SUMMARY
Few studies have examined the relationship between viral activity and bacterial invasive disease, considering both influenza virus and respiratory syncytial virus (RSV). This study aimed to assess the potential relationship between invasive pneumococcal disease (IPD), meningococcal disease (MD), and influenza virus and RSV activity in The Netherlands. Correlations were determined between population-based data on IPD and MD during 1997–2003 and influenza virus and RSV surveillance data. Incidence rate ratios of disease during periods of high influenza virus and RSV activity over the peri-seasonal and summer baseline periods were calculated. The analyses comprised 7266 and 3072 cases of IPD and MD. When data from all seasons were included, the occurrence of pneumococcal bacteraemia and MD correlated significantly with influenza virus and RSV activity both in children and adults. Periods of increased influenza virus and RSV activity showed higher rates of pneumococcal bacteraemia in older children and adults than the peri-season period. Rates of MD in children were also higher during periods of increased influenza virus activity; the same appeared true for MD in older children during periods of increased RSV activity. Although no causal relationship may be inferred from these data, they support a role for influenza virus and RSV in the pathogenesis of IPD and MD.
INTRODUCTION
Streptococcus pneumoniae and Neisseria meningitidis are leading causes of life-threatening invasive bacterial disease, like meningitis and septicaemia. Both invasive pneumococcal disease (IPD) and meningococcal disease (MD) show seasonal patterns with higher incidences during the winter months [1–4]. As well as climatic conditions, the circulation of respiratory viruses has been postulated to play a role in this seasonal increase. Viral infections may predispose to bacterial infection by enhancing bacterial adherence, e.g. by disrupting the respiratory tract epithelium barrier or by increased bacterial-cellular interaction [5]. In particular influenza viruses and respiratory syncytial virus (RSV) with their clear epidemic and seasonal activity during winters are suggested to be causally involved [6–14].
Few ecological studies have examined the epidemiological relationship between invasive bacterial disease and respiratory viral activity in the community, looking at influenza virus and RSV separately [1, 10, 11]. Since both viruses often co-circulate, it is usually difficult to differentiate influenza virus-active periods from RSV-active periods. In The Netherlands during 1997–2003, however, viral surveillance data revealed largely separate peaks of influenza virus and RSV activity. In the present study, we aimed to assess the potential relationship between IPD, MD, and influenza virus and RSV activity.
METHODS
Viral surveillance
In The Netherlands, a national representative laboratory-based surveillance for various viruses is conducted by the Weekly Sentinel System of the Dutch Working Group on Clinical Virology throughout the whole year. On a weekly basis they provide the absolute number of reported patients either hospitalized or visiting outpatient clinics that tested positive for a certain virus. Data on influenza virus and RSV during the period 1997–2003 were used in the present study. During the study period, most of the laboratory diagnoses of influenza and RSV infections were made by virus isolation on cell culture or by rapid antigen tests. The great majority of diagnoses were made by rapid antigen detection methods – immunofluorescence tests directly on patients' specimens or rapid slide tests. The weekly virological reports adequately reflect trends in national viral activity [15].
Pneumococcal and meningococcal isolates
The Netherlands Reference Laboratory for Bacterial Meningitis (NRLBM, Academic Medical Center/National Institute of Public Health and the Environment, Amsterdam, The Netherlands) collects isolates nationwide from cerebrospinal fluid (CSF) and blood from hospitalized patients with proven bacterial meningitis (CSF and possibly blood culture-positive), or bacteraemia with suspected meningitis (blood culture-positive only), bacteraemia without suspected meningitis, or isolates from other normally sterile bodily fluids. From week 27 of 1997 to week 26 of 2003 all blood and CSF samples sent to the NRLBM and positive for S. pneumoniae were considered. IPD and MD were defined as all cases in which S. pneumoniae or N. meningitidis was isolated from blood and/or CSF. IPD was further subdivided in bacteraemia (blood culture-positive only) and meningitis (CSF and possibly blood culture-positive).
Dutch immunization guidelines
For MD the last season (2002–2003) was excluded from the analysis because nationwide vaccination against serogroup C meningococci commenced for all individuals aged between 1 and 18 years in 2002. Pneumococcal vaccination has not been routinely recommended for elderly persons in The Netherlands, and consequently uptake of the 23-valent pneumococcal polysaccharide vaccine has been negligible [16]. Heptavalent pneumococcal conjugate vaccination has only recently (in 2006) been introduced into the national immunization programme for infants. During our study period, influenza vaccination was recommended for persons with high-risk medical conditions (such as chronic pulmonary or renal disease, and immunodeficiencies) and for all persons aged ⩾65 years irrespective of the presence of these conditions. Influenza vaccination coverage of the total population during 1997–2003 in age groups 16–44, 45–64, 65–74 and ⩾75 years was on average 5%, 15%, 70% and 80%, respectively (Statistics Netherlands, Voorburg/Heerlen, The Netherlands). Annual figures for children were unavailable, but coverage was 3% for the 0–4 years age group and 5–6% for the 5–14 years age group in 2003 [17].
Analyses
The relationship between the weekly rate of IPD and MD with the weekly number of laboratory identifications of influenza viruses and RSV was assessed by calculating the Spearman correlation coefficient correcting for long-term trends, with SAS statistical software version 8.02 (SAS Institute, Cary, NC, USA). In an attempt to quantify the potential relationship between invasive bacterial disease and the circulation of influenza viruses and RSV, the methods of Izurieta et al. were applied with minor modifications [18]. Influenza virus-active periods were defined for each winter season from week 40 to week 20, as periods of at least two consecutive weeks in which each week accounted for at least 5% of the season's total number of influenza virus isolates [18]. Periods of influenza virus predominance were defined as the influenza virus-active weeks with <5% of the season's total number of positive tests for RSV [18]. Similarly, the RSV-active period was defined as periods of at least two consecutive weeks in which each week accounted for at least 5% of the season's total number of positive tests for RSV. Periods of RSV predominance were defined as the RSV-active weeks with <5% of the season's total number of influenza virus isolates. Peri-seasonal baseline periods were defined as those periods of at least two consecutive weeks with each week accounting for <5% of the season's total number of influenza virus isolates and positive tests for RSV during the winter season (week 40 to week 20). Finally, the summer baseline period was defined as weeks 21–39. Unlike Izurieta and colleagues' study, weeks in which parainfluenza viruses were isolated were not excluded from the study as (sporadic) isolates were reported throughout the year. For the same reason, weeks in which sporadic isolates of influenza virus and RSV were reported during the summer baseline period were not excluded.
Age-specific incidence rate ratios with 95% confidence intervals of MD and IPD during periods of influenza virus and RSV predominance compared to the peri-seasonal and summer reference period were calculated with Episheet (K. Rothman, Episheet: Spreadsheets for the analysis of epidemiological data, 2002).
RESULTS
In total 7266 episodes of IPD were recorded in the 6-year period. Isolated bacteraemia accounted for the majority of episodes (81%); meningitis was diagnosed in 19% (1391 episodes). With regard to MD (3072 episodes during the 5-year surveillance period), meningitis accounted for 60% of episodes, isolated bacteraemia for 39% of cases, and other MD for 1%.
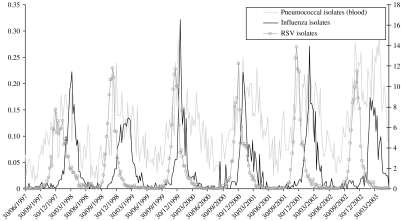
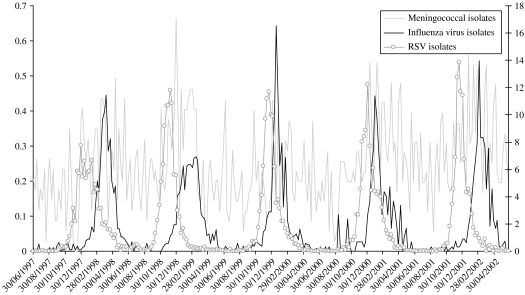
Figure 1 demonstrates that the occurrence of pneumococcal bacteraemia peaked when influenza viruses and RSV circulated each year, although invasive bacterial disease had already started to rise before the peaks of influenza virus and RSV activity. This seasonality was also present for MD in children, although less pronounced (Fig. 2).
Fig. 1.
Invasive pneumococcal disease in all ages together in relation to the circulation of influenza viruses and RSV in the community.
Fig. 2.
Invasive meningococcal disease in children in relation to the circulation of influenza viruses and RSV in the community.
According to the definitions, there were 92 influenza virus-active and/or RSV-active weeks; 46 weeks of influenza predominance, 42 weeks of RSV predominance, and only 4 weeks of both influenza virus and RSV activity during 1997–2003. Significant positive correlations were found between weekly rates of invasive bacterial disease and the weekly number of laboratory identifications of influenza viruses and RSV in both children and adults (Table). During periods of influenza virus predominance, rates of pneumococcal bacteraemia in older children and adults were significantly higher than those during the peri-season (Table); MD rates were significantly higher in children but not in adults. During periods of RSV predominance, rates of MD were higher only in older children, and IPD rates were significantly higher in adults but not in children.
Table.
Incidence of invasive pneumococcal and meningococcal disease in relation to influenza virus and RSV circulation in the community
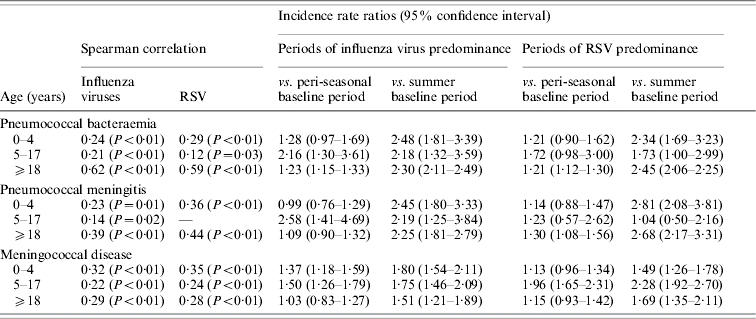
RSV, Respiratory syncytial virus.
DISCUSSION
In this 6-year retrospective study with largely separate peaks of influenza virus and RSV activity in The Netherlands, IPD and MD were significantly associated with the circulation of these viruses in the community in all age groups when data from all seasons were included. However, when analysis was restricted to the period from week 40 of one year until week 20 of the next, in an attempt to adjust for other seasonal effects, the results were variable. During periods of influenza virus predominance, rates of MD in children were significantly higher than those during the peri-season, and rates of pneumococcal bacteraemia were higher in older children and adults but not in young children. IPD rates in adults were higher during periods of RSV predominance, while rates of MD only appeared higher in older children.
Previous studies demonstrated that patients with severe pneumococcal pneumonia and MD were more likely than control subjects to show serological evidence of recent influenza virus infection [7, 19]. Madhi & Klugman showed in a double-blind, randomized, placebo-controlled trial that pneumococcal conjugate vaccination appeared to reduce the incidence of hospitalization for pneumonia associated with influenza A virus and RSV in children [20]. These data also suggest that these viral infections predispose to pneumococcal co-infection in the lung. Additionally, some ecological studies reported a significant correlation between influenza virus and RSV activity and IPD in adults [1, 10], although others did not confirm this [11]. In children significant correlations were also reported between IPD and RSV activity [1, 10, 11]. The temporal relationship between IPD and influenza virus activity in children appeared less clear [10, 11], only one study reported a significant correlation after incorporation of a time lag [1]. Regarding MD, a significant relationship with influenza virus activity in infants was reported by one study [13]. In contrast, various other studies assessing the relationship between MD and RSV, concluded that no convincing evidence could be found for a causal association between RSV and MD [9, 14]. In the present study, IPD and MD in both children and adults correlated directly with influenza virus and RSV activity. We did not incorporate a time lag, since our viral surveillance data originated merely from a hospital population.
In the present study, IPD rates already seemed to rise before influenza virus and RSV activity did. The same appeared to be true for MD. This suggests that apart from viral activity, other seasonal factors may be important in the aetiology of invasive bacterial disease and may imply that correction for seasonality is necessary.
A previous study attempted to unravel the seasonality of IPD by comparing rates of IPD and seasonal phenomena within seven different states of the United States [2]. In this study, IPD was found to be correlated inversely with the temperature (r=−0·82 with a 1-week lag, P<0·01), although the lowest rates of IPD were found in the coldest states and no threshold temperature could be identified. This study also reported that the seasonal pattern of IPD correlated directly with the sinusoidal variations in photoperiod (r=0·85 with a 5-week lag) throughout the states and therefore hypothesized that nationwide seasonal changes such as photoperiod-dependent variation, i.e. the variation in hours of daylight throughout the seasons, influences host susceptibility and may underlie the seasonality of IPD [2, 21]. For MD, wind speed and humidity have been suggested to play roles in seasonality [22].
The concept of whether or not to correct for seasonality is, however, very challenging. In the multifactorial aetiology of invasive bacterial disease, several environmental factors such as a shortened photoperiod and lower environmental temperature along with host- and virus-specific factors may play a role. The shortened photoperiod and lower environmental temperature may induce crowding, thereby enhancing transmission of pathogens (viruses) in the community [23], they may also affect host susceptibility to (viral) infection. In this dynamic interaction of factors, viruses may play an intermediate role by contributing to an increased susceptibility to secondary bacterial infection [23]. In case all of these factors might affect the incidence of invasive bacterial disease independently of each other, it would be justified to correct for seasonality in order to determine the influence of the increased circulation of influenza viruses and RSV in the community. It is, however, unlikely that all these factors operate independently. Moreover, there is, for example, still no convincing evidence that either low environmental temperature or shortened photoperiod are indeed factors in the aetiology of bacterial invasive disease. It may still be that the length of the photoperiod ‘just’ parallels the occurrence of invasive bacterial disease. Keeping this in mind, correction for seasonality should be done with caution to avoid the possibility of overcorrection and subsequent disturbance of the potential relationship between influenza virus and RSV activity, and invasive bacterial disease. In the present study we attempted to correct for other potential seasonal factors in general, by applying the peri-seasonal baseline period as reference, encompassing the rather extended period in which rates of invasive bacterial disease tended to be higher.
To appreciate the results of the present study, some possible limitations should be discussed. It may be that our observed pattern in viral activity incorporated a delay since most of our surveillance data were derived from hospitalized patients. Therefore, viral activity in the community may already have started to rise somewhat earlier. In addition, we demonstrated positive incidence rate ratios of IPD and MD during influenza virus- and RSV-active periods, but no causal relationship can be inferred from these data since direct evidence for involvement of the viruses in the pathology of the infection is lacking. However, an assessment of the impact of viral seasons solely based on patients with IPD or MD that also tested positive for influenza virus or RSV would lead to an underestimation since viruses may have long gone in cases of bacterial super-infection. The temporal relationship we found between influenza virus and RSV isolation and IPD or MD activity might also have been affected by other seasonally active viruses. However, as previously stated, by taking the peri-seasonal baseline period as the reference period, we aimed to limit this possibility. Moreover, other respiratory viruses (rhinovirus, parainfluenza virus, and adenovirus) either demonstrated no clear seasonal circulation or very large periods of marginally increased activity (data available from corresponding author), also limiting their disturbing role in our study. It should be noted that the observed relationship between influenza virus infection and invasive bacterial disease in the elderly may have been underestimated since routine annual influenza vaccination has been recommended since 1996 in The Netherlands, with vaccination coverage around 70–80% during the study period (Statistics Netherlands, Voorburg/Heerlen, The Netherlands).
A major strength of the present study is that it assessed IPD and MD nationwide, comprising a period of 6 years with different viral attack rates. Furthermore, since all years showed largely separate peaks of influenza virus and RSV activity, this allowed the potential relationship between IPD, MD, and influenza virus/RSV activity to be explored separately. Moreover, as The Netherlands is a small but densely populated country, population characteristics are relatively homogenous nationwide and viral circulation is more or less simultaneous across the country, making ecological studies more reliable. Additionally, due to nationwide surveillance our study included a large number of cases enabling subset analysis by age.
In conclusion, the present study demonstrated that during periods of increased influenza virus and RSV activity higher rates of pneumococcal bacteraemia in older children and adults were found than during the peri-season. Rates of MD in children were also higher during periods of increased influenza virus activity; the same appeared true for older children during periods of increased RSV activity. This supports a role for RSV and influenza virus in the multifactorial pathogenesis of these diseases, however, it provides no evidence for a causal relationship. Regarding the accumulating evidence for viral-bacterial interaction and given the broad range of bacterial antigens for which vaccines would be needed, additional approaches to the prevention of invasive bacterial disease may be useful, including viral vaccines.
ACKNOWLEDGEMENT
This study was funded by The Netherlands Organization for Health Research and Development ZONMW (grant number 2200.0121). We thank the Dutch Working Group on Clinical Virology for providing the viral surveillance data.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Kim PE et al. Association of invasive pneumococcal disease with season, atmospheric conditions, air pollution, and the isolation of respiratory viruses. Clinical Infectious Diseases. 1996;22:100–106. doi: 10.1093/clinids/22.1.100. [DOI] [PubMed] [Google Scholar]
- 2.Dowell SF et al. Seasonal patterns of invasive pneumococcal disease. Emerging Infectious Diseases. 2003;9:573–579. doi: 10.3201/eid0905.020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connolly M, Noah N. Is group C meningococcal disease increasing in Europe? A report of surveillance of meningococcal infection in Europe 1993–6. Epidemiology and Infection. 1999;122:41–49. doi: 10.1017/s0950268898001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah AP et al. Seasonality of primarily childhood and young adult infectious diseases in the United States. Chronobiology International. 2006;23:1065–1082. doi: 10.1080/07420520600920718. [DOI] [PubMed] [Google Scholar]
- 5.Hament JM et al. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunology and Medical Microbiology. 1999;26:189–195. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 6.Young LS et al. A simultaneous outbreak of meningococcal and influenza infections. New England Journal of Medicine. 1972;287:5–9. doi: 10.1056/NEJM197207062870102. [DOI] [PubMed] [Google Scholar]
- 7.Cartwright KA et al. Influenza A and meningococcal disease. Lancet. 1991;338:554–557. doi: 10.1016/0140-6736(91)91112-8. [DOI] [PubMed] [Google Scholar]
- 8.Hubert B et al. Meningococcal disease and influenza-like syndrome: a new approach to an old question. Journal of Infectious Diseases. 1992;166:542–545. doi: 10.1093/infdis/166.3.542. [DOI] [PubMed] [Google Scholar]
- 9.Stuart JM, Cartwright K, Andrews NJ. Respiratory syncytial virus infection and meningococcal disease. Epidemiology and Infection. 1996;117:107–111. doi: 10.1017/s0950268800001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talbot TR et al. Seasonality of invasive pneumococcal disease: temporal relation to documented influenza and respiratory syncytial virus circulation. American Journal of Medicine. 2005;118:285–291. doi: 10.1016/j.amjmed.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Watson M et al. The association of respiratory viruses, temperature, and other climatic parameters with the incidence of invasive pneumococcal disease in Sydney, Australia. Clinical Infectious Diseases. 2006;42:211–215. doi: 10.1086/498897. [DOI] [PubMed] [Google Scholar]
- 12.Grabowska K et al. Occurrence of invasive pneumococcal disease and number of excess cases due to influenza. BMC Infectious Diseases. 2006;6:58. doi: 10.1186/1471-2334-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen ES et al. A 20-year ecological study of the temporal association between influenza and meningococcal disease. European Journal of Epidemiology. 2004;19:181–187. doi: 10.1023/b:ejep.0000017659.80903.5f. [DOI] [PubMed] [Google Scholar]
- 14.Kneyber MCJ, van Vught AJ. Respiratory syncytial virus infection and invasive meningococcal disease: is there an association? European Journal of Pediatrics. 2003;162:352–353. doi: 10.1007/s00431-003-1185-z. [DOI] [PubMed] [Google Scholar]
- 15.Van den Brandhof WE et al. Reporting virological diagnostics in The Netherlands. Representativity of data from the weekly viral reports [in Dutch] Infectieziekten Bulletin. 2002;13:110–113. [Google Scholar]
- 16.De Greeff SC et al. Two pneumococcal vaccines: the 7-valent conjugate vaccine (Prevenar) for children up to the age of 5 years of age and the 23-valent polysaccharide vaccine (Pneumo 23) for the elderly and specific groups at risk [in Dutch] Nederlands Tijdschrift voor Geneeskunde. 2007;151:1454–1457. [PubMed] [Google Scholar]
- 17.Tacken M 2004. Monitoring influenza vaccination campaign 2003 [in Dutch]. NIVEL .
- 18.Izurieta HS et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. New England Journal of Medicine. 2000;342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien KL et al. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clinical Infectious Diseases. 2000;30:784–789. doi: 10.1086/313772. [DOI] [PubMed] [Google Scholar]
- 20.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nature Medicine. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerging Infectious Diseases. 2001;7:369–373. doi: 10.3201/eid0703.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altizer S et al. Seasonality and the dynamics of infectious diseases. Ecology Letters. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 23.Lofgren E et al. Influenza seasonality: underlying causes and modeling theories. Journal of Virology. 2007;81:5429–5436. doi: 10.1128/JVI.01680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]