SUMMARY
For jurisdictions implementing measles elimination strategies, a minimum surveillance benchmark of 1/100 000 population per year measles-like illness (MLI) cases initially notified, but then rejected based on laboratory testing was proposed. We used this standard to assess the quality of the Victorian enhanced measles surveillance between 1998 and 2003. Victorian enhanced measles surveillance includes interviews with notified cases and confirmatory laboratory testing for notifications. We found 72% (918/1281) of measles notifications were discarded after testing. The median annual rate of discard was 2·9/100 000. The annual discard rate was inversely associated with the age of the notifications, and measles negative with no other diagnosis made was the most common laboratory outcome. The annual rates of discarded notifications in Victoria were consistently above the minimum recommended standard. The rate of discarded MLIs as a surveillance threshold should be useful in measles endemic regions, but may require modification where disease elimination has occurred.
INTRODUCTION
In 1997 the Victorian Department of Human Services (DHS) in Australia enhanced measles surveillance by endeavouring to follow-up and obtain laboratory confirmation of every notification in the state [1]. Measles control strategies have been very effective in Victoria with high vaccine coverage (at 30 June 2006, 95% of children had received the first dose of measles–mumps–rubella (MMR) vaccine scheduled at 12 months of age by their second birthday [2]) and long periods with no endemic disease punctuated by virus importation with usually limited local spread and occasional larger outbreaks [3–5]. Examination of circulating viruses in Victoria also shows increasingly rapid turnover of virus genotypes [6]. The Western Pacific Region of the World Health Organization (WHO), including Australia, has nominated a target date of 2012 for measles elimination [7]. The combination of the genotype data, the absence of endemic measles transmission and the lack of sustained transmission following importation provide evidence that measles has been eliminated from Victoria [8].
As part of the poliomyelitis eradication strategy, the rate of acute flaccid paralysis (AFP) notifications is used to assess the performance of the surveillance system [9, 10]. Based on the non-polio AFP model, Harpaz & Papania proposed the quality of measles surveillance systems be assessed by calculating the annual rate of notified measles-like illness (MLI) investigated and discarded following laboratory testing [11]. For jurisdictions that are implementing measles elimination strategies and enhanced surveillance, the recommended minimum standard of annual rate of MLI investigated is one discarded case per 100 000 population per year [11]. Harpaz & Papania used this standard to assess surveillance in over 80 surveillance programmes in the United States, other countries in the Americas and other WHO regions [11].
We assessed the quality of the Victorian enhanced measles surveillance system using the annual rate of discard of MLI from 1998 to 2003. In addition, we provide comparisons of the discard rates by age group and determined if rates differ between epidemic and inter-epidemic periods.
METHODS
Victoria is the second most populous state in Australia with more than 4·8 million people in an area just smaller than the United Kingdom [12]. Infectious disease surveillance and response programmes are centred in the capital, Melbourne. Doctors and laboratories are required to report presumptive measles cases to the DHS under the Health (Infectious Diseases) Regulations [13]. The enhanced measles surveillance system has been described previously [1]. Briefly, telephone interviews and home-based serological testing, if serology has not already been performed, are offered for every notified measles case. Specimens negative for measles-specific IgM are routinely tested for rubella and parvovirus B19-specific IgM, with serological tests for other viruses as clinically indicated.
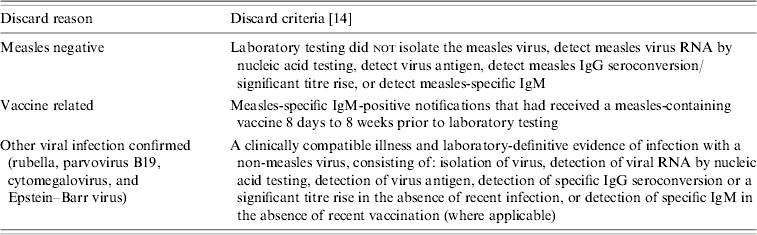
We reviewed all measles notifications received by the DHS between 1998, when enhanced surveillance commenced, and 2003. Our analysis focused only on discarded notifications and excluded notifications that were either not laboratory tested or were MLI cases that were epidemiologically linked to a laboratory-confirmed case in the absence of laboratory testing. We classified discarded notifications in two ways: first, by whether a diagnosis resulting in discard could be based on laboratory criteria (Table 1) [14] and, second, whether the notification was received in an epidemic or inter-epidemic period.
Table 1.
Discard classification for laboratory-investigated notifications of presumptive measles
We defined an epidemic period as beginning from the date a confirmed case of measles was reported to the DHS and ending at 28 days, approximately two measles incubation periods [15], after rash onset of the last confirmed case in the period. In our analysis, one or more confirmed cases was defined as the beginning of an epidemic period. Inter-epidemic periods commenced the day after an epidemic period concluded and ended the day prior to the date of notification of the next confirmed measles case. We examined the proportion of notifications that had follow-up with laboratory testing in inter-epidemic and epidemic periods.
Calculations of the discard rates were based on methods described by Harpaz & Papania [11]. The median discard rate and range by inter-epidemic and epidemic periods and age group are provided.
The enhanced measles surveillance data were stored on the Notifiable Infectious Diseases Surveillance version 1.3.0 (Department of Human Services, Victoria, Australia) system using a Microsoft Access 2000 (Microsoft Corporation, Redmond, WA, USA) database. The data were analysed using stata 8.0 (Stata Corporation, College Station, TX, USA). Estimated mid-year annual population values for Victoria were obtained from the Australian Bureau of Statistics [16].
RESULTS
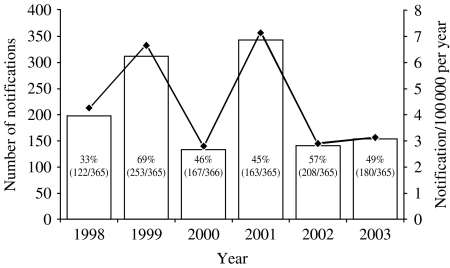
The DHS received 1281 measles notifications between 1998 and 2003 at a median annual rate of 3·7 notifications/100 000 population (range 2·9–7·1/100 000) across the state (Fig. 1). Out of the 1281 notifications, 251 were laboratory confirmed or epidemiologically linked to a laboratory-confirmed case. Of the remaining 1030 notifications, 112 (11%) did not have sufficient laboratory information to allow them to be discarded, leaving 918 (89%) notifications discarded on the basis of laboratory testing. Laboratory testing was performed for 90% (1153/1281) of all notifications received in the review period. Of the 918 notifications discarded on the basis of laboratory testing, 369 (40%) were received in inter-epidemic periods and 549 (60%) were received in epidemic periods.
Fig. 1.
Number and rate of measles notifications received and the proportion of days in each year in an epidemic period, Victoria, 1998–2003 (n=1281). (Victorian population based on estimated annual mid-year data; Australian Bureau of Statistics [16].) □, Number; –◆–, rate.
From 1998 to 2003 the median annual rate of measles notifications discarded in Victoria was 2·9/100 000 (Table 2). Each year contained epidemic and inter-epidemic periods (Fig. 1, Table 2) with the median annual rate of discard higher in epidemic periods (3·3/100 000) than inter-epidemic periods (2·6/100 000).
Table 2.
Annual rate of discard (per 100 000 population) by inter-epidemic and epidemic periods, 1998–2003, Victoria*
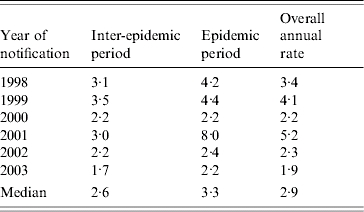
Victorian population based on estimated annual mid-year data (Australian Bureau of Statistics [16]).
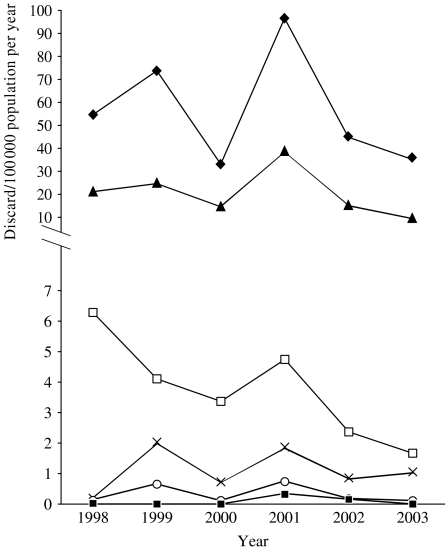
There was an inverse relationship between the median annual rate of discard and the age of the case at the time of notification (Fig. 2). The annual rates of discard in the review period were above 1/100 000 for all age groups aged <16 years. The median annual rate of discard was highest for cases in the <1 year age group (49·8/100 000, range 33·1–96·6/100 000). For older age groups, 16–35 years, 36–64 years, and ⩾65 years, some annual figures fell below the 1/100 000 discard rate (Fig. 2).
Fig. 2.
Annual rate of measles discard by age group, 1998–2003, Victoria. (Victorian population based on estimated annual mid-year data; Australian Bureau of Statistics [16].) –◆–, <1 year; –▲–, 1–4 years; –□–, 5–15 years; –×–, 16–35 years; –○–, 36–64 years; –■–, ⩾65 years.
The two most common laboratory outcomes for discarded notifications were measles negative (median annual rate 2·6/100 000, range 1·7–4·8/100 000), that is no laboratory definitive evidence of measles and no other organisms identified, followed by parvovirus B19 infection (median annual rate 0·2/100 000, range 0·1–0·5/100 000). Other common laboratory outcomes were, in order of frequency, rubella, cytomegalovirus, and Epstein–Barr virus (all notified at a median annual rate of <0·05/100 000). Measles negative was also the most common reason for discard in the <1 year age group (median annual rate 57·2/100 000, range 41·9–104·9/100 000). Discards due to the diagnosis of parvovirus B19 were most commonly found in the 1–4 years (median annual rate 0·8/100 000, range 0·0–1·2/100 000) and the 5–15 years age groups (median annual rate 0·8/100 000, range 0·0–1·7/100 000). Discards associated with recent vaccination (Table 1) was most commonly found in the 1–4 years age group (median annual rate 2·0/100 000, range 1·2–3·2/100 000).
DISCUSSION
We applied the method proposed by Harpaz & Papania in assessing the quality of the Victorian measles surveillance system and found the annual rates of reported MLI investigated and discarded in Victoria between 1998 and 2003 have consistently been above the minimum recommended standard of case follow-up and laboratory testing, with a median annual discard rate of 2·9 discards/100 000 population [11]. Additionally, the proportion (90%) of notifications in the state that underwent laboratory testing was above the target 80% recommended by the WHO for regions in measles elimination phase [17].
The median rate of MLI investigated and discarded was higher in epidemic than inter-epidemic periods reflecting a higher overall rate of notification in epidemic periods. At times when measles is circulating locally, the likelihood of true measles cases increases and doctors and health authorities may be more likely to report and laboratory-test patients presenting with MLI.
There was age-group variation in the rate of discard during the review period. Year-by-year the discard rate in the younger age groups remained above the threshold level, up to the 5–15 years age group. The median rate of discard was highest in infants and decreased with increasing age, reflecting the pattern of MLI. We found the most common reason for discard was measles negative with no other diagnosis after appropriate laboratory testing. However, unlike the rare but very dramatic AFP associated with poliomyelitis, MLI is common, particularly in younger age groups, and can be caused by a variety of pathogens that are difficult to differentiate clinically without laboratory guidance. In order of frequency, other common viral causes of rash-like illness – parvovirus B19, rubella, cytomegalovirus, and Epstein–Barr virus – were identified in our study. The ages for which notifications were discarded following laboratory diagnosis of parvovirus B19 were consistent with the ages at which protective parvovirus antibodies were detected in a previous Victorian seroprevalence study [18]. Serological testing of MLI notifications was routinely restricted to measles, rubella and parvovirus B19, with further tests performed as guided by the clinical scenario. It is likely that a proportion of the illnesses in the discarded notifications and in the notifications without laboratory testing were caused by other viral exanthemata, such as human herpesvirus-6 associated with roseola infantum [19]. Another common reason for discards was recent vaccination, particularly in the 1–4 years age group, reflecting the recommended ages (12 months and 4 years) of administration of the first two doses of the MMR vaccine in Australia [20].
Despite efforts and resources being put into performing enhanced measles surveillance and laboratory testing of notified cases, our median rate of annual discard puts Victoria at the tail end of the curve for all sites listed by Harpaz & Papania [11]. Calculation of the rate of MLIs discarded relies on two components: the rate of notification of suspected measles cases and the rate of laboratory testing of these notifications. Variation in either component will affect the final figure. In Victoria routine one- and two-dose MMR vaccine coverage is high and population coverage was improved by the one-off nationwide Measles Control Campaign in 1998 [2, 21]. Further, there is good evidence from surveillance data, laboratory-testing data, and genotyping of circulating viruses, to suggest that measles has been eliminated. Local clinicians may be more aware that endemic measles has been halted and that epidemic circulation of the virus is uncommon. The reduced likelihood of diagnosing measles in the community may subsequently result in fewer clinicians notifying cases of MLI as measles [3, 22]. These factors are likely to have contributed to the lower rate of measles notification by clinicians in 2000, 2002, and 2003, when MLI discard rates in Victoria were at their lowest.
As Victoria does not have similar enhanced surveillance for rubella or other MLI, the proportion of all reported MLI in the community being investigated is unlikely to alter in the short-term. If the rate of measles notification continues to decline in Victoria, despite obtaining laboratory testing for the same proportion of notifications, the rate of MLI discarded and investigated in the state is likely to fall below the proposed benchmark. For example, using 2003 figures for population size, proportion of notifications laboratory tested, and proportion of these positive, halving the annual measles notification figure in Victoria would see the state-wide annual discard rate drop below 1/100 000. During a recent WHO Global Measles Management meeting, a yet higher measles surveillance benchmark was recommended: a minimum reporting of two suspected measles cases per 100 000 population per country [23]. Victoria, with a highly organized and relatively expensive measles surveillance system, and certainly countries with a less sophisticated system will probably find it difficult to meet the new WHO surveillance target. We suggest as measles control improves globally with measles notifications becoming much less common, a more sophisticated assessment of measles surveillance will be required in addition to the discard parameter suggested by Harpaz & Papania or similar. Surveillance assessment will need to rely more heavily on laboratory-testing figures and genotyping of isolated viruses, in the first instance, than surveillance of discarded MLI notifications [24, 25]. In areas where acute rubella surveillance is undertaken, combined notifications of rash illness or febrile-rash illness may be used to assess surveillance quality using discarded cases.
We found the guidelines for assessment of measles surveillance system proposed by Harpaz & Papania using the minimum rate of notifications investigated and discarded were a useful, practical, and quick method for assessing the quality of measles surveillance in Victoria. The annual rate of measles notifications investigated and discarded over the study period was consistently above 1·0/100 000 However, the method of assessment is specific for identifying weak measles surveillance programmes rather than the best performing programmes [11, 26, 27]. Maintenance and regular performance assessment of the enhanced surveillance system are important for progression towards measles eradication. Further consideration needs to be given to refining the method of assessing measles surveillance, with particular focus on laboratory testing and genotyping (to confirm the lack of a circulating endemic strain and to potentially identify a source country of importation), as larger regions approach or reach measles elimination.
ACKNOWLEDGEMENTS
We acknowledge Pauline Lynch and the General Surveillance & Control Program, Victorian Department of Human Services who were responsible for conducting the surveillance; Victorian Infectious Diseases Reference Laboratory which was responsible for confirmatory laboratory testing; and Debbie Gercovich who was responsible for the home phlebotomy services. Yung-Hsuan J. Wang was a Master of Applied Epidemiology scholar at the Australian National University and was funded by the Australian Government Department of Health and Ageing.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.The Enhanced Measles Surveillance Working Party. Implementing a system of enhanced surveillance for measles in Victoria. Communicable Diseases Intelligence. 1999;23:51. [PubMed] [Google Scholar]
- 2.National Centre for Immunisation Research and Surveillance http://www.ncirs.usyd.edu.au/research/r-acir-3rdquart.html. http://www.ncirs.usyd.edu.au/research/r-acir-3rdquart.html . Childhood immunisation coverage-assessment date 30 June 2006 ( ). Accessed 11 January 2007.
- 3.Lambert SB et al. Enhanced measles surveillance during an interepidemic period in Victoria. Medical Journal of Australia. 2000;172:114–118. doi: 10.5694/j.1326-5377.2000.tb127934.x. [DOI] [PubMed] [Google Scholar]
- 4.Davidson N et al. A measles outbreak among young adults in Victoria, February 2001. Communicable Diseases Intelligence. 2002;26:273–278. [PubMed] [Google Scholar]
- 5.Lambert SB et al. Measles outbreak in young adults in Victoria, 1999. Medical Journal of Australia. 2000;173:467–471. doi: 10.5694/j.1326-5377.2000.tb139297.x. [DOI] [PubMed] [Google Scholar]
- 6.Chibo D et al. Molecular characterization of measles viruses isolated in Victoria, Australia, between 1973 and 1998. Journal of General Virology. 2000;81:2511–2518. doi: 10.1099/0022-1317-81-10-2511. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization – Western Pacific Regional Office. Progress towards national plans for measles elimination. Measles Bulletin. 2006;8:1. [Google Scholar]
- 8.World Health Organization, United Nation's Children's Fund Geneva: 2001. . Measles mortality reduction and regional elimination strategic plan 2001–2005. : World Health Organization, ; Report no.: WHO/V&B/01.13 Rev. 1. [Google Scholar]
- 9.de Quadros CA et al. Eradication of wild poliovirus from the Americas: acute flaccid paralysis surveillance, 1988–1995. Journal of Infectious Diseases. 1997;175:S37–S42. doi: 10.1093/infdis/175.supplement_1.s37. (Suppl. 1): [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Geneva: WHO; 2003. . Poliomyelitis: recommended surveillance standards. [Google Scholar]
- 11.Harpaz R, Papania MJ. Can a minimum rate of investigation of measleslike illnesses serve as a standard for evaluating measles surveillance? Journal of Infectious Diseases. 2004;189:S204–S209. doi: 10.1086/378776. (Suppl. 1): [DOI] [PubMed] [Google Scholar]
- 12.Australian Bureau of Statistics Canberra: Australian Bureau of Statistics; 2003. . Demography, Australia 2003. [Google Scholar]
- 13.Health (Infectious Diseases) Regulations 2001 2001. . S.R. No. 41/2001 ( ; amended 30 January 2004).
- 14.Communicable Diseases Network Australia 2004. . Interim surveillance case definitions for the Australian national notifiable diseases surveillance system. Australian Government Department of Health and Ageing, (version 1:43–44).
- 15.Guidelines for the control of measles outbreaks in Australia. Canberra: 2000. : Commonwealth Department of Health and Aged Care, (Technical Report Series No. 5). [Google Scholar]
- 16.Australian Bureau of Statistics Canberra: Australian Bureau of Statistics; 2002. p. 130. . 2001 Census of Population and Housing: selected social and housing characteristics, Australia. , pp. [Google Scholar]
- 17.World Health Organization Geneva: WHO; 2001. . Module on best practices for measles surveillance. ; Report no.: WHO/V&B/01.43. [Google Scholar]
- 18.Kelly HA et al. The age-specific prevalence of human parvovirus immunity in Victoria, Australia compared with other parts of the world. Epidemiology and Infection. 2000;124:449–457. doi: 10.1017/s0950268899003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamanishi K et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988;i:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 20.Australian Technical Advisory Group on Immunisation. The Australian Immunisation Handbook. 8th edn. Canberra: National Health and Medical Research Council; 2003. pp. 106–113. , pp. [Google Scholar]
- 21.Turnbull FM et al. The Australian measles control campaign, 1998. Bulletin of the World Health Organisation. 2001;79:882–888. [PMC free article] [PubMed] [Google Scholar]
- 22.Lambert S. Measles in Victoria 1992 to 1996: the importance of laboratory confirmation. Communicable Diseases Intelligence. 1998;22:17–22. [PubMed] [Google Scholar]
- 23.World Health Organization – Western Pacific Regional Office. Global measles management meeting September 2006. Measles Bulletin. 2006;11:2–4. [Google Scholar]
- 24.Andrews R. Measles outbreak among young adults in Victoria. Communicable Diseases Intelligence. 2001;25:12. [PubMed] [Google Scholar]
- 25.Riddell MA et al. Measles case imported from Europe to Victoria, Australia, March 2006. Eurosurveillance. 2006;11(5) doi: 10.2807/esw.11.20.02959-en. ): E0605182. [DOI] [PubMed] [Google Scholar]
- 26.Durrheim DN, Speare R, Ogunbanjo GA. Elimation programs: monitoring the effectiveness of surveillance [Letter] Journal of Infectious Diseases. 2004;190:2195–2196. doi: 10.1086/425427. [DOI] [PubMed] [Google Scholar]
- 27.Harpaz R, Papania MJ. Reply to Durrheim et al. [Letter] Journal of Infectious Diseases. 2004;190:2196–2197. [Google Scholar]