection of large sequence polymorphisms. Journal of Clinical Microbiology. 2006;44:881–887. doi: 10.1128/JCM.44.3.881-887.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Amand AL et al. Use of specific rRNA oligonucleotide probes for microscopic detection of Mycobacterium avium complex organisms in tissue. Journal of Clinical Microbiology. 2005;43:1505–1514. doi: 10.1128/JCM.43.4.1505-1514.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggio MP et al. Search for Mycobacterium paratuberculosis DNA in orofacial granulomatosis and oral Crohn's disease tissue by polymerase chain reaction. Gut. 1997;41:646–650. doi: 10.1136/gut.41.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kruiningen HJ et al. Experimental disease in infant goats induced by a mycobacterium isolated from a patient with Crohn's disease. A preliminary report. Digestive Diseases and Sciences. 1986;31:1351–1360. doi: 10.1007/BF01299814. [DOI] [PubMed] [Google Scholar]
- Van Kruiningen GJ, Ruiz B, Gumprecht L. Experimental disease in young chickens induced by a Mycobacterium paratuberculosis isolate from a patient with Crohn's disease. Canadian Journal of Veterinary Research. 1991;55:199–202. [PMC free article] [PubMed] [Google Scholar]
- Schwartz D et al. Use of short-term culture for identification of Mycobacterium avium subsp. paratuberculosis in tissue from Crohn's disease patients. Clinical Microbiology and Infection. 2000;6:303–307. doi: 10.1046/j.1469-0691.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- Greenstein RJ et al. On the action of methotrexate and 6-mercaptopurine on M. avium subspecies paratuberculosis. PLoS ONE. 2007;24:e161. doi: 10.1371/journal.pone.0000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danze PM et al. Association of HLA class II genes with susceptibility to Crohn's disease. Gut. 1996;39:69–72. doi: 10.1136/gut.39.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson HJ. Keeping Crohn's disease quiet. New England Journal of Medicine. 1998;334:1599–1600. doi: 10.1056/NEJM199606133342409. [DOI] [PubMed] [Google Scholar]
- Sartor RB. Current concepts of the aetiology and pathogenesis of ulcerative colitis and Crohns disease. Gastroenterology Clinics of North America. 1995;24:475–507. [PubMed] [Google Scholar]
- Strober W, Neurath MF, Rich RR. Clinical Immunology, Principles and Practice. St. Louis: Mosby; 1996. pp. 1401–1428. , pp. [Google Scholar]
- Van Hogezand RA, Verspaget HW. Selective immunomodulation in patients with inflammatory bowel disease-future therapy or reality. Netherlands Journal of Medicine. 1996;48:64–67. doi: 10.1016/0300-2977(95)00091-7. [DOI] [PubMed] [Google Scholar]