SUMMARY
Vaginal complaints compel an evaluation of bacterial vaginosis (BV), however, many cases of BV are asymptomatic. We evaluated the sensitivity and specificity of vaginal symptoms in the diagnosis of BV and examined the utility of creating a BV screening tool using clinical, behavioural and demographic characteristics. A total of 1916 pregnant women were included in this analysis. In total, 757 women screened positive for BV and over one third of BV-positive women presented without any lower genital tract symptoms (39·4%). African American race, abnormal vaginal odour, and smoking were independently related to BV positivity. A BV screening tool including these three factors was fairly predictive of BV status with the area under the ROC curve equal to 0·669. This three-item prediction rule may be useful in identifying high- risk pregnant women in need of BV screening and, given the high specificity, accurately identify the group of BV-negative pregnant women.
INTRODUCTION
The self-report of vaginal complaints commonly compels a provider to evaluate the presence of a lower genital tract condition, the most prevalent being bacterial vaginosis (BV) [1–6]. However, more than 50% of cases of BV are asymptomatic thus a screening tool for BV cannot depend on symptom report alone [7].
BV involves a reduction in the normal amount of hydrogen peroxide producing Lactobacillus and an overgrowth of anaerobic and Gram-negative or Gram-variable bacteria, including Mycoplasma hominis, Bacteroides spp., Mobiluncus spp., and Gardnerella vaginalis [8, 9]. During pregnancy, BV has been associated with an increased risk of preterm labour, preterm delivery, premature rupture of the membranes, and miscarriage [10–19]. The clinical diagnostic test for BV is the Amsel criteria, which involves an assessment of four conditions (vaginal pH >4·5, malodorous secretion following potassium hydroxide, presence of clue cells, and self-reported vaginal discharge) with the existence of three or more conditions corresponding to a positive diagnosis of BV [20, 21]. A second, laboratory-based diagnostic test for BV involves a Gram stain of vaginal fluid, the quantification of the amount of three morphotypes (Lactobacillus, Mobiluncus and Gardnerella) and the use of the Nugent criteria to define a case of BV [22]. Since the first stage in initiating BV screening using either method is commonly dependent on pre-emptive self-reported symptoms, many asymptomatic cases of BV are not detected or treated. Moreover, women reporting many of the symptoms characteristic of BV may instead have a number of other lower genital tract conditions such as Chlamydia or gonococcal cervicitis, trichomonos, or candida. Thus, the development of an alternative BV screening tool is warranted.
In this study, we described the prevalence of symptomatic and asymptomatic BV among women in the first trimester of pregnancy; assessed the agreement between individual self-reported vaginal symptoms and BV status; and examined the utility of creating a BV screening tool incorporating clinical, demographic and symptom parameters to identify BV-positive women.
METHODS
Study design
During the period September 2001 to June 2004, pregnant women presenting for their first prenatal care visit to two obstetrical practices at the Hospital of the University of Pennsylvania (HUP), who had completed ⩽12 weeks gestation based on self-reported last menstrual period (LMP) and resided within the city of Philadelphia were eligible to participate in the project. To ensure the generalizability of the study results, one of the recruitment sites was a private obstetrical practice providing care to insured women, and the other recruitment site was a publicly funded obstetric clinic. Nurse interviewers specifically trained for the project approached eligible women in the office, explained the purpose and practice of the study, and obtained informed consent. The nurse interviewers facilitated universal vaginal swab collection for BV assessment, obtained urine samples for toxicological confirmation of cigarette, marijuana, and cocaine use; collected contact information to inform follow-up activities; performed a comprehensive abstraction of the prenatal record to collect information on current and subsequent BV diagnosis and treatment; and completed the standardized baseline questionnaire. Eighty-seven percent of eligible women participated in the project. The protocol and consent forms were approved by the Institutional Review Board of the University of Pennsylvania.
Specific questions concerning vaginal symptoms were included within the baseline questionnaire to assess current vaginal itching or burning, vaginal swelling, vaginal pain, abnormal discharge, and/or abnormal odour since LMP. Information on the consistency of the discharge (i.e. thicker than normal, thin/watery or same consistency) and colour of the discharge using visual aids was also noted. Information on the presence and magnitude of vaginal bleeding was also collected. The questionnaire was administered in a confidential location within the office by a female nurse interviewer.
Assessment of BV
At the enrolment visit, provider-collected (53% of the sample) or self-collected (47% of the sample) vaginal swabs were obtained for each woman. We have previously documented excellent validity and reliability in BV assessment, among this cohort, using these two methods of swab collection [23]. Vaginal secretions were put on a glass slide, air dried and the slides were sent weekly to the Clinical Microbiology Laboratory at the HUP. A single microbiologist Gram-stained and blindly interpreted the samples for BV status using the criteria described by Nugent et al. [22]. A summary BV score was computed for each participant ranging from 0 to 10; this score was dichotomized with values of ⩾7 indicating a case of BV. This dichotomized scoring system has been used extensively in both clinical and epidemiological studies of BV positivity and has been found to be a reliable method of detecting overall BV positivity [24]. The interviewer and obstetrician were blinded to the study-related Gram stain results. The remainder of prenatal care was under the direction of the woman's health-care provider. Any provider-directed BV screening during the course of prenatal care, primarily due to patient symptom report, was not used in determining study-related BV status. We did abstract the prenatal care record of all women participating in the study and collected information on subsequent BV diagnosis and treatment.
Data analysis
Self-reported vaginal symptoms (yes/no) were based on the vaginal complaints captured in the baseline interview. Women were considered to have self-reported symptoms if they reported having at least one symptom since LMP. We calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each of these vaginal symptoms as predictors of BV.
In the univariable analysis testing the association of various risk factors for BV positivity, Pearson's χ2 or Fisher's exact tests were used to analyse categorical variables, and t tests were used for continuous comparisons. Gestational age was determined by LMP and confirmed by ultrasound prior to 20 weeks. Perceived stress was measured using the Cohen's perceived stress scale [25]. We created a multivariable logistic regression model to determine the association between self-reported symptoms, identified risk factors, and BV positivity. The model included variables easily collected in the clinical setting that were significantly associated with BV positivity in the univariate analyses at P⩽0·05. The following variables were included in the model; self-reported vaginal odour (y/n), self-reported abnormal discharge (y/n), African American race (yes/other), cigarette smoking (y/n), vaginal douching (y/n), and age (<20 years, ⩾20 years). Parity and age were highly correlated, thus only age was included in the model. Vaginal douching since LMP was dropped from the model due to a high number of missing values and ever douching was modelled instead. Cigarette and marijuana use were also highly correlated and cigarette use was included in the model. Stepwise logistic regression was performed by removing each least-significant factor and then refitting the model with P<0·05 considered significant. The goodness of fit of the final regression model was assessed using Wald χ2 and logistic regression diagnostics.
The screening tool was developed by assigning a score depending on the presence or absence of the three identified significant risk factors for BV using the results from the final multivariate regression model. A total risk factor score was determined for each woman by adding the number of individual risk factors present. For example, an African American woman who reported smoking cigarettes received a total risk score equal to 2. We used receiver-operator-characteristic (ROC) analysis to determine the optimal score cut-off point for prediction of BV with the maximal sensitivity while maintaining a reasonable specificity. The goal of the predictive model was to identify as many BV-positive women as possible; therefore, maximizing the sensitivity of the predictive rule was of importance. The SAS statistical package version 8.0 (SAS Institute Inc., Cary, NC, USA) was used.
RESULTS
The study enrolled 1948 women from September 2001 to June 2004 and 1916 women completed a baseline questionnaire, had a Gram stain result and were included in this analysis (98·4%). Forty percent of pregnant women screened positive for BV at the time of enrolment in the study (n=757) and over 20% reported a prior case of BV. Almost three quarters of the women were African American (72%), 17% were Caucasian, 6% Asian/Pacific Islander and 5% were of other racial groups. The average age at enrolment was 25±6·1 years and the average gestational age at enrolment at the time of BV assessment was 10±2·6 weeks. Sixty percent of women reported ever douching and, of these women, almost 20% reported current vaginal douching. The prevalence of any prior sexually transmitted disease (STD) was 78·7%. Over 10% of women reported current cigarette use and a slightly higher proportion of pregnant women reported marijuana use (13·2%).
Over one third (39·4%) of women with BV presented without any lower genital tract symptoms. About one-half (55%) of women without BV were asymptomatic. A slightly higher proportion of BV-positive compared to BV-negative women presented with abnormal discharge or odour (23·7% vs. 16·5% and 15·7% vs. 7·1%, respectively); however, the report of vaginal burning/itching, pain or swelling was similar between the two groups (13·0% vs. 13·4%; 5·8% vs. 5·3%; and 2·2% vs. 2·7%, respectively). As shown in Table 1, the report of abnormal odour [relative risk (RR) 1·62, 95% confidence interval (CI) 1·42–1·85] and abnormal vaginal discharge (RR 1·29, 95% CI 1·14–1·46) were significantly related to BV status in the univariate analysis.
Table 1.
Bivariate analysis of selected factors for bacterial vaginosis (BV) positivity among pregnant women
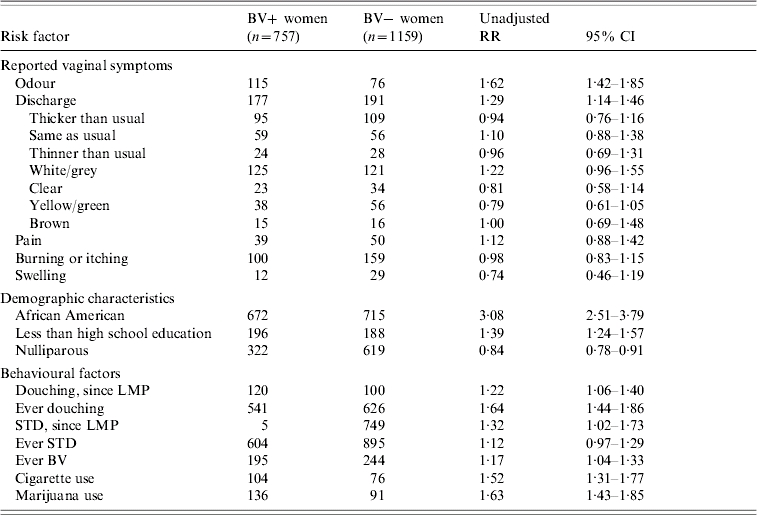
RR, Relative risk; CI, confidence interval; LMP, last menstrual period; STD, sexually transmitted disease.
Several demographic, behavioural and past/current obstetric factors were associated with BV. Women with BV were significantly younger than BV-negative women (24 years vs. 27 years; P<0·0001). In addition, African American race (RR 3·08, 95% CI 2·51–3·57), less than a high school education (RR 1·39, 95% CI 1·24–1·57) and nulliparity (RR 0·84, 95% CI 0·78–0·91) were related to BV status (Table 1). Many behavioural factors were related to BV positivity. BV-positive pregnant women were significantly more likely to report current or ever douching (RR 1·22, 95% CI 1·06–1·40 and RR 1·68, 95% CI 1·44–1·86, respectively), more likely to report a prior diagnosis of BV (RR 1·17, 95% CI 1·04–1·33), a current STD (RR 1·32, 95% CI 1·02–1·73) and to report current cigarette or marijuana use (RR 1·52, 95% CI 1·31–1·77 and RR 1·63, 95% CI 1·43–1·85, respectively) compared to BV-negative women. In addition, the mean stress score, using the Cohen perceived stress instrument, was higher among BV-positive compared to BV-negative women (5·1 vs. 4·9 respectively, P<0·001).
As shown in Table 2, none of the individual symptom reports had high sensitivity with corresponding high specificity for correctly classifying BV status. Reports of abnormal discharge and vaginal odour had low sensitivity but relatively high specificity, while reports of white/grey discharge had higher sensitivity but very low specificity. The report of vaginal pain or swelling also had very high specificity (95·7% and 97·5%, respectively) but very low individual sensitivity (5·2% and 1·6%, respectively).
Table 2.
Predictive characteristics of individual vaginal symptoms
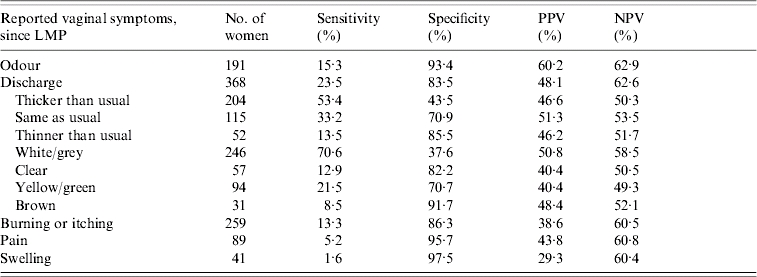
LMP, Last menstrual period; PPV, Positive predictive value; NPV, Negative predictive value.
The multivariate model included the significant factors related to BV positivity in the univariate analysis: African American race (RR 4·36, 95% CI 3·34–5·71), abnormal vaginal odour (RR 2·12, 95% CI 1·49–2·99), and smoking (RR 2·11, 95% CI 1·51–2·93). Abnormal vaginal discharge (RR 1·16, 95% CI 0·89–1·51) was not related to BV status after adjusting for the other demographic and behavioural factors. In addition, significant relationships did not remain for age, or current douching (Table 3). Adding stress to the model did not change these results. The goodness-of-fit statistic revealed that this final explanatory model fitted well (Wald χ2 statistic=188·7, P value <0·0001).
Table 3.
Final explanatory logistic regression model for factors related to bacterial vaginosis (BV) among pregnant women
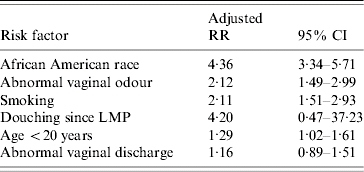
RR, Relative risk; CI, confidence interval; LMP, last menstrual period.
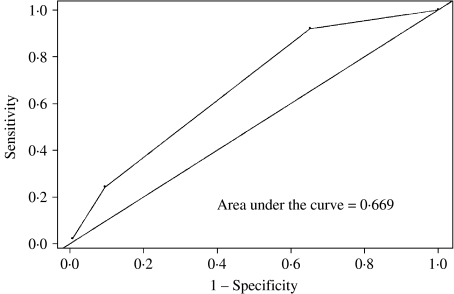
The Figure presents the ROC curve for the three-item scoring system which included African American race, abnormal vaginal odour and current smoking. A total risk score was determined for each woman by adding the number of risk factors present. The model was most ‘predictive’ of BV positivity when a score of ⩾2 was obtained. The area under the ROC curve was 0·669 (Fig.). As shown in Table 4, a score of ⩾2 resulted in the highest sensitivity with corresponding high specificity.
Fig.
Receiver-operator-characteristic curve for the three-item scoring system in predicting BV positivity. Score includes: African American race, abnormal vaginal odour and current smoking.
Table 4.
Characteristics of three-item scoring system in predicting bacterial vaginosis (BV) positivity
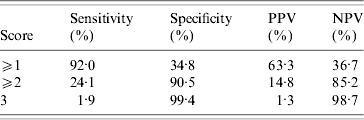
PPV, Positive predictive value; NPV, negative predictive value.
Risk factor scoring system: African American race, abnormal vaginal odour, and smoking.
DISCUSSION
Although universal screening for BV among low-risk pregnant women is not currently indicated given the ineffectiveness of treatment options in reducing these adverse outcomes, the diagnosis of BV is still important in this population [26, 27]. BV has been related to many adverse gynaecological conditions including: pelvic inflammatory disease, post-hysterectomy vaginal cuff cellulites, post-abortal endometritis, postpartum endometritis; and many adverse pregnancy outcomes including amniotic fluid infection, preterm delivery, preterm labour, preterm premature rupture of the membranes (PPROM), and spontaneous abortion [10–14, 28]. Given the high proportion of asymptomatic BV-positive women, we attempted to develop a screening tool which incorporated not only symptom reports, but demographic and behavioural and health factors in an effort to classify all cases of BV-positive pregnant women enrolled in our cohort.
As expected, we found that a high proportion of BV-positive pregnant women presented without vaginal symptoms and the classic lower genital tract complaints of recent abnormal vaginal discharge or odour were not accurate sensitive individual predictors of BV status. These findings are similar to other reports which conclude that abnormal odour and vaginal discharge are not useful independent markers in identifying BV-positive non-pregnant women [7]. We did find that the combination of abnormal vaginal odour, African American race and smoking were predictive of BV status and the absence of these factors was extremely useful in the identification of BV-negative women.
Our BV screening tool which includes abnormal odour, African American race, and smoking had high sensitivity (92%) for predicting BV positivity in the presence of two or more of these factors; although the specificity was modest (35%). However, the specificity of the screening tool with all three of the factors was very high (99·4%) indicating that the likelihood of BV positivity among women without any of these three factors upon presentation is very low. When used in our population of pregnant women with a prevalence of 40% for BV, the PPV using a cut-off of ⩾2 was 63% and the NPV was 36·7%.
Others have attempted to develop screening tools to identify BV status [29, 30]. Pastore et al. developed a six-item BV risk scoring system including vaginal pH >4·5, African American race, condom use during pregnancy, antenatal BV, absences of semen on smear, and no history of STD, among a population of pregnant women with a lower prevalence of BV (18%). The authors reported a sensitivity and specificity of 77% among women with scores ⩾4. Two of the risk factors found to be predictive among this study population (i.e. STD and prior antenatal BV), were not predictive in our model perhaps due to the different demographic characteristics of the study populations. We did not collect information on vaginal pH or semen at the time of BV assessment; it is unknown whether these characteristics dramatically influence the test characteristics of our tool.
Several limitations in this study design deserve mention. In addition to not measuring vaginal pH at the time of BV assessment, an assessment of other lower genital tract conditions characteristic of symptom reports among pregnant women were not measured (i.e. trichomonas, candida, and gonococcal or chlamydial cervicitis). Moreover, given the study population enrolled in the cohort and the high prevalence of BV (40%), these results may not be generalizable to other rural or primarily white pregnant women.
In this report, we conducted a comprehensive assessment to identify the behavioural, demographic and/or symptom reports most predictive of BV status. These results are useful in understanding the contribution of demographic, behavioural and clinical factors on BV and illustrate that although many providers rely on the report of abnormal odour or discharge to pursue further BV screening, we have shown that these symptoms are not sensitive in detecting BV. These results indicate that a three-item prediction rule including the report of vaginal odour, in combination with race and smoking status, is most sensitive in predicting BV-positive women. In a busy obstetric clinic, universal screening for BV may be problematic and time consuming. We have developed and tested the utility of a three-item BV screening tool which may be useful in designing targeting screening programmes among pregnant women to facilitate selective BV screening, treatment and reduction of disease burden among high-risk pregnant women. In addition, this screening tool includes easy-to-measure self-reported factors and was also effective in identifying a low-risk group of BV-negative pregnant women with a high predictive value.
ACKNOWLEDGEMENTS
The authors thank Sara Kietzman and Lena Flowers who interviewed all the women enrolled in this study and Jill Wadlin for the BV assessment.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Rein MF, Holmes KK. Non-specific vaginitis, vulvovaginal candidiasis, and trichonomasis clinical features, diagnosis and management. Current Clinical Topics in Infectious Diseases. 1983;4:281–315. [Google Scholar]
- 2.Fleury FS. Adult vaginitis. Clinical Obstetrics and Gynecology. 1981;24:407–438. doi: 10.1097/00003081-198106000-00008. [DOI] [PubMed] [Google Scholar]
- 3.McGregor JA, French CM. Bacterial vaginosis in pregnancy. Obstetrical & Gynecological Survey. 2000;55:1–19. doi: 10.1097/00006254-200005001-00001. [DOI] [PubMed] [Google Scholar]
- 4.Holzman C et al. Factors linked to bacterial vaginosis in nonpregnant women. American Journal of Public Health. 2001;91:1664–1670. doi: 10.2105/ajph.91.10.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cristiano L et al. Bacterial vaginosis: prevalence in outpatients, association with some micro-organisms and laboratory indices. Genitourinary Medicine. 1989;65:382–387. doi: 10.1136/sti.65.6.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yen S et al. Bacterial vaginosis in sexually experienced and non-sexually experienced young women entering the military. Obstetrics and Gynaecology. 2003;102:927–933. doi: 10.1016/s0029-7844(03)00858-5. [DOI] [PubMed] [Google Scholar]
- 7.Klebanoff MA et al. Vulvovaginal symptoms in women with bacterial vaginosis. Obstetrics and Gynaecology. 2004;104:267–272. doi: 10.1097/01.AOG.0000134783.98382.b0. [DOI] [PubMed] [Google Scholar]
- 8.Eschenbach DA et al. Prevalence of hydrogen peroxide producing lactobacillus species in normal women and women with bacterial vaginosis. Journal of Clinical Microbiology. 1989;27:251–256. doi: 10.1128/jcm.27.2.251-256.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiegel CA et al. Anaerobic bacteria in nonspecific vaginitis. New England Journal of Medicine. 1980;303:601–607. doi: 10.1056/NEJM198009113031102. [DOI] [PubMed] [Google Scholar]
- 10.Abner KP et al. Risk factors for plasma cell endometritis among women with cervical Neisseria gonorrhea, cervical Chlamydia trachomatis, or bacterial vaginosis. American Journal of Obstetrics and Gynecology. 1998;178:987–990. doi: 10.1016/s0002-9378(98)70536-8. [DOI] [PubMed] [Google Scholar]
- 11.Meis PJ et al. The preterm prediction study: significance of vaginal infections. American Journal of Obstetrics and Gynecology. 1995;173:1231–1235. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 12.Hillier SL et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. New England Journal of Medicine. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 13.Kurki T et al. Bacterial vaginosis in early pregnancy and pregnancy outcome. Obstetrics and Gynaecology. 1992;80:173–177. [PubMed] [Google Scholar]
- 14.Hay PE et al. Abnormal bacterial colonization of the genital tract and subsequent preterm delivery and late miscarriage. British Medical Journal. 1994;308:295–298. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peipert JF et al. Bacterial vaginosis as a risk factor for upper genital tract infection. American Journal of Obstetrics and Gynecology. 1997;177:1184–1187. doi: 10.1016/s0002-9378(97)70038-3. [DOI] [PubMed] [Google Scholar]
- 16.Goffinet F et al. Bacterial vaginosis: prevalence and predictive value for premature delivery and neonatal infection in women with preterm labour and intact membranes. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2003;108:146–151. doi: 10.1016/s0301-2115(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 17.Hauth JC et al. Early pregnancy threshold vaginal pH and Gram stain scores predictive of subsequent preterm birth in asymptomatic women. American Journal of Obstetrics and Gynecology. 2003;188:831–835. doi: 10.1067/mob.2003.184. [DOI] [PubMed] [Google Scholar]
- 18.Meis PJ et al. The preterm prediction study: significance of vaginal infections. American Journal of Obstetrics and Gynecology. 1995;173:1231–1235. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 19.Oakeshott P et al. Bacterial vaginosis and preterm birth: a prospective community-based cohort study. British Journal of General Practice. 2004;54:119–122. [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtman R, Duran P, Lichtman R, Papera S. The Vulva and Vagina. Norwalk, CT:: Appleton & Lange; 1990. Well woman care, chapter 11. [Google Scholar]
- 21.Eschenbach DA et al. Diagnosis and clinical manifestations of bacterial vaginosis. American Journal of Obstetrics and Gynecology. 1988;158:819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 22.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of Clinical Microbiology. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson DB et al. Self-collected versus provider-collected vaginal swabs for the diagnosis of bacterial vaginosis: an assessment of validity and reliability. Journal of Clinical Epidemiology. 2003;56:862–866. doi: 10.1016/s0895-4356(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 24.Joesoef MR et al. Reproducibility of a scoring system for gram stain diagnosis of bacterial vaginosis. Journal of Clinical Microbiology. 1991;29:1730–1731. doi: 10.1128/jcm.29.8.1730-1731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 26.Carey JC et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. New England Journal of Medicine. 2000;342:534–540. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 27.Berghella V et al. Sexual intercourse association with asymptomatic bacterial vaginosis and Trichomonas vaginalis treatment in relationship to preterm birth. American Journal of Obstetrics and Gynecology. 2002;187:1277–1282. doi: 10.1067/mob.2002.127134. [DOI] [PubMed] [Google Scholar]
- 28.Eschenbach DA et al. Diagnosis and clinical manifestations of bacterial vaginosis. American Journal of Obstetrics and Gynecology. 1988;158:819–828. doi: 10.1016/0002-9378(88)90078-6. [DOI] [PubMed] [Google Scholar]
- 29.Pastore LM et al. Risk score for antenatal bacterial vaginosis: BV PIN points. Journal of Perinatology. 2002;22:125–132. doi: 10.1038/sj.jp.7210654. [DOI] [PubMed] [Google Scholar]
- 30.Pastore LM et al. Prospective validation of a perinatal bacterial vaginosis screening risk score. Journal of Perinatology. 2004;24:735–742. doi: 10.1038/sj.jp.7211179. [DOI] [PubMed] [Google Scholar]