SUMMARY
Acute respiratory infections (ARIs) are the most important infectious cause of death, but there is less information of their burden in the community. This study describes the burden of ARI in a cohort of 50 Iranian families visited weekly over 2 months. Eighty-one out of 113 (72%) children and 29/103 (28%) adults had a total of 124 episodes of ARI. Seventy-five per cent of the episodes occurring in children were primary/co-primary compared to 40% of those in adults (P<0·01). Children were more likely to be the first symptomatic cases and infections were frequently transmitted within the family. Frequencies were lowest among adults, low among infants aged <6 months and highest among children aged <5 years (P<0·01). Winter episodes occurred more frequently in January (P<0·01). The high frequency and apparent transmissibility of ARI in this cohort highlights its enormous burden in the community.
INTRODUCTION
Acute respiratory infections (ARIs) are the most important cause of childhood mortality and morbidity worldwide causing approximately one fifth of all childhood deaths [1, 2]. Although community-based studies in Latin America and Africa have described the largest burden of community-acquired ARI in developing countries as occurring in infants and young children [3–7], there is a paucity of information of the epidemiology of these infections in the Middle East. This geographical area has very contrasting epidemiological features, varying from high to low infant mortality, often in neighbouring countries, and infectious diseases still cause significant morbidity and mortality [8].
The data presented here describe the frequency, intra-familial transmission and clinical characteristics of ARI in a community-based cohort of nuclear, stable families resident in Rasht, in northern Iran. This information is critical to highlight the burden of ARI in the community.
METHODS
The study was based in a cohort of families registered in two primary health-care centres (Golsar and Rashtian) in Rasht city in northern Iran. These centres are attended by mostly high- and middle-income families residing in stable economic and housing conditions with good access to preventive and curative services. The medical records of the centres were screened consecutively to construct a list of families with at least two children, one of whom was <1 year old. The first 50 families fulfilling these criteria were visited at home by three teams of nurses and family health-care technicians. After explaining the purpose of the study, families were invited to participate and to provide informed verbal consent. Eight families who refused to participate were replaced with the next consecutive families selected from the centres. All families were enrolled from mid-November to mid-December 2003, after a pilot study to identify potential logistical problems. Each family was then followed for 2 months and allowed to drop out at the end of their 2-month follow-up.
After collection of baseline data, including the household and family characteristics, families were visited on a weekly basis for 2 months to ascertain the presence of ARI. If a family experienced an ARI episode, visits were increased to twice weekly to identify other cases in the household, to describe the incidence and transmission within the family and the burden of ARI in this community. An upper respiratory infection was defined as the presence of acute respiratory symptoms at the time of visiting the household, including cough, nasal congestion, rhinorrhoea, conjunctivitis, sore throat, hoarseness and fever. Only upper respiratory episodes with at least two of these symptoms, as verified by the investigators, were included. An acute lower respiratory infection was defined as the presence of respiratory signs and symptoms of <7 days' duration and followed the World Health Organisation (WHO) diagnostic protocol, which is based on the respiratory rate and the presence of cough and chest indrawing [9].
Primary ARI was defined as the first symptomatic case in the family, irrespective of disease severity. Co-primary ARI was defined as any other case developing signs and symptoms within 2 days after the primary case. Secondary ARI was defined as any second, third, fourth or more individuals in the household who developed symptoms ⩾3 days after the start of a primary case. Each individual with an ARI episode was followed to establish the duration of illness. New cases occurring ⩾1 week after all ARI episodes had resolved in a family were classified as new primary infections.
Statistical analyses included summary descriptive statistics [median, interquartile ranges (IQR), percentages]. The frequency of respiratory episodes in adults and children were compared using χ2 tests. Parametric and non-parametric tests were used to compare normally and not normally distributed data, respectively. P values <0·05 were considered as significant.
Ethical approval for the study was obtained from the Research Ethics Committees of the Liverpool School of Tropical Medicine and Guilan University of Medical Sciences. Informed consent to participate was obtained from all families. Participants were free to decline or withdraw at any time without suffering any disadvantage or prejudice.
RESULTS
The 50 families selected comprised 113 children aged <16 years and 103 adults, including 100 parents and three grandmothers. There were no single-parent families. The median number of children was two, with a range of 2–5. The median age was 3 (IQR 1–9) years for the children and 35 (IQR 31–38) years for the adults. The number of bedrooms ranged from one to four with a median of two. All families had piped water supplies and only one (2%) kept animals (chicken) at home. Forty-six (92%) of the 50 families experienced an ARI during the follow-up period. There were no statistical differences between the general characteristics of the families with and without ARI. In total, 124 ARI episodes were identified, resulting in an average of 1·2 episodes per family/month. Eighty-one of the 113 (72%) children and 29/103 (28%) of the adults had one or more episodes and 94/124 episodes occurred in 81 children and 30 episodes occurred in 29 adults. Children with ARI were slightly older (median 3, IQR 1–7 years) than children without ARI (2, IQR 1–10 years) and adults with ARI were younger than those without ARI (33, IQR 29–35 and 35, IQR 32–40 years respectively), but these differences were not statistically significant. Similar proportions of children and adults with and without ARI were male, although slightly more women than men experienced ARIs (P=0·07).
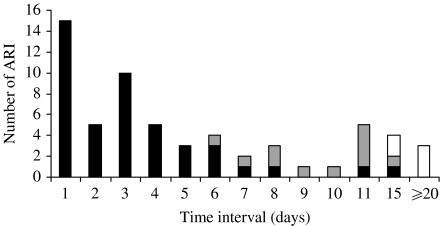
Seventy-one (75%) of the episodes in children were defined as primary or co-primary and 23 (24%) as secondary cases, while 12/30 (40%) of the episodes in adults were primary or co-primary and 18/30 (60%) secondary cases, resulting in a larger proportion of episodes in children being classified as primary or co-primary than in adults (P<0·01), as shown in Table 1. The time gaps between the start of the first and subsequent episodes in the family are shown in Figure 1. Most of the second episodes of ARI in the household occurred within 6 days, most of third episodes occurred within 6–14 days after the initial episodes and most of the fourth episodes occurred 15 days after the first case was identified, suggesting several waves of transmission with delay in onset of symptoms of between 1 and 6 days from the previous case.
Table 1.
Type and duration of acute respiratory infection (ARI) in children and adults
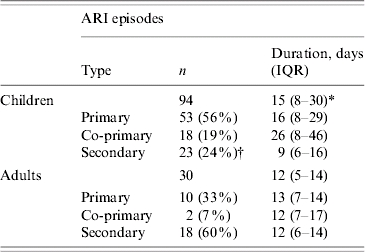
IQR, Interquartile range.
P<0·05
P<0·01 when compared with adults.
Fig. 1.
Time interval between the first and subsequent cases of acute respiratory infection (ARI) occurring within a household. ■, Second cases; , third cases; □, fourth cases.
, third cases; □, fourth cases.
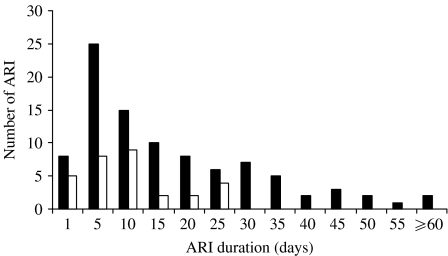
The distribution of ARI by age is shown in Table 2. Infants aged <6 months had the lowest and children aged 6 months to 5 years the highest frequencies among children, while adults had the lowest attack rates (P<0·01). Most episodes were mild, presenting with nasal congestion, cough and hoarseness. Five (5%) children had skin rashes and one was hospitalized. Sore throat was more frequent in adults than children [17 (51%) vs. 20 (21%) respectively, P<0·01], although this might reflect the difficulty in assessing this sign in children. The duration of ARI are shown in Figure 2. Most (70%) children were breastfed, one third were breastfeeding on enrolment and 43% attended a nursery. Nearly all children and more than half of the adults had experienced significant ARI in the preceding 6 months and wheezing was the most common medical complaint in children. One third of the children had been hospitalized, mostly due to neonatal icterus, gastroenteritis or pneumonia. A high proportion of the adults (46%) had high education and smoking was relatively rare, with only 12% of the adults reporting smoking. None of these factors were associated with an increased incidence of ARI.
Table 2.
Frequency of Acute respiratory infections (ARI) by age
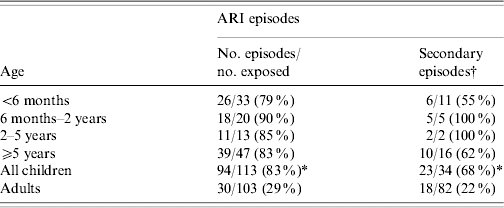
P<0·01 when compared with adults.
Number of participants experiencing secondary episodes among participants not experiencing primary or co-primary episodes.
Fig. 2.
Duration of acute respiratory infection (ARI) episodes in children and adults. ■, Children; □, adults.
The frequencies of ARI in November and December were similar [14 episodes out of 58 (24%) participants and 44 episodes out of 216 (20%) participants, respectively], increased in January [49 episodes out of 146 (34%) participants, P<0·01] and decreased in February [17 episodes out of 89 (19%) participants, P<0·05], suggesting that episodes occurred more frequently in January during the winter season.
DISCUSSION
This study highlights the high burden of ARI in the community and its rapid and high frequency of transmission within the family. Similar to reports from other geographical areas, children were more frequently affected than adults [3, 6].
The transmission in children and adults differed, with children being mostly primary and co-primary cases, while adults were mostly secondary cases. Although the study included small numbers of children and caution is necessary when interpreting the data, the patterns observed suggest that children were more likely to be infected outside the household, playing an important role in the spread of infection within the family. The high proportion of episodes among adults confirms that adults were susceptible to the infections, suggesting that previous infections had resulted in incomplete protection [10]. Case definitions, however, were based on the development of signs and symptoms, and an alternative hypothesis is that adults were more likely to experience asymptomatic infections [11], with parents bringing the infections into the household through asymptomatic carriage, transmitting the infection to their immunologically naive children who in turn, were more likely to develop symptoms. The pattern observed, therefore, could reflect either increased susceptibility to overt disease or to infection, although as pathogens also change over time, adults and children are all immunologically naive at some point.
There were no significant variations in the age distribution of ARI in children suggesting that, although younger children are more vulnerable to severe episodes, the incidence is similar across all young age groups. This is in agreement with previous studies describing that age does not so much affect incidence of ARI but rather severity and mortality [12], while studies in industrialized countries, where the mortality of ARI is low, still report similar ARI incidence as studies in developing countries, where the fatality rates are still high [13, 14].
Despite most episodes having a mild clinical presentation, episodes in children had longer duration than in adults. This might be due to children having mostly primary and co-primary infections while parents experienced mostly secondary episodes and were thus able to develop faster immune responses [15].
The seasonality of ARI varies with geography and climate [16], with more than one yearly peak occurring in some countries [17]. These differences can be due to year on year variations of the particular pathogens, environmental factors, population behaviour and immunity [18]. Despite the short duration of the study, there was a higher frequency of ARI, with a peak activity in January. This might reflect the high frequency of ARI during the winter season, and is in agreement with a 10-year study describing the incidence of influenza-associated ARI in Iran as being higher from November to April, with a peak between January and February [19].
ARI are due to a large number of different pathogens, each with a specific epidemiological pattern. However, their clinical presentations are often indistinguishable from each other unless aetiological studies are conducted. This is often difficult in community-based studies, where most episodes are mild and are treated symptomatically. Despite this community being stable, well educated and with good housing conditions, ARI still represents a high burden of disease. The high frequency of ARI highlights that it is not only a major cause of hospitalization, but also a high burden in the community. Even if only a fraction of these infections become life threatening, it results in large number of patients presenting to the health services with significant associated costs. Preventive measures, such as the development of specific vaccines [20] and nutritional supplements that decrease disease severity such as vitamin A and zinc [21], should be given research priority in countries with a high burden of ARI.
ACKNOWLEDGEMENTS
This study was supported by the Iranian Ministry of Health and Medical Education through a study scholarship for Dr M. Naghipour.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Lanata CF et al. Methodological and quality issues in epidemiological studies of acute lower respiratory infections in children in developing countries. International Journal of Epidemiology. 2004;33:1362–1372. doi: 10.1093/ije/dyh229. [DOI] [PubMed] [Google Scholar]
- 2.Rudan I et al. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bulletin of the World Health Organization. 2004;82:895–903. [PMC free article] [PubMed] [Google Scholar]
- 3.Barata Rde C et al. Gastroenteritis and acute respiratory infections among children under 5 years old in an area of southeastern Brazil, 1986–1987. Revista de Saúde Pública. 1996;30:553–563. doi: 10.1590/s0034-89101996000600010. [DOI] [PubMed] [Google Scholar]
- 4.Hortal M et al. A community-based study of acute respiratory tract infections in children in Uruguay. Reviews of Infectious Diseases. 1990;12:S966–73. doi: 10.1093/clinids/12.supplement_8.s966. [DOI] [PubMed] [Google Scholar]
- 5.Lopez Bravo IM, Sepulveda H, Valdes I. Acute respiratory illnesses in the first 18 months of life. Pan American Journal of Public Health. 1997;1:9–17. doi: 10.1590/s1020-49891997000100003. [DOI] [PubMed] [Google Scholar]
- 6.Oyejide CO, Osinusi K. Acute respiratory tract infection in children in Idikan Community, Ibadan, Nigeria: severity, risk factors, and frequency of occurrence. Reviews of Infectious Diseases. 1990;12:S1042–1046. doi: 10.1093/clinids/12.supplement_8.s1042. [DOI] [PubMed] [Google Scholar]
- 7.Vaahtera M et al. Epidemiology and predictors of infant morbidity in rural Malawi. Paediatric and Perinatal Epidemiology. 2000;14:363. doi: 10.1046/j.1365-3016.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- 8.Shawky S. Infant mortality in Arab countries: sociodemographic, perinatal and economic factors. Eastern Mediterranean Health Journal. 2001;7:956–965. [PubMed] [Google Scholar]
- 9.Pio A. Standard case management of pneumonia in children in developing countries: the cornerstone of the acute respiratory infection programme. Bulletin of the World Health Organization. 2003;81:298–300. [PMC free article] [PubMed] [Google Scholar]
- 10.Hacking D, Hull J. Respiratory syncytial virus, viral biology and the host response. Journal of Infection. 2002;45:18–24. doi: 10.1053/jinf.2002.1015. [DOI] [PubMed] [Google Scholar]
- 11.van Gageldonk-Lafeber AB et al. A case-control study of acute respiratory tract infection in general practice patients in The Netherlands. Clinical Infectious Diseases. 2005;41:490–497. doi: 10.1086/431982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acharya D et al. Acute respiratory infections in children: a community based longitudinal study in south India. Indian Journal of Public Health. 2003;47:7–13. [PubMed] [Google Scholar]
- 13.Klig JE, Chen L. Lower respiratory infections in children. Current Opinion in Pediatrics. 2003;15:121–126. doi: 10.1097/00008480-200302000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Henrickson KJ et al. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatric Infectious Disease Journal. 2004;23:S11–18. doi: 10.1097/01.inf.0000108188.37237.48. [DOI] [PubMed] [Google Scholar]
- 15.Simoes EA. Respiratory syncytial virus infection. Lancet. 1999;354:847–852. doi: 10.1016/S0140-6736(99)80040-3. [DOI] [PubMed] [Google Scholar]
- 16.Robertson SE et al. Respiratory syncytial virus infection: denominator-based studies in Indonesia, Mozambique, Nigeria and South Africa. Bulletin of the World Health Organization. 2004;82:914–922. [PMC free article] [PubMed] [Google Scholar]
- 17.Nokes DJ et al. Respiratory syncytial virus epidemiology in a birth cohort from Kilifi district, Kenya: infection during the first year of life. Journal of Infectious Diseases. 2004;190:1828. doi: 10.1086/425040. [DOI] [PubMed] [Google Scholar]
- 18.Weber A, Weber M, Milligan P. Modeling epidemics caused by respiratory syncytial virus (RSV) Mathematical Biosciences. 2001;172:95–113. doi: 10.1016/s0025-5564(01)00066-9. [DOI] [PubMed] [Google Scholar]
- 19.Mokhtari-Azad T et al. Influenza surveillance in the Islamic Republic of Iran from 1991 to 2001. Eastern Mediterranean Health Journal. 2004;10:315–321. [PubMed] [Google Scholar]
- 20.Moore ML, Peebles RS., Jr. Respiratory syncytial virus disease mechanisms implicated by human, animal model, and in vitro data facilitate vaccine strategies and new therapeutics. Pharmacology & Therapeutics. 2006;112:405–424. doi: 10.1016/j.pharmthera.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Cuevas LE, Koyanagi A. Zinc and infection: a review. Annals of Tropical Paediatrics. 2005;25:149–160. doi: 10.1179/146532805X58076. [DOI] [PubMed] [Google Scholar]