SUMMARY
We observed an outbreak of necrotizing fasciitis associated with Streptococcus agalactiae infection in a group of juvenile saltwater crocodiles (Crocodylus porosus). We undertook screening of crocodiles and the environment to clarify the source of the outbreak and evaluated the isolates cultured from post-mortem specimens with molecular methods to assess clonality and the presence of known group B streptococcal virulence determinants. The isolates were indistinguishable by pulsed-field gel electrophoresis. They were a typical serotype Ia strain with the Cα-like protein gene, epsilon (or alp1), the mobile genetic elements IS381 ISSag1 and ISSag2, and belonged to multi-locus sequence type (ST) 23. All of these characteristics suggest they were probably of human origin. We review the medical and veterinary literature relating to S. agalactiae necrotizing fasciitis, epidemiology and virulence determinants.
INTRODUCTION
Necrotizing fasciitis is a severe infection involving the superficial fascia and subcutaneous tissues, which is associated with early toxin-mediated, systemic toxicity, and has a described mortality in humans of 30–60% [1, 2]. The underlying pathogenic process involves the production of destructive enzymes and toxins by the bacterial organisms that allow evasion of the host immune defences and the spread of the bacteria through the tissue planes resulting in rapid tissue necrosis [3]. The main causal pathogens are Lancefield group A streptococci (GAS) and clostridia, although several other organisms, such as Klebsiella pneumoniae, Pseudomonas aeruginosa and Vibrio vulnificus have been described in monomicrobial infections and polymicrobial necrotizing fasciitis is also recognized [4]. In 1984, group C and G streptococci were reported for the first time to cause necrotizing fasciitis in humans [5].
Streptococcus agalactiae, a group B streptococcus (GBS) well known for causing pneumonia, meningitis and sepsis in human neonates [6], has also been reported as a rare cause of monomicrobial necrotizing fasciitis, both in infants and adults [4, 7–18]. Whilst S. agalactiae is one of the most important causes of bovine mastitis and has been isolated from other animals including dogs, cats, goats, elephants, fish, and frogs [19–23], there has previously been only two reported cases of GBS necrotizing fasciitis in animals, which occurred in captive bottlenose dolphins [24, 25]. The few cases of necrotizing fasciitis that have been reported in domestic animals have mainly involved group G streptococci, with severe dermal, fascial, and muscular necrosis recognized in dogs infected with S. canis [26, 27]. Although there are no specific reports of S. agalactiae-associated necrotizing fasciitis in reptiles, GBS septicaemia due to serotype V has been reported in three subadult, captive bred, emerald monitors (Varanus prasinus) which died suddenly, after being fed with contaminated mice [28].
Although various virulence mechanisms in GAS are well characterized, those causing invasive disease in GBS are not as well understood.
It has been noted that the severe manifestations of GBS disease resemble GAS infection, and one author managed to isolate a novel pyrogenic exotoxin from a patient who died with toxic-shock syndrome associated with S. agalactiae [29]. Despite common elements defined with recent genome sequencing of both organisms [30], there has never been reported transfer of enterotoxin genes from GAS to GBS. However, variations in the capsular polysaccharide antigens, variable surface proteins and numerous mobile genetic elements are known to contribute to virulence in GBS [31].
We report a clonal outbreak of necrotizing fasciitis due to S. agalactiae in a group of juvenile saltwater crocodiles (Crocodylus porosus) and we describe virulence determinants and the molecular typing profile of the responsible bacterium. We also review both the literature of GBS infections in animals and the potential virulence determinants of GBS.
METHODS
Captive-raised male juvenile crocodiles from several rearing pens at the Darwin Crocodile Farm, 40 km south of Darwin in the tropical Northern Territory of Australia, became unwell over a 3-month period between November 2005 and January 2006. Affected animals were aged between 6 and 11 months, were lethargic and displayed necrotic skin lesions involving either the ventral body wall or limbs or one or multiple swollen limbs. The majority of affected crocodiles either died within a few days or became moribund and were euthanized for post-mortem examination. Crocodile farming in the Northern Territory is intensive, with high stocking densities. The diet at the time of the outbreak consisted of kangaroo, horse and buffalo meat, supplied frozen from a local pet meat supplier, with a vitamin/mineral supplement. The first cases occurred following an inventory count on the farm (stressful to the crocodiles), and there was a recent history of what were assumed to be traumatic skin lesions due to fighting in the affected tanks.
There were 29 deaths recorded during the outbreak and full post-mortem examinations were performed on seven animals. Skin, muscle and internal organ tissues were prepared for histological examination, Gram stains and bacterial culture. In December 2005, mouth swabs from five crocodiles from pens containing affected animals, water samples from two affected pens, and two bores and a water heater supplying the pens were cultured using enrichment media for streptococcal species. In January 2006, at the end of the outbreak, ten more mouth swabs were taken for culture.
Streptococcal isolates from the crocodiles were observed for haemolysis on tryptone soy agar with sheep blood and catalase reaction. Lancefield serogrouping was performed with a latex agglutination test system (Streptococcal Grouping Reagent, Oxoid, Basingstoke, Hants, UK) and identification was completed with rapid ID 32 Strep (bioMérieux, Marcy l'Etoile, France). Antibiotic susceptibility testing was performed by agar disc diffusion according to the method of the Clinical Laboratory Standards Institute (CLSI).
Macrorestriction analysis of the chromosomal DNA of isolates was performed using the enzyme SmaI and subsequent separation of the fragments by pulsed-field gel electrophoresis (PFGE). Chromosomal DNA was prepared using a modification of published methods [32, 33]. Slices of DNA-containing agarose plugs were digested overnight with 20 U SmaI (New England BioLabs, Genesearch, Arundel, Queensland, Australia) at 25°C, then electrophoresed on a CHEF DRIII system (Bio-Rad Laboratories, Hercules, CA, USA) for 25 h at 6 V/cm, with ramped pulse times of 5–40 s in a 1·2% SeaKem Gold agarose gel (BioWhittaker Molecular Applications, Rockland, ME, USA) and 0·5× TBE electrophoresis buffer containing 100 μm thiourea. Lambda DNA concatemer PFGE markers (Promega, Madison, WI, USA) were used as DNA size standards. The gel was stained with ethidium bromide (0·5 μg/ml, Sigma, Castle Hill, NSW, Australia) for 45 min, destained in 0·5× TBE for 45 min, and photographed under UV illumination.
Genotyping was performed, using a GBS molecular typing system which identifies serotype, protein antigens, mobile genetic elements and seven antibiotic resistance genes (including three erythromycin, two tetracycline and two aminoglycoside resistance genes) [31, 34, 35]. Three of nine representative S. agalactiae isolates from three of seven different crocodiles were assessed. Multi-locus sequence typing (MLST) using the seven house-keeping genes that define the GBS MLST scheme was performed on one isolate as previously described [36], after DNA had been extracted using the Qiagen DNeasy kit (Qiagen, Doncaster, Victoria, Australia).
RESULTS
Gross lesions varied from swelling of one or multiple limbs and/or the ventral or lateral body wall to sloughing of the skin of these regions to reveal underlying necrotic subcutaneous tissue and skeletal muscle (Fig. 1). Histopathological lesions of affected body wall and limbs generally revealed large regions of muscle and subcutaneous tissue that had undergone coagulation necrosis, classically with wave-like margins of abundant Gram-positive cocci surrounded by variable numbers of inflammatory cells, including phagocytes containing abundant Gram-positive cocci. Histopathological findings in other organs were limited to subtle lesions suggestive of septicaemia, including mild mixed inflammatory cell infiltrates associated with the epicardium and multifocal acute splenic necrosis. Mixed Gram-negative rods and Gram-positive cocci were visible in internal organs of some affected crocodiles.
Fig. 1.
Post-mortem photograph of an affected crocodile, showing typical lesions of inflammation, necrosis and sloughing of the soft tissue of the ventral neck, thorax and distal right forelimb (arrow, 10 cm length).
S. agalactiae was isolated from all seven crocodiles; in six from the skin or affected subcutaneous tissue and in one from the liver. Two additional isolates were obtained from one crocodile from the lung and spleen. In addition to S. agalactiae, various Gram-negative bacteria including Salmonella spp., Providencia rettgeri, Pseudomonas aeruginosa and Edwardsiella tarda were also recovered from internal organs as well as in some of the body wall and limb lesions. These were considered secondary invaders.
Streptococcal colonies were β-haemolytic, catalase negative, grouped to Lancefield serological group B and showed the typical biochemical properties of this species in ID 32 Strep test kits. The culture of one of five mouth swabs from live crocodiles taken in December yielded S. agalactiae. S. agalactiae was not recovered from any of the five water samples taken in December or from ten live crocodile mouth swabs taken at the end of the outbreak in January. It should be noted, however, that the mouth swabs and water samples were taken after a 2-week period of antibiotic treatment administered orally in the crocodile's food (Sulprim, Ilium Veterinary Products Troy Laboratories, Smithfield, NSW, Australia).
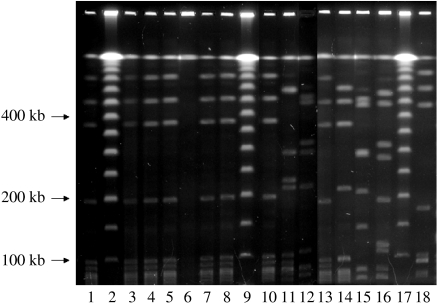
PFGE of the clinical S. agalactiae isolates demonstrated that all eight isolates from six of the seven crocodiles had identical banding patterns (Fig. 2); the ninth isolate from the seventh animal did not resolve with PFGE. This suggested the cases represented an outbreak caused by the same strain. Genotyping was performed on three of nine isolates, from different crocodiles. All isolates demonstrated a genotype consistent with serotype Ia, with protein alp1 (or, epsilon) and insertion sequences IS1381, ISSag1 and ISSag2; tetM was the only antibiotic resistance gene detected. MLST showed that they belonged to ST 23. The isolates were all susceptible to penicillin, erythromycin, clindamycin, and vancomycin, but resistant to tetracycline and oxacillin.
Fig. 2.
Composite photograph of SmaI macrorestriction pattern of GBS isolates from crocodiles and miscellaneous human clinical specimens. Lanes 2, 9, 17, phage lambda DNA ladder; lanes 1, 3–8, 10, 13, isolates of crocodile origin (lane 6 unresolved); lanes 11, 12, 14–16, 18 various human clinical isolates (kb, kilobases).
DISCUSSION
GBS are increasingly recognized as virulent human pathogens and 22 human cases of necrotizing fasciitis associated with this organism have been reported in the literature [4, 7–18]. Five of these cases occurred in children and three of these were infants aged <12 weeks [7–10]. Of note, S. agalactiae necrotizing fasciitis in adults has a tendency to occur in patients with diabetes and other comorbidities resulting in relative immunosuppression, with a high mortality [12, 18]. The virulence of the organism in these cases has led authors to postulate that it has ‘GAS-like behaviour’. Although no molecular typing or analysis of virulence factors was reported on isolates from any of the human cases, one author described the purification of a novel pyrogenic exotoxin in separate case of S. agalactiae toxic shock syndrome without necrotizing fasciitis [29].
The most notable contribution of S. agalactiae to disease in animals is due to its predilection to colonize mammary glands of various ruminants, where it can survive for extended periods, causing clinical and subclinical mastitis and affecting milk quality [37]. It is also a major piscine pathogen, causing morbidity among freshwater, estuarine and marine fishes, such that a vaccine has been developed and trialled in this context [38]. In the one previously reported case of GBS necrotizing fasciitis in animals, from a 15-year-old female common bottlenose dolphin, the marine mammal presented with vomiting, renal failure and liver dysfunction [24]. The only known risk factor was skin lesions sustained from fighting with a male. Septicaemia and endotoxic shock, possibly due to clostridial infection, were suspected and ceftriaxone and dexamethasone were given. Despite this, the dolphin died 2 h after the onset of clinical signs.
The cases reported here of necrotizing fasciitis in C. porosus juveniles represent the first clonal outbreak of S. agalactiae necrotizing fasciitis described in a reptile. This is notable because both necrotizing fasciitis and infection with S. agalactiae are very unusual in reptiles. This is despite various bacteria often being identified in ulcers of crocodiles, subsequent to bite injuries. However, while Gram-negative bacteria are common aetiological agents of septicaemia in juvenile crocodiles, they have generally not been observed to cause lesions of fasciitis. The only potential suggestion of a possible case of bacterial necrotizing fasciitis in the reptile literature came from one large study of skin lesions in crocodiles. A C. porosus juvenile that died after not eating and being listless for several days had extensive necrosis of the subcutis with lifting of the entire scales. Extensive subcutaneous oedema, myolysis and large colonies of Gram-positive bacteria were seen at necropsy, as well as organ findings consistent with fulminating bacterial septicaemia, but no culture findings were given [39]. Moreover, an earlier study from 1993 noted that β-haemolytic streptococci were isolated from two of nine frozen tail meat samples from Nile crocodiles (C. niloticus), but identified these isolates as S. equisimilis (probably the currently recognized S. dysgalactiae var. equisimilis [either group C or G]) [40].
Another major point of interest highlighted by the cases we have described, is the fact that these cases occurred when the animals were still aged <1 year. In the only other reported case of invasive GBS disease in reptiles, a clonal outbreak of group B septicaemia in emerald monitors, the animals were also described as ‘subadult’, but no further detail was given regarding age [28]. Similar infections in humans have occurred in both adults and infants, so it is unclear whether younger age could potentially increase susceptibility to overwhelming GBS infection in animals. There may be other factors more important than age, such as the high stocking densities increasing the potential for bite wounds and the stress of captivity, which was also considered a possible predisposing factor in the reported case of necrotizing fasciitis in a dolphin [24].
The source of the outbreak reported in emerald monitors was proven to relate to contamination of mice used for feeding, when nine of 165 remaining dead mice had GBS isolated from the gastrointestinal tract, with an identical banding pattern on PFGE to that of the infected monitors [28]. In contrast, although, cutaneous lesions from fighting may have provided a portal of entry for the organism, the source of infection in our outbreak remains uncertain. None of the water samples taken at the time of the outbreak had positive culture results. Culture of one of a total of 15 mouth swabs from live crocodiles in the same pens as affected animals yielded S. agalactiae, suggesting that the organism may inhabit mucous membranes. The various dietary items fed to the crocodiles at the time were not cultured, therefore, the possibility of infection through the feed remains uninvestigated.
Interestingly, when considering the potential origin of the GBS, the genotyping results demonstrate a profile more consistent with streptococci of human rather than bovine origin. Bovine isolates are often non-typable, but of those that are typable a high proportion belong to serotype III [41]. Unlike isolates from humans and unlike the strains reported in this outbreak, bovine isolates are usually susceptible to tetracycline [19], and only a minority have mobile genetic elements, including ISSag1 and ISSag2. When present, such mobile genetic elements probably encode a pathogenicity-like island which determines virulence for humans and possibly other animals such as crocodiles [42]. GBS adhesin genes scpB, and lmb, coding for C5a peptidase and laminin-binding protein, respectively, are part of a 16-kb composite transposon flanked by the ISSag2 insertion sequences [43]. These two genes are 98% identical with their counterparts in GAS and all human GBS isolates examined to date contain them, whereas they are present in only 20% of animal GBS isolates, suggesting possibly a specific adaptation to colonization of the human host [44].
In view of the rarity of necrotizing fasciitis in animals and indeed, of S. agalactiae as a cause of this disease in humans, further consideration should be given to the genotypic characteristics of the aggressive clone responsible for this outbreak. Of the nine different GBS serotypes, capsular type Ia, although frequently isolated in cases of neonatal sepsis, is not as significantly associated with invasive human neonatal disease as is serotype III [45]. However, reported serotypes causing necrotizing fasciitis in humans vary from Ib and III, to VI [7, 9, 13]. Similarly, GAS necrotizing fasciitis has been shown to occur from a wide diversity of GAS strains and not just the notably virulent M1 serotype [46, 47]. Interestingly, S. agalactiae of serotype Ia has previously been isolated from the inner organs of a monkey which died of sepsis [21]. Whilst capsular polysaccharide has an important role in allowing the organism to evade phagocytosis, other virulence factors are important including haemolysin, C5a peptidase, superoxide dismutase, lipoteichoic acid and laminin-binding protein. The surface protein antigen epsilon, identified in our isolates, is one of a group of variable antigens that contribute to virulence and elicit protective immunity [31, 48].
Other factors previously identified as being involved in the complex virulence mechanisms of GBS include the CovS/CovR two-component global regulatory system, which is involved in regulating the expression of virulence genes [49]. Sequencing and comparison of a serotype III and serotype V whole genome sequence revealed that the genomes were very similar, with the only major difference being the capsulation loci and mobile genetic elements. This suggested that the capsulation loci or pathogenicity islands are the main determinants of serotype III strains in causing invasive disease [50]. Further research has focused on the role of insertion sequences. The IS1548 insertion sequence in the hylB gene has been associated with strains identified from patients with endocarditis [51], and from neonatal CSF isolates [45], but was not found in either the S. agalactiae isolates from the emerald monitors with sepsis [28] or in our isolates from C. porosus. Ia-alp1-IS1381 is the commonest protein and mobile genetic element profile of human isolates of serotype Ia and, based on a limited comparison between our genotyping method and MLST, is usually associated with ST 23 [52]. IS1381 is found in most serotypes except the hypervirulent type III subtype (typically ST 17), and was present in our isolates [31, 52]. In addition to factors related to the organism, host factors may affect the pathogenesis of GBS infection in both humans and animals. Crocodiles can be expected to have many of the same components of the immune system as mammals. Specifically, they have been shown to have serum complement activity, with a main difference in reptiles being temperature dependence of immune function. [53].
The MLST type ST 23 identified in our outbreak strain of GBS, can be considered in view of the information it may provide, both in terms of virulence and the origin of the organism. Prior attempts to define correlations between S. agalactiae MLST and virulence have revealed only that ST 17 appears to be a hypervirulent lineage associated with neonatal sepsis, in keeping with the reputed aggressive nature of serotype III [34, 54]. Despite an initial model postulating an animal origin of the hypervirulent clone [55], Brochet et al. compared the genome content of ST 17 (human) and ST 61 (bovine) strains, which are closely related by MLST and concluded that the genome of the common ancestor was probably closer to that of human ST 17 strains than to that of the ST 61 strains of bovine origin [56]. A prior study which performed MLST analysis on human and bovine isolates also examined four disease-causing isolates from other animals (one elephant, two dogs and a goat). Three of the 50 strain types found were present in both human and bovine isolates, whilst four unique strain types were present in the other animals [55]. In our case, a clonal outbreak was caused by ST 23. MLST shows that isolates with the same sequence type can have different capsular serotypes. Both serotypes Ia and III have been represented by ST 23, but the majority are Ia, consistent with our genotype findings of Ia in this study [34, 57]. This sequence type has been reported as one of two major genotypes from cattle [58]. However, ST 23 was also found to be a common sequence type in a collection of human isolates from maternal carriage and fetal invasive disease [57]. Although found to be a prevalent sequence type both in the latter study and in MLST analysis of the global human S. agalactiae [36], there has been no specific association demonstrated between this sequence type and invasive disease in humans. Another study found ST 23 to be less commonly represented among invasive human adult and neonatal isolates of S. agalactiae than ST 17 and ST 19 [59]. Our findings in this clonal outbreak of necrotizing fasciitis, suggest that the hypervirulent nature of the GBS involved may not relate to the sequence type, and the MLST results do not unequivocally confirm a human source for the outbreak. However, if this disease outbreak was indeed from a human source, it has implications for safe husbandry and handling of such animals under conditions of intensive farming.
Current approaches to vaccine development in humans aim to overcome serotype-specificity by targeting vaccine candidates other than capsular polysaccharide, including C5a peptidase, Sip and the Β-component of the C protein, which have elicited protective immunity in mice [60]. In fish, another species in which the possibility of vaccination against S. agalactiae is being investigated; a vaccine prepared by concentrating the extracellular products from clarified killed S. agalactiae produced a significant degree of protection against experimental infection [38].
In conclusion, this is the first clonal outbreak of necrotizing fasciitis from S. agalactiae described in a reptile species. Notably, genetic profiling and MLST suggest this may potentially have been caused by a strain of human origin, being serotype Ia MLST 23, with serotype Ia known to be the second most common invasive serotype in neonates and a capable pathogen, as demonstrated in this case. Although necrotizing fasciitis related to S. agalactiae remains a rare clinical problem in humans and animals, reptile keepers should be aware of this novel, severe cause of morbidity and mortality, which potentially has a predilection for juveniles and occurrence in circumstances of intensive husbandry.
ACKNOWLEDGEMENTS
We are grateful to Mrs Ann Palmer and Dr Sally Isberg from Darwin Crocodile Farm for their support with the investigation. Dr Greg Brown edited the background and scale for Figure 1. Daniel Godoy and Brian Spratt acknowledge support from the Wellcome Trust.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Wong CH et al. Necrotising fasciitis: clinical presentation, microbiology and determinants of mortality. Journal of Bone and Joint Surgery of America. 2003;85:1454–1460. [PubMed] [Google Scholar]
- 2.Headley AJ. Necrotising soft tissue infections: a primary care review. American Family Physician. 2003;68:323–328. [PubMed] [Google Scholar]
- 3.Giuliano A et al. Bacteriology of necrotising fasciitis. American Journal of Surgery. 1977;134:52–56. doi: 10.1016/0002-9610(77)90283-5. [DOI] [PubMed] [Google Scholar]
- 4.Liu Yuag-Meng et al. Microbiology and factors affecting mortality in necrotising fasciitis. Journal of Microbiology Immunology and Infection. 2005;38:430–435. [PubMed] [Google Scholar]
- 5.Gaunt N et al. Necrotising fasciitis due to group C and G haemolytic streptococcus after chiropody. Lancet. 1984;1:516. doi: 10.1016/s0140-6736(84)92888-5. [DOI] [PubMed] [Google Scholar]
- 6.Gotoff SP. Group B streptococcal infections. Pediatric Reviews. 2002;23:381–385. [PubMed] [Google Scholar]
- 7.Lang ME, Vaudry W, Robinson JL. Case report and literature review of late-onset group B streptococcal disease manifesting as necrotising fasciitis in preterm infants: is this a new syndrome? Clinical Infectious Diseases. 2003;37:e132–135. doi: 10.1086/378892. [DOI] [PubMed] [Google Scholar]
- 8.Bomar WE, Ferlauto JJ, Wells DH. An unusual presentation of a β-hemolytic group B streptococcal infection. Journal of Pediatric Surgery. 1980;15:683–685. doi: 10.1016/s0022-3468(80)80528-8. [DOI] [PubMed] [Google Scholar]
- 9.Ramamurthy RS, Srinivasan G, Jacobs NM. Necrotising fasciitis and necrotising cellulitis due to group B streptococcus. American Journal of Diseases of Childhood. 1977;131:1169–1170. doi: 10.1001/archpedi.1977.02120230115022. [DOI] [PubMed] [Google Scholar]
- 10.Vianni RM, Lewis A, Bradley JS. Postoperative group B streptococcal necrotising fasciitis in a previously healthy child. Pediatric Infectious Disease Journal. 1997;16:630–631. doi: 10.1097/00006454-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Crum NF, Wallace MR. Group B streptococcal necrotising fasciitis and toxic shock-like syndrome: A case report and review of the literature. Scandanavian Journal of Infectious Diseases. 2004;35:878–881. doi: 10.1080/00365540310016691. [DOI] [PubMed] [Google Scholar]
- 12.Riefler J et al. Necrotising fasciitis in adults due to group B streptococcus. Report of a case and review of the literature. Archives of Internal Medicine. 1988;148:727–729. [PubMed] [Google Scholar]
- 13.Matsubara K et al. Three fatal cases of invasive serotype VI group B streptococcal infection. Journal of Infection. 2006;53:e139–142. doi: 10.1016/j.jinf.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Tang WM et al. Report of 2 fatal cases of adult necrotising fasciitis and toxic shock syndrome caused by Streptococcus agalactiae. Clinical Infectious Diseases. 2000;31:e15–17. doi: 10.1086/318148. [DOI] [PubMed] [Google Scholar]
- 15.Gardam MA et al. Group B streptococcal necrotising fasciitis and streptococcal toxic shock-like syndrome in adults. Archives of Internal Medicine. 1998;158:1704–1708. doi: 10.1001/archinte.158.15.1704. [DOI] [PubMed] [Google Scholar]
- 16.Sutton GP et al. Group B streptococcal necrotising fasciitis arising from an episiotomy. Obstetrics and Gynecology. 1985;66:733–736. [PubMed] [Google Scholar]
- 17.Holmstrom B, Grimsley EW. Necrotising fasciitis and toxic shock syndrome caused by group B streptococcus. Southern Medical Journal. 2000;93:1096–1098. [PubMed] [Google Scholar]
- 18.Wong CH, Kurup A, Tan KC. Group B Streptococcus necrotising fasciitis: an emerging disease? European Journal of Clinical Microbiology and Infectious Diseases. 2004;23:573–575. doi: 10.1007/s10096-004-1154-0. [DOI] [PubMed] [Google Scholar]
- 19.Wibawan IWT, Lautrou Y, Lammler C. Antibiotic resistance patterns and pigment production of streptococci of serological group B isolated from bovines and humans. Journal of Veterinary Medicine. 1991;38:731–736. doi: 10.1111/j.1439-0450.1991.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 20.Yildirim AO et al. Pheno- and genotypic properties of streptococci of serological group B of canine and feline origin. FEMS Microbiology Letters. 2002;212:187–192. doi: 10.1111/j.1574-6968.2002.tb11265.x. [DOI] [PubMed] [Google Scholar]
- 21.Lammler C, Abdulmawjood A, Weib R. Properties of serological group B streptococci of dog, cat and monkey origin. Journal of Veterinary Medicine. 1998;45:561–566. doi: 10.1111/j.1439-0450.1998.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 22.Evans JJ et al. Characterization of β-haemolytic Group B Streptococcus agalactiae in cultured seabream, Sparus auratus L., and wild mullet, Liza klunzingeri (Day) in Kuwait. Journal of Fish Diseases. 2002;25:505–513. [Google Scholar]
- 23.Amborski RL et al. A non-hemolytic, group B streptococcus infection in cultured bullfrogs, Rana catesbeiana, in Brazil. Journal of Wildlife Diseases. 1983;19:180–184. doi: 10.7589/0090-3558-19.3.180. [DOI] [PubMed] [Google Scholar]
- 24.Zappulli V et al. Fatal necrotising fasciitis and myositis in a captive common bottlenose dolphin (Tursiops truncates) associated with Streptococcus agalactiae. Journal of Veterinary Diagnostic Investigation. 2005;17:617–622. doi: 10.1177/104063870501700620. [DOI] [PubMed] [Google Scholar]
- 25.Evans JJ et al. First report of Streptococcus agalactiae and Lactococcus garvieae from a wild bottlenose dolphin (Tursiops truncatus) Journal of Wildlife Diseases. 2006;42:561–569. doi: 10.7589/0090-3558-42.3.561. [DOI] [PubMed] [Google Scholar]
- 26.De Winter LM, Prescott JF. Relatedness of Streptococcus canis from canine streptococcal toxic shock syndrome and necrotising fasciitis. Canadian Journal of Veterinary Research. 1999;63:90–95. [PMC free article] [PubMed] [Google Scholar]
- 27.Miller CW et al. Streptococcal toxic shock syndrome in dogs. Journal of the American Veterinary Medical Association. 1996;209:1421–1426. [PubMed] [Google Scholar]
- 28.Hetzel U et al. Septicaemia in emerald monitors (Varanus prasinus Schlegel 1839) caused by Streptococcus agalactiae acquired from mice. Veterinary Microbiology. 2003;95:283–293. doi: 10.1016/s0378-1135(03)00184-6. [DOI] [PubMed] [Google Scholar]
- 29.Schlievert PM, Gocke JE, Deringer JR. Group B streptococcal toxic shock-like syndrome: report of a case and purification of an associated pyrogenic toxin. Clinical Infectious Diseases. 1993;17:26–31. doi: 10.1093/clinids/17.1.26. [DOI] [PubMed] [Google Scholar]
- 30.Green NM et al. Genome sequence of a serotype M28 strain of group A Streptococcus: potential new insights into puerperal sepsis and bacterial disease specificity. Journal of Infectious Diseases. 2005;192:760–770. doi: 10.1086/430618. [DOI] [PubMed] [Google Scholar]
- 31.Kong F et al. Towards a genotyping system for Streptococcus agalactiae (group B streptococcus): use of mobile genetic elements in Australasian invasive isolates. Journal of Medical Microbiology. 2003;52:337–344. doi: 10.1099/jmm.0.05067-0. [DOI] [PubMed] [Google Scholar]
- 32.Elliot JA et al. Sudden increase in isolation of group B streptococci, serotype V, is not due to emergence of a new pulsed-field gel electrophoresis type. Journal of Clinical Microbiology. 1998;36:2115–2116. doi: 10.1128/jcm.36.7.2115-2116.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fasola E et al. Molecular analysis of multiple isolates of the major serotypes of group B streptococci. Journal of Clinical Microbiology. 1993;31:2616–2620. doi: 10.1128/jcm.31.10.2616-2620.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng X et al. Evaluation of a multiplex PCR based reverse line blot hybridisation (mPCR/RLB) for identification or serotype and surface protein antigens of Streptococcus agalactiae (group B streptococcus, GBS) Journal of Clinical Microbiology. 2006;44:3822–3825. doi: 10.1128/JCM.01232-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng X et al. Simultaneous detection of nine antibiotic resistance-related genes in Streptococcus agalactiae using multiplex PCR and reverse line blot hybridization assay. Antimicrobial Agents and Chemotherapy. 2006;50:204–209. doi: 10.1128/AAC.50.1.204-209.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones N et al. Multilocus sequence typing system for group B streptococcus. Journal of Clinical Microbiology. 2003;41:2530–2536. doi: 10.1128/JCM.41.6.2530-2536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keefe GP. Streptococcus agalactiae mastitis: a review. Canadian Veterinary Journal. 1997;38:429–437. [PMC free article] [PubMed] [Google Scholar]
- 38.Pasnik DJ, Evans JJ, Klesius PH. Duration of protective antibodies and correlation with survival in Nile tilapia Oreochromis niloticus following Streptococcus agalactiae vaccination. Diseases of Aquatic Organisms. 2005;66:129–134. doi: 10.3354/dao066129. [DOI] [PubMed] [Google Scholar]
- 39.Buenviaje GN, Ladds PW, Martin Y. Pathology of skin diseases in crocodiles. Australian Veterinary Journal. 1998;76:357–363. doi: 10.1111/j.1751-0813.1998.tb12368.x. [DOI] [PubMed] [Google Scholar]
- 40.Madsen M. Microbial flora of frozen tail meat from captive Nile crocodiles (Crocodylus niloticus) International Journal of Food Microbiology. 1993;18:71–76. doi: 10.1016/0168-1605(93)90009-6. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Z et al. Molecular serotype identification of Streptococcus agalactiae of bovine origin by multiplex PCR-based reverse line blot assay. FEMS Microbiology Letters. 2006;263:236–239. doi: 10.1111/j.1574-6968.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- 42.Franken C et al. ISSag1 in streptococcal strains of human and animal origin. International Medical Microbiology. 2004;294:247–254. doi: 10.1016/j.ijmm.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Herbert MA, Beveridge CJE, Saunders NJ. Bacterial virulence factors in neonatal sepsis: group B streptococcus. Current Opinion in Infectious Diseases. 2004;17:225–229. doi: 10.1097/00001432-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Franken C et al. Horizontal gene transfer and host specificity of beta-haemolytic streptococci: the role of a putative composite transposon containing scpB and lmb. Molecular Microbiology. 2001;41:925–935. doi: 10.1046/j.1365-2958.2001.02563.x. [DOI] [PubMed] [Google Scholar]
- 45.Rolland K et al. Genetic features of Streptococcus agalactiae strains causing severe neonatal infection as revealed by pulsed-field gel electrophoresis and hylB gene analysis. Journal of Clinical Microbiology. 1999;37:1892–1898. doi: 10.1128/jcm.37.6.1892-1898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassell M et al. Streptococcal necrotising fasciitis from diverse strains of Streptococcus pyogenes in tropical northern Australia and comparison with the literature. BMC Infectious Diseases. 2004;4:60–67. doi: 10.1186/1471-2334-4-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlaminckx BJ et al. Specific manifestations of invasive group A streptococcal disease: type distribution and virulence determinants. Journal of Clinical Microbiology. 2003;41:4941–4949. doi: 10.1128/JCM.41.11.4941-4949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindahl G, Stalhammar-Carlemalm M, Areschoug T. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clinical Microbiology Reviews. 2005;18:102–127. doi: 10.1128/CMR.18.1.102-127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamy M-C et al. CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Molecular Microbiology. 2004;54:1250–1268. doi: 10.1111/j.1365-2958.2004.04365.x. [DOI] [PubMed] [Google Scholar]
- 50.Herbert MA et al. Genetic islands of Streptococcus agalactiae strains NEM316 and 2603 VR and their presence in other Group B streptococcal strains. BMC Microbiology. 2005;5:31–43. doi: 10.1186/1471-2180-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Granlund M et al. Identification of a novel insertion element, IS1548, in group B streptococci, predominantly in strains causing endocarditis. Journal of Infectious Diseases. 1998;177:967–976. doi: 10.1086/515233. [DOI] [PubMed] [Google Scholar]
- 52.Sun Y et al. Comparison of a three-set genotyping system with multilocus sequence typing for Streptococcus agalactiae (group B streptococcus) Journal of Clinical Microbiology. 2005;43:4704–4707. doi: 10.1128/JCM.43.9.4704-4707.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merchant M, Britton A. Characterization of serum complement activity of saltwater (Crocodylus porosus) and freshwater (Crocodylus johnstoni) crocodiles. Comparative Biochemistry and Physiology. 2006;143:488–493. doi: 10.1016/j.cbpa.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 54.Musser JM et al. Identification of a high-virulence clone of type III Streptococcus agalactiae (group B Streptococcus) causing invasive neonatal disease. Proceedings of the National Academy of Sciences USA. 1989;86:4731–4735. doi: 10.1073/pnas.86.12.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bisharat N et al. Hyperinvasive neonatal group B streptococcus has arisen from a bovine ancestor. Journal of Clinical Microbiology. 2004;42:2161–2167. doi: 10.1128/JCM.42.5.2161-2167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brochet M et al. Genomic diversity and evolution within the species Streptococcus agalactiae. Microbes and Infection. 2006;8:1227–1243. doi: 10.1016/j.micinf.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 57.Bisharat N et al. Population structure of group B streptococcus from a low-incidence region for invasive neonatal disease. Journal of Clinical Microbiology. 2005;151:1875–1881. doi: 10.1099/mic.0.27826-0. [DOI] [PubMed] [Google Scholar]
- 58.Bohnsack JF et al. Serotype III Streptococcus agalactiae from bovine milk and human neonatal infections. Emerging Infectious Diseases. 2004;10:1412–1419. doi: 10.3201/eid1008.030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luan SH et al. Multilocus sequence typing of Swedish invasive group B streptococcus isolates indicates a neonatally associated genetic lineage and capsule switching. Journal of Clinical Microbiology. 2005;43:3727–3733. doi: 10.1128/JCM.43.8.3727-3733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johri AK et al. Group B Streptococcus: global incidence and vaccine development. Nature Reviews. 2006;4:932–942. doi: 10.1038/nrmicro1552. [DOI] [PMC free article] [PubMed] [Google Scholar]