SUMMARY
There are no data on the serotypes of rotaviruses prevalent in Kuwait, which has a large expatriate population and hence a focal point for transmission of pathogens. The serotype information will contribute to the fund of knowledge on the world epidemiology of rotavirus serotypes and will predict the outcome of vaccination in Kuwait. Of the 75 rotavirus-positive samples from 172 children (aged <5 years) with severe diarrhoea, 69 were genotyped. The distribution of genotypes was G1 (63·8%) followed by G9 (10·2%), G2 (7·3%), G4 (7·3%) and G3 (4·4%). Among the P types, P[8] was the most common type found across all G types. By fluorescent focus neutralization test, serum antibodies to genotypes G1 (94%), G4 (68%) and G9 (46%) were found in 120 other children. These results show that G1 is the predominant serotype in Kuwait and that a vaccine that contains G1 will be most effective.
INTRODUCTION
Rotavirus (RV) is one the most significant causes of infantile diarrhoea throughout the world. In developing countries, 1 in every 100–200 children dies of RV disease by 5 years of age [1, 2]. In industrialized countries, the burden of RV is underlined by the high rate of hospitalization, and clinic and emergency-room visits [3]. An economic analysis in the United States estimated that RV disease costs the economy more than one billion dollars annually [4]. The goal to reduce the number of deaths in developing countries, and to cut the high cost of hospitalization in developed countries, can only be achieved by introducing a RV vaccine into the childhood vaccination programme [5, 6]. However, since there are differences in the regional and temporal distribution of RV sero/genotypes [7], it is important to conduct surveys on the prevalence of RV antibodies and determine the genotypes present in a given area.
Three of the seven RV groups are known to infect the humans. Among them, the most dominant is group A, which causes diarrhoeal diseases worldwide [8].
RV is a triple-layered, non-enveloped virus with a double-stranded, segmented RNA genome. Of the 11 genomic segments, segment 6 codes for the most abundant viral protein, VP6. This is the major antigenic determinant of group A reactivity and the target of common diagnostic assays. Segments 7, 8 or 9 codes for the major glycoprotein, VP7, which is the basis for RV serotyping. There are 14 G serotypes and 11 of them have been recovered from humans. The majority of human RVs belong to serotypes G1–G4 and to the newly emerged G9. G sero/genotypes are synonymous. Segment 4 codes for a protease-sensitive protein, VP4, which is the basis for P-serotyping. Out of the 26 P genotypes reported, only 12 of them have been recovered from humans [9]. Thus, genotyping of RVs targets regions of segment 9 (G genotype) and segment 4 (P genotype).
Since the genomic segments of RVs can be exchanged, emergence of new, reassortant strains is a real possibility. This may adversely affect the efficacy of the vaccines being developed for the prevention of RV infection [10, 11].
A RV vaccine is planned for introduction in Kuwait. However, there are no data on the prevalence of RV genotypes and serotype-specific antibodies in children in Kuwait. Therefore, the aim of this study was to determine the prevalence of RV genotypes and serotype-specific antibodies to two common RV serotypes (G1, G4) and an emerging RV serotype (G9) in children up to 5 years of age in Kuwait.
METHODS
Samples for genotyping
Faecal samples were collected from 172 children, aged 0–5 years, admitted with diarrhoea from October 2005 to April 2006 in Al-Amiri and Al-Adan hospitals, Kuwait. A single, fresh stool sample, from each patient, was sent to the Virology Laboratory of the Faculty of Medicine, Kuwait University, in a cool box. The sample was kept at 4°C after addition of glycerol until tested. All of the 172 samples were screened by RV-ELISA (Dako A/S, Glostrup, Denmark) for the presence of RV. The ELISA-positive samples were processed for genotyping.
Samples for antibody detection
Blood samples collected from 120 children admitted with minor, non-gastrointestinal ailments from February to December 2005 in Mubarak Al-Kabeer Hospital, Kuwait, were selected for studying the prevalence of RV-specific antibodies to two common RVs (G1, G4) and an emerging RV (G9) reported to be circulating in this region [12, 13]. From the blood samples sent to the Virology Laboratory, sera were separated and stored at −20°C until tested. Samples represented the following age groups: (a) 0–1 year (n=38), (b) 2–3 years (n=46, and (c) 4–5 years (n=36).
Virus strains
Reference strains belonging to group A RV, representing sero/genotypes G1 (Wa), G2 (DS-2), G3 (SA-11), G4 (ST3) and G9 (W161), were obtained from the Centers for Disease Control and Prevention (CDC, Atlanta, GA, USA).
Cell culture
MA104 cell line obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) was used for virus propagation and titration and performing the fluorescent focus neutralization (FFN) assay. Cells were cultured in plastic flasks (Nunclon, Roskilde, Denmark) in a growth medium, Parker 199 with 10% fetal calf serum (Invitrogen, Taastrup, Denmark).
Propagation of RV strains
Before inoculating MA104 cells grown in 25 ml plastic flasks, RV strains were activated with 1 μg/ml trypsin (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) for 30 min at 37°C. Each virus strain was then inoculated into two flasks (0·5 ml/flask). After an incubation period of 30 min at 37°C, the medium (10 ml/flask) containing 0·5 μg/ml trypsin was added to the flasks and the culture was incubated at 37°C in a 5% CO2 incubator for 48–72 h, until the virus disrupted the monolayer. The flasks were then frozen at −80°C until used.
Virus titration
Aliquots of RV types G1, G4 and G9 were diluted twofold from 1/5 to 1/40 in Dulbecco's minimal essential medium (Invitrogen). Each dilution of the three RV types was inoculated (100 μl/well) into MA104 cells grown in 96-well cell culture plates (Nunclon) using three parallel wells per dilution. After an overnight incubation at 37°C in a CO2 incubator, the cells were scraped from the three wells (per dilution) and transferred to an Eppendorf tube. Cell suspensions were centrifuged at 11 000 g for 1 min in an Eppendorf centrifuge (model number 5415C, Eppendorf, Hamburg, Germany) and washed with 300 μl PBS (pH 7·2) by centrifugation at 3600 g for 1 min. Then the cells in each Eppendorf tube were suspended in 300 μl PBS. The cell suspension was then distributed onto 10-spot immunofluorescent (IF) microscopic slides (50 μl/spot), using two spots for each dilution of the viruses. The slides were allowed to dry and then fixed in chilled acetone for 10 min. After drying, 50 μl/spot of the first antibody (anti-RV IgG of rabbit origin (Dako A/S) diluted 1/5 in PBS was added to the slides and incubated at 37°C for 30 min in a moist atmosphere. The slides were then washed and the second antibody, fluorescein isothiocynate (FITC)-conjugated anti-rabbit IgG (Dako A/S, Denmark) diluted 1/5 in PBS was added. The slides were viewed by a fluorescent microscope to allow counting of the number of fluorescent foci. The last dilution with at least one clear focus was considered to contain 1 focus-forming unit (f.f.u.) in 50 μl. In the FFN assay, a working dilution containing 10 f.f.u./100 μl of G1, G4 and G9 viruses was used.
Detection of RV-specific antibodies
For antibody detection, a virus neutralization test, the FFN assay [14, 15] was used. To determine the antibody prevalence, 1/10 dilution of the serum samples were tested against 10 f.f.u. of G1, G4 and G9 serotypes. From the dilution of each serum sample to be tested, 0·1 ml/well was added to six parallel wells of 96-well cell culture plates (Nunclon). Then 0·1 ml from the dilutions containing 10 f.f.u. of each virus strain (G1, G4, and G9) was added to two parallel wells of each serum sample. After 1 h incubation at 3°C, 10 000 MA104 cells (0·1 ml/well) were added to the wells containing the serum-virus mixtures and to the control wells (virus-medium mixture, serum-medium mixture and medium only). After a 24 h incubation at 37°C in a CO2 incubator, cells were scraped, collected by centrifugation, suspended in PBS and transferred to 10-spot IF microscope slides using two spots/sample. The cells were then fixed, dried and treated as described for the virus titration procedure. The number of fluorescent foci was counted first in the control spots and then in the test spots. When the number of the fluorescent foci was reduced by ⩾50%, it was taken as the indication of the presence of RV-specific antibodies.
Genomic amplification of RVs by the multiplex One-Step RT–PCR (OS-RT–PCR) for G-typing
The viral RNA was extracted from the reference RV strains and the stool samples by Qiagen RNA isolation kit (Qiagen Inc., Valencia, CA, USA). The method recommended by the manufacturer was followed. The primers used in the genotyping assay were those described by Das et al. [16]. The primer 9Con1: TAGCTCCTTTTAATGTATGG is a generic primer, which hybridizes to the conserved region close to the 5′-end of the VP7 gene segment of all group A RVs. The genotype-specific primers 9T1-1 (for G1): TCTTGTCAAAGCAAATAATG; 9T1-2 (for G2): GTTAGAAATGATTCTCC ACT; 9T-3P (for G3): GTCCAGTTGCAGTGTAGC; 9T-4 (for G4): GGGTCGATGGA AAATTCT; and 9T-9B (for G9): TATAAAGTCCATTGCAC, hybridize specifically to their respective RV genotypes. The amplification included the generic primer 9Con1, together with the genotype-specific primers which produced amplified products of expected length to the corresponding genotypes as follows: G1, 158 bp; G2, 244 bp; G3, 466 bp; G4, 403 bp; G9, 110 bp.
Four microlitres of extracted RNA was preheated in the presence of 1 μl primer mix. The mixture was heated at 97°C for 5 min and immediately cooled on ice. For amplification, the Superscript OS-RT–PCR kit (Invitrogen) which combines reverse transcription and PCR in one step was used. The master mix contained the following: 12·5 μl of the 2× reaction mix (buffer and MgCl2), 0·5 μl enzyme mixture (reverse transcriptase and Taq DNA polymerase), and 7 μl water. To each preheated sample (5 μl), 20 μl of the master mix was added to make a final volume of 25 μl (the concentration of each primer was 0·4 μm in the final reaction volume). cDNA synthesis was performed at 42°C for 1 h. The PCR amplification conditions were as follows: initial denaturation at 94°C for 3 min, followed by 40 cycles (denaturation at 94°C for 30 s, annealing at 42°C for 30 s, extension at 72°C for 1 min). A final extension step at 72°C for 10 min was also included. The amplified products were separated in 2% agarose gel (Amersham Pharmacia Biotech AB, Uppsala, Sweden).
RV P-typing
Following G-typing, the samples were P-typed based on the VP4 region using the primers and protocol of Gentsch et al. [17]. The P-typing was done as a two-step PCR using Superscript II RT–PCR protocol (Invitrogen). The first RT–PCR amplification was done using the generic primers, con2, 5′-ATTTCGGACCATTTATAACC-3′ [nucleotides (nt) 868–887] and con3, 5′-TGGCTTCG CCATTTTATAGACA-3′ (nt 11–32). The extracted viral RNA was preheated as given in the G-typing protocol except that the primers used were con2 and con3. The reaction mixture for RT–PCR contained 12·5 μl reaction buffer, 0·5 μl enzyme mixture and 5 μl preheated RNA containing 0·4 μm each of primers con2 and con3, in a final reaction volume of 25 μl. The cDNA synthesis was done at 42°C for 1 h and the enzyme inactivated by heating to 95°C for 2 min. The thermal cycling conditions for PCR amplification were as follows: denaturation at 94°C for 1 min, annealing at 45°C for 1 min followed by extension at 72°C for 1 min repeated for 20 cycles.
The second round of amplification was done on the product of the first round RT–PCR using the primers specific for the three most common RV P types: 1T-1, 5′-TCTACTTGGATAACGTGC-3′ (nt 339–356) for P[8]; 2T-1, 5′-CTATTGTTAGAGGTTAGAGTC (nt 474–494) for P[4]; and 3T1 5′-TGTToGATTAGTTGGATTCAA (nt 259–278) for P[6]. The reaction mixture contained 12·5 μl reaction buffer, 0·5 μl enzyme mixture, 0·8 μm each of primers con3 and one of the P-type-specific primers (1T-1, 2T-1, 3T-1) and 2 μl of the first round RT–PCR product in a final volume of 25 μl. The thermal cycling conditions were as follows: initial denaturation at 95°C for 2 min followed by 35 cycles of denaturation at 94°C for 1 min, annealing of the primers at 37°C for 1 min and extension at 72°C for 1 min. A final extension step at 72°C for 10 min was also included. The expected products for the respective P types were as follows: P[8], 345 bp; P[6], 267 bp; P[4], 483 bp.
Statistical test
The difference in prevalence of antibodies between two groups was calculated by χ2 test. A P value of ⩽0·05 was considered significant.
RESULTS
Prevalence of RV G1-, G4-, and G9-specific antibodies in sera of children
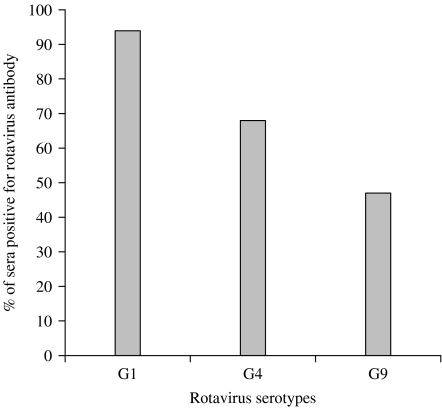
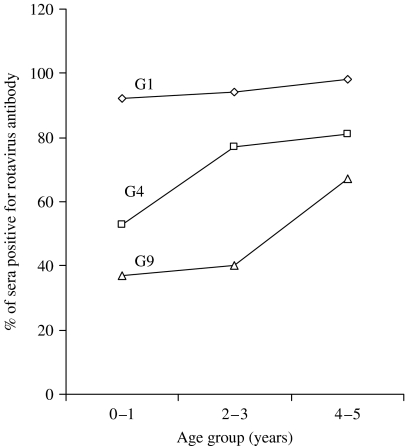
Serum samples of 120 children were tested for the presence of RV-specific antibodies by the FFN assay. Combining the three age groups of children (from 0 to 5 years), the prevalence rate was the highest to G1 serotype (94%), followed by G4 (68%) and G9 (46%) (Fig. 1). No difference in the prevalence of RV antibodies to G1 serotype in the three groups was found. The differences in the prevalence of antibodies for G1 and G4, G4 and G9, and G1 and G9 were all significant (P⩽0·001). The prevalence of RV antibodies by age groups of children (0–1, 2–3 and 4–5 years) is shown in Fig. 2. The differences were significant for G4 between 0–1 years and 4–5 years; and for G9 between 2–3 years and 4–5 years, and between 0–1 years and 4–5 years (P=0·025 for all three comparisons). The other comparisons were not significant.
Fig. 1.
Prevalence of rotavirus-specific antibodies in sera of children to serotypes G1, G4 and G9.
Fig. 2.
Prevalence of rotavirus-specific antibodies by age groups to three serotypes of rotaviruses.
Genotypes of RV-ELISA positive samples
The distribution of the genotypes is shown in the Table. Of the 172 stool samples tested, 75 (43·6%) were positive in RV-ELISA. These RV-positive samples were subsequently processed for genotyping for G and P types. Of the 75 RV-ELISA-positive samples, 69 (92%) were G-typed. Sixty-four of the 69 G-typed samples were subjected to P-typing and 57 (89%) could be typed. The majority, 44 (63·8%) of RVs belonged to genotype G1. Genotypes G2, G3 and G4 could be detected in 7·3%, 4·4% and 7·3% strains respectively. The emerging genotype, G9 was also present and comprised 10·2% of the strains, while mixed genotypes were detected in 7·7% of the samples. Six (8%) of the 75 ELISA-positive samples could not be genotyped. Among the P types, P[8] was the most frequent type which combined mostly with G1 type (91%). P[8] was also found in combination with G2, G3, G4 and G9. There was only a single P[6] type in combination with G4. The proportions of P types were P[8], 89%; P[4], 9%; and P[6], 2%.
Table.
Distribution of RV genotypes identified in Kuwait

NT, Non-typable.
DISCUSSION
G-typing done on 75 ELISA-positive samples of children suffering from diarrhoea in Kuwait by the multiplex OS-RT–PCR showed that apart from G1 genotype, other genotypes such as G2 and G4 and even the emerging genotype, G9 are present. P-typing showed that P[8] is the most common type spread across all G types. Our data also showed the dominance of G1 genotype in Kuwait since more than half (63·8%) of the RV strains belonged to this genotype. The high RV antibody prevalence rate (94%) detected for the G1 genotype also supports this view. However, the relatively high frequency (11%) of genotype G9 is noteworthy. In fact, the lowest antibody prevalence was detected for this RV. Compared to antibody acquisition to G1 and G4 types, the acquisition of antibody to G9 type was steeper from age group 2–3 years to age group 4–5 years (Fig. 2). This may indicate late infection with this G type. The antibody prevalence for genotype G4, which comprised only 6·3% of the RV strains genotyped, was higher (68%) than that for genotype G9 (47%). This could be due to the fact that G4 is an already established genotype that has been circulating in different parts of the world for a long time, while the G9 serotype has only recently emerged. Moreover, the presence of antibodies to these serotypes may be a consequence of heterotypic immune responses as both genotypes possessed a heterotypic VP4 antigen, P[8], which was shared by other G types, in particular G1. The property of eliciting heterotypic antibody responses in addition to homotypic antibodies, in infection with some RV strains [18], has been exploited in vaccine preparation. Indeed, the monovalent, Rotarix vaccine which contains type G1 only, has prevented 70% of all and 80% of severe RV diarrhoeal cases in a trial involving 63 000 children in Latin America. Vaccine efficacy was observed against severe RV gastroenteritis caused by G1 and non-G1 types including the emerging G9 type [19], although in one study, the vaccine afforded less protection against heterotypic strain, such as G2P[4] [20]. In Kuwait, the G1 serotype seems to be, at least in part, responsible for the development of heterotypic immunity. This may be an important factor in relation to vaccination. It should be noted that serum antibody response is a marker of protection against RV diarrhoea [21].
Previous studies conducted in the Gulf Region have provided valuable data on the role of RV in the causation of diarrhoea. These data showed that RVs are the major aetiological agents of diarrhoeal diseases in children aged <5 years being detected in between 21% and 40% of diarrhoeal cases [22–30]. In Saudi Arabia, where serotyping was performed by using monoclonal antibodies, RV G1 was found to be the most prevalent serotype (53·5%) [31, 32] which is in agreement with our data obtained by genotyping. A recent genotyping study showed that in Iran, as in Kuwait, G1 was the predominant genotype [12]. Molecular characterization of RV strains in Iraqi Kurdistan showed that the emerging G9 strain was also present in that part of the world [13]. The proportion of G9 genotype (11%) was the same as we have detected in our present study. Thus, the relative distribution of different G and P types in Kuwait is in general agreement with data from this region [12, 13] and other parts of the world [7]. The fact that RV is the causative agent in about half of the children (aged <5 years) hospitalized with severe diarrhoea, underscores the importance of RV infection in Kuwait. The majority of severe RV diarrhoeal cases were caused by genotype G1 RV, but other types including the emerging G9 were also present. Both genotypic identification of viruses and antibody surveillance have provided us with background information for the planned introduction of the Rotarix vaccine in Kuwait. Since there are temporal variations in the distribution of RV serotypes, long-term surveillance for serotypes is necessary.
Kuwait is at the crossroads of countries where a large expatriate population from different parts of the world lives and works and hence a focal point for transmission and acquisition of microbial pathogens including RVs. Our data will contribute to the fund of knowledge on the epidemiology of RV, which is truly a global paediatric pathogen.
ACKNOWLEDGEMENTS
We thank Susan Silpi Kurian for excellent technical assistance in screening samples for the presence of rotavirus. This work was supported by Kuwait University, Research Grant number, MK01/04.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Parashar UD et al. Rotavirus and severe childhood diarrhea. Emerging Infectious Diseases. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parashar UD et al. Global illness and deaths caused by rotavirus disease in children. Emerging Infectious Diseases. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pediatric Rotavirus European Committee (PROTECT) The paediatric burden of rotavirus disease in Europe. Epidemiology and Infection. 2006;134:908–916. doi: 10.1017/S0950268806006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tucker AW et al. Cost-effectiveness analysis of a rotavirus immunization program for the United States. Journal of the American Medical Association. 1998;279:1371–1376. doi: 10.1001/jama.279.17.1371. [DOI] [PubMed] [Google Scholar]
- 5.Dennehy PH. Rotavirus vaccines: an update. Current Opinion in Pediatrics. 2005;17:88–92. doi: 10.1097/01.mop.0000147907.30720.04. [DOI] [PubMed] [Google Scholar]
- 6.Glass RI et al. Rotavirus vaccines: past, present and future. Archives de Pédiatrie. 2005;12:844–847. doi: 10.1016/j.arcped.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 7.Santos N, Hoshino Y. Global distribution of rotavirus serotypes and genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Reviews in Medical Virology. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 8.Estes MK, Knipe DM, Howley PM. Fields Virology. Philadelphia: Lippincott-Raven; 2001. Rotaviruses and their replication; pp. 1747–1785. , pp. [Google Scholar]
- 9.Martella V et al. Identification of a novel VP4 genotype carried by a serotype G5 porcine rotavirus strain. Virology. 2006;346:301–311. doi: 10.1016/j.virol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Bányai K et al. Eight-year survey of human rotavirus strain demonstrates circulation of unusual G and P types in Hungary. Journal of Clinical Microbiology. 2004;42:393–397. doi: 10.1128/JCM.42.1.393-397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentsch JR et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. Journal of Infectious Diseases. 2005;1:S146–S159. doi: 10.1086/431499. (Suppl. 192): [DOI] [PubMed] [Google Scholar]
- 12.Khalili B et al. Epidemiology of rotavirus diarrhea in Iranian children. Journal of Medical Virology. 2004;73:309–312. doi: 10.1002/jmv.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed HM et al. Molecular characterization of rotavirus gastroenteritis strains, Iraqi Kurdistan. Emerging Infectious Diseases. 2006;12:A24–A26. doi: 10.3201/eid1205.051422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulson BS. Longitudinal studies of neutralizing antibody responses to rotavirus in stools and sera of children following severe rotavirus gastroenteritis. Clinical and Diagnostic Laboratory Immunology. 1998;5:897–901. doi: 10.1128/cdli.5.6.897-901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson M, Sigstam G, Svensson L. Antibody prevalence and specificity to group C rotavirus in Swedish sera. Journal of Medical Virology. 2000;60:210–215. doi: 10.1002/(sici)1096-9071(200002)60:2<210::aid-jmv17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Das BK et al. Characterization of rotavirus strain from newborns in New Delhi, India. Journal of Clinical Microbiology. 1994;32:1820–1822. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentsch JR et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. Journal of Clinical Microbiology. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richardson SC, Grimwood K, Bishop RF. Analysis of homotypic and heterotypic serum immune responses to rotavirus proteins following primary rotavirus infection by using the radioimmunoprecipitation technique. Journal of Clinical Microbiology. 1993;31:377–385. doi: 10.1128/jcm.31.2.377-385.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Vos B et al. A rotavirus vaccine for prophylaxis of infants against rotavirus gastroenteritis. Pediatric Infectious Disease Journal. 2004;23:S179–S182. doi: 10.1097/01.inf.0000142370.16514.4a. (Suppl. 10): [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Palacios GM et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. New England Journal of Medicine. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 21.Velasquez FR et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. Journal of Infectious Diseases. 2000;182:1602–1609. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- 22.Al-Nakib W et al. Rotavirus and non-bacterial infantile gastroenteritis in Kuwait. International Journal of Epidemiology. 1980;9:355–359. doi: 10.1093/ije/9.4.355. [DOI] [PubMed] [Google Scholar]
- 23.Sethi SK et al. Acute diarrhoea and rotavirus infections in young children in Kuwait. Annals of Tropical Pediatrics. 1984;4:117–121. doi: 10.1080/02724936.1984.11748321. [DOI] [PubMed] [Google Scholar]
- 24.Al-Bwardy MA et al. Bacterial, parasitic and viral enteropathogens associated with diarrhoea in Saudi children. Annals of Tropical Pediatrics. 1988;8:26–30. doi: 10.1080/02724936.1988.11748533. [DOI] [PubMed] [Google Scholar]
- 25.El-Assouli SM et al. Genetic and antigenic analysis of human rotavirus prevalent in Al-Taif, Saudi Arabia. Journal of Tropical Pediatrics. 1996;4:211–219. doi: 10.1093/tropej/42.4.211. [DOI] [PubMed] [Google Scholar]
- 26.El-Assouli SM et al. Rotavirus infection in children in Saudi Arabia. American Journal of Tropical Medicine and Hygiene. 1992;46:272–277. doi: 10.4269/ajtmh.1992.46.272. [DOI] [PubMed] [Google Scholar]
- 27.Batikhi MN. Epidemiological study on Jordanian patients suffering from diarrhoea. New Microbiology. 2002;25:405–412. [PubMed] [Google Scholar]
- 28.Youssef M et al. Bacterial, viral and parasitic enteric pathogens associated with acute diarrhea in hospitalized children from northern Jordan. FEMS Immunology and Medical Microbiology. 2000;28:257–263. doi: 10.1111/j.1574-695X.2000.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 29.Amini S et al. Rotavirus infection in children with acute diarrhea in Tehran. Medical Journal of Islamic Republic of Iran. 1990;1:25–28. [PubMed] [Google Scholar]
- 30.Ismaeel AY et al. Causative pathogens of severe diarrhea in children. Saudi Medical Journal. 2002;23:1064–1069. [PubMed] [Google Scholar]
- 31.El-Assouli SM. Inter-relationships among subgroups, serotypes and electropherotypes of rotaviruses isolated from humans. Journal of Diarrheal Disease Research. 1996;14:201–206. [PubMed] [Google Scholar]
- 32.El-Sheikh SM, El-Assouli SM. Prevalence of viral, bacterial, and parasitic enteropathogens among young children with acute diarrhoea in Jeddah, Saudi Arabia. Journal of Health, Population and Nutrition. 2001;1:25–30. [PubMed] [Google Scholar]