To The Editor:
The occurrence of the common cold and influenza shows clear seasonality. The cold and influenza season corresponds to the season of vitamin D insufficiency. This association was highlighted in the recent article in this journal by Cannell et al. [1], which proposed that the lack of vitamin D during the winter may be a ‘seasonal stimulus’ to the infectivity of the influenza virus.
Vitamin D is produced in the skin when sunlight is absorbed. Thus, vitamin D levels, or serum 25-hydroxyvitamin D (25-OHD), fluctuate seasonally. At latitudes above 37° N and below 37° S, sunlight is insufficient to induce cutaneous vitamin D synthesis during the winter months so 25-OHD levels are low [2]. New evidence has accumulated that vitamin D can have important functions in the immune system, specifically the innate immune system. Is our susceptibility to colds and influenza related to vitamin D insufficiency in the wintertime? Furthermore, would vitamin D supplementation boost our immune system and work as a preventive measure for colds and influenza?
We considered these questions after reviewing the adverse events of a vitamin D3 supplementation trial that we completed [3]. The design and primary results of the study have been published and are summarized here. We conducted a 3-year randomized controlled trial to test the hypothesis that vitamin D3 supplementation would prevent bone loss in calcium-replete, African-American post-menopausal women. A total of 208 women were randomized to receive vitamin D3 (n=104) or placebo (n=104). After 2 years, the vitamin D3 dose was increased to 50 μg/d (2000 IU) in the active group. None of the patients had a history of chronic obstructive pulmonary disease, congestive heart failure, or myocardial infarction. Few patients had a history of asthma and seasonal allergies with no significant difference between the vitamin D3 group and the placebo group.
The study was approved by the Institutional Review Board of Winthrop University Hospital. After randomization, patients were followed up every 6 months for 3 years. At each visit, information on URI symptoms was obtained by first asking the participants, ‘Have you been well?’ If the participant answered ‘No’, then she was asked, ‘Have you had any colds or influenza?’ A report of cold and influenza was recorded as an adverse event.
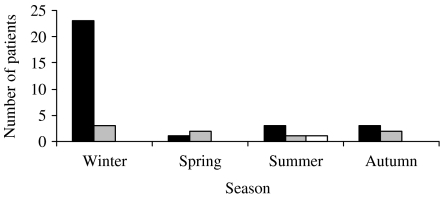
After 3 years, a total of 34 patients reported cold and influenza symptoms, eight in the vitamin D3 group vs. 26 in the placebo group (P<0·002). When we examined the seasonality of the symptoms, we found that the placebo group had cold/influenza symptoms mostly in the winter. The vitamin D group had symptoms throughout the year while on 20 μg/d, whereas only one subject had a cold/influenza while on 50 μg/d (Fig.). None of the 34 patients with reported cold and influenza symptoms had significant comorbidities.
Fig.
Incidence of reported cold/influenza symptoms according to season. The placebo group reported more cold/influenza symptoms in the winter. Only one subject had cold/influenza symptoms while taking high doses of vitamin D (50 μg/d). ■, Placebo;  , 20 μg/d vitamin D; □, 50 μg/d vitamin D.
, 20 μg/d vitamin D; □, 50 μg/d vitamin D.
Vitamin D supplementation, particularly at higher doses, may protect against the ‘typical’ winter cold and influenza. A major flaw in this analysis is that the data on viral URI symptoms were collected in an insensitive and imprecise way. From a 3-year trial on 208 subjects, we would expect at least 139 colds, but we only collected 39 reports of viral URI symptoms, indicating the incomplete capture of data. However, given the double-blinded, randomized nature of this trial, the responses should not have been biased.
The physiological basis of the protective effect of vitamin D lies in its ability to stimulate innate immunity and to moderate inflammation. The active form of vitamin D, 1,25-dihydroxyvitamin D (1,25-OH2D) stimulates the genetic expression of antimicrobial peptides (AMPs) in human monocytes, neutrophils, and epithelial cells [4]. Recognition of microbial particles by toll-like receptors (TLRs) induces expression of antimicrobial peptides, such as defensins and cathelicidins. These peptides have a broad range of actions against microorganisms, including bacteria, fungi and viruses. Liu et al. showed that stimulation of TLR 2/1 engages a vitamin D-dependent intracellular circuit that results in the expression of cathelicidin, enhancing the microbicidal capability of the monocyte [5]. Remarkably, the authors also observed that sera from African-American individuals, who are known to have substantially lower serum vitamin D levels than whites, were inefficient in inducing genetic expression of cathelicidin. When the authors supplemented the sera with 25-OHD, cathelicidin levels increased to levels observed in monocytes collected from whites. Wang et al. showed that 1,25-OH2D induces expression of cathelicidin and defensin β2 genes [4]. Defensin β2 has been shown to have inhibitory effects on adenovirus and HIV-1 [6, 7]. Defensins can block viral infection by directly acting on the virion or by affecting the target cell and thereby indirectly interfering with viral infection [8]. One of the defensins called retrocyclin-2 inhibits influenza virus infection by blocking membrane fusion mediated by the viral hemagglutinin [9].
These reports provide a rationale for vitamin D supplementation in the prevention of colds and influenza. Since there is an epidemic of vitamin D insufficiency in the United States, the public health impact of this observation could be great. These findings should be confirmed by randomized, placebo-controlled trials.
Declaration of Interest
This research was supported by the National Institutes of Aging (RO1 AG15325), National Institutes of Health, Bethesda, MD.
References
- Cannell JJ. et al. Epidemic influenza and vitamin D. Epidemiology and Infection. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AT, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D; exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. Journal of Clinical Endocrinology and Metabolism. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- Aloia JF. et al. A randomized controlled trial of vitamin D3 supplementation in African American women. Archives of Internal Medicine. 2005;165:1618–1623. doi: 10.1001/archinte.165.14.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. et al. 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Journal of Immunology. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Liu PT. et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Bastian A, Schafer H. Human α-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regulatory Peptides. 2001;103:1516–1521. doi: 10.1016/s0167-0115(01)00282-8. [DOI] [PubMed] [Google Scholar]
- Sun L. et al. Human β-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. Journal of Virology. 2005;79:14318–14329. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotman ME, Chang TL. Defensins in innate antiviral immunity. Immunology. 2006;6:447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- Leikina E. et al. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nature Immunology. 2005;6:995–1001. doi: 10.1038/ni1248. [DOI] [PubMed] [Google Scholar]