SUMMARY
Rotavirus infections are the main cause of gastroenteritis in infants and children and it is expected that by the age of 5 years, nearly every child will have experienced at least one episode of rotavirus gastroenteritis. While severe cases are hospitalized, milder disease is either treated at home or by the GP, and as such the true prevalence of rotavirus infection in the community, and the burden of disease, is unknown. This paper reports the results of a cost-of-illness study which was conducted alongside a structured community surveillance study. Forty-eight percent of our sample was found to have rotavirus acute gastroenteritis; and the average total cost of a child presenting with rotavirus gastroenteritis ranged between £59 and £143 per episode, depending on the perspective. Given the prevalence and severity of the disease, the estimated burden of rotavirus gastroenteritis to society is £11.5 million per year.
INTRODUCTION
Gastroenteritis is a common childhood illness. Estimates suggest that a child aged <5 years will experience between 2·2 and 3·2 episodes per year [1–3]. The symptoms of diarrhoea and vomiting vary in their severity, but death can occur, and is not uncommon in developing countries, accounting for an estimated 13–21% of all deaths of children aged <5 years, between 1·4 million and 2·5 million children per year globally [3, 4]. Rotavirus infections are the main cause of gastroenteritis in infants and young children and it is expected that by the age of 5 years, nearly every child will have experienced at least one episode of rotavirus gastroenteritis [5]. The symptoms of rotavirus gastroenteritis are generally more severe than other viral and non-specific causes, and as such rotavirus has a greater (clinical) disease burden [6, 7].
Children with severe diarrhoeal disease are often hospitalized and rotaviruses are mostly identified as the cause through laboratory tests performed on faecal samples collected from these cases. The available data on the incidence of illness is, therefore, only known for a small sample of the more severe cases. In contrast, a child with milder disease is either treated at home or by their general practitioner (GP), and thus the true prevalence of rotavirus infection in the community is unknown. This implies that the burden of disease is also underestimated. It is important to correctly estimate this burden for two reasons. First, two new vaccines have recently been developed and are now approved for use in a number of countries [8] and in order to estimate whether such vaccines are cost-effective, it is necessary to know the cost of illness. Second, it is important to quantify the burden experienced by parents and families. Such indirect costs are often ignored in economic analyses [9], and while they are often less than the direct costs of care and treatment they are important to the parents of those affected. The nature of gastroenteritis means that much of the burden falls on parents, having to take time off work or away from other activities to care for their ill child.
This paper reports the results of a cost-of-illness study which was conducted alongside a structured community surveillance study. The epidemiological and virological surveillance set out to establish the prevalence of different viral causes of gastroenteritis in children up to 5 years of age, the distribution of different genotypes of rotaviruses circulating in the community, and the symptoms and severity of disease attributable to the different viruses. In addition to this, information on resource use, expenditure and lost income was collected, such that the costs, both direct and indirect, associated with infantile viral gastroenteritis in the community could be estimated.
METHODS
Study population
This study of cost was conducted as an integral part of a structured surveillance, epidemiological and virological study. Full details of the methods and results of the virological surveillance are reported separately [10]. The study consisted of twenty GPs in East Anglia, over three rotavirus seasons (the winter months between December 2000 and April 2003), collecting faecal specimens from children aged ⩽5 years who presented with symptoms of gastroenteritis, where gastroenteritis was defined as the presence of vomiting and/or diarrhoea. Parents or carers of these children were asked to complete a questionnaire (available from authors on request) detailing severity of illness, health-care resource use, personal medical expenditure, changes in child-care patterns and associated costs, time off work and lost income due to their child's illness. The faecal samples were tested for the presence of enteric viruses (including rotavirus) using polymerase chain reaction (PCR).
Cost methodology
Following standard convention [11–14] the cost of illness was estimated from all possible perspectives. Costs incurred by the health service were estimated by combining information on resource use (as collected in the questionnaires) with unit cost data from published sources; while costs incurred by parents and families were estimated from information on out-of-pocket expenditure and reported days of lost work and lost income. This allowed the cost of an episode of gastroenteritis, and specifically an episode of rotavirus gastroenteritis to be calculated.
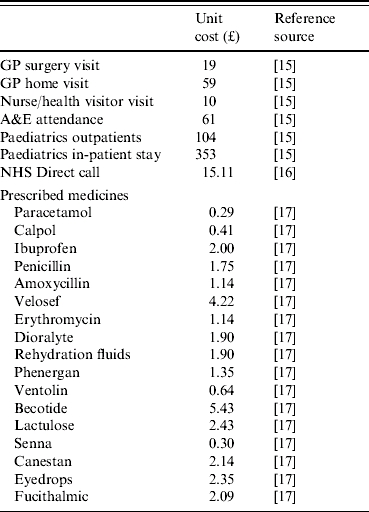
Health-care resource use, including GP surgery and home visits, nurse or health visitor consultations, prescriptions, NHS Direct telephone advice, hospital attendances and in-patient stays, were valued using published data on the unit cost of each service (see Table 1) [15–17]. It should be noted, however, that greater qualification of the prescribed medicines was required because parents simply listed the type of medication their child was prescribed, and did not provide information on the brand or the dose. Therefore, in order to determine the cost for each of these, three GPs (independent of the study) were asked to consider each of the listed medicines in more detail, and state what they would prescribe to a child aged ⩽5 years. The resulting unit costs (derived from the BNF [17]) for these prescribed medicines are also reported in Table 1. The cost of the laboratory tests in the cohort study were not included in the cost analysis as it is not universal practice to test stool samples from patients in the community.
Table 1.
Unit costs of health care
Costs incurred by society were estimated from the value of lost productivity. Lost productivity was valued by multiplying the number of days off work due to a child's illness by average gross daily earnings (estimated to be £74.20, as gross weekly earnings were £371) [18].
All costs are presented in 2001/2002 £ sterling. Costs pertaining to previous years were inflated using the using the Hospital and Community Health Services pay and price index for health-care services, and the retail price index for other costs [15].
Analysis
The cohort data were analysed as a whole to establish the cost of illness of infantile gastroenteritis and separately, to assess the contribution of rotavirus gastroenteritis. Ideally the analysis would also assess the burden of other enteric viruses, however, small sample sizes (particularly for rare events like hospitalization) make this statistically invalid, thus instances of viral (but non-rotavirus) gastroenteritis have been grouped with cases where no aetiological agent was found; this group is labelled non-specific. Complete case analysis was undertaken, whereby only those with complete available data were included in the analysis. All analysis was undertaken using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). Means of continuous data were compared using a Student's t test or the Mann–Whitney U test if the distribution was non-normal. The χ2 test was used to compare proportions.
RESULTS
Study samples
Over a period of 3 years there were 223 stool samples returned. Each parent received a questionnaire, of which 136 were returned, giving a response rate of 61%. Thus, the laboratory results and demographics are for a sample of 223, while the analysis of the questionnaire data (results on severity, resource use and cost) are for a sample of 136.
There is some possibility of selection bias between these two groups. Table 2 presents a comparative analysis of the characteristics of these two groups. It shows that both samples had relatively equal numbers of boys and girls, and similar mean ages of approximately 2 years old; although the children of parents who completed the questionnaire were marginally older. There is notable difference in the proportion of samples collected each year, significantly more parents completed questionnaires in year 1 of the study compared with year 2, however, this is not expected to bias the results and the cohort is considered representative of all those sampled.
Table 2.
Characteristics and demographics of the cohort and non-questionnaire completers, n (%) or mean (range)
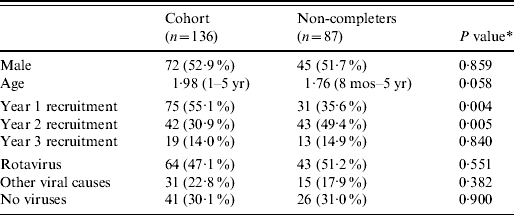
This is the P value of a Mann–Whitney U test (due to a non-normal distribution) of the difference in means or a χ2 test of the difference in proportions.
Prevalence
A large proportion of those tested had rotavirus (49% of the whole sample, 48% of those who completed the questionnaire), while other viruses were also present, predominantly norovirus and adenovirus. Twenty children tested positive for more than one virus, the most common combination being rotavirus and norovirus; while for 67 children who consulted the GP with symptoms of vomiting and/or diarrhoea no aetiological agent was detected. For a more detailed analysis of the virological data see Iturriza Gómara et al. [10].
Severity of illness
It was found that significantly more children with rotavirus reported experiencing vomiting, and they experienced on average a greater number of vomiting episodes and a greater duration of vomiting, than those who did not have a (any) virus. This was also reflected in the estimated severity scores, with rotavirus gastroenteritis having a greater severity score than other viruses and no virus [10].
Health-care resource use
Table 3 reports the health-care resource use for the full cohort (those presenting to a GP with diarrhoea and vomiting) and separately for the subgroups in which rotavirus was (n=64) and was not (n=72) detected. Resource use was recorded according to health-care visits (to both primary and secondary providers), telephone advice and medications taken (prescription and over-the-counter). Table 4 provides fuller details on the types of medications and their frequencies for the whole cohort.
Table 3.
Health-care resource use

OTC, Over-the-counter.
This is the P value of a Mann–Whitney U test (due to a non-normal distribution) of the difference in means between rotavirus and not rotavirus.
Table 4.
Frequencies of medication, prescribed and over-the-counter (OTC) purchases
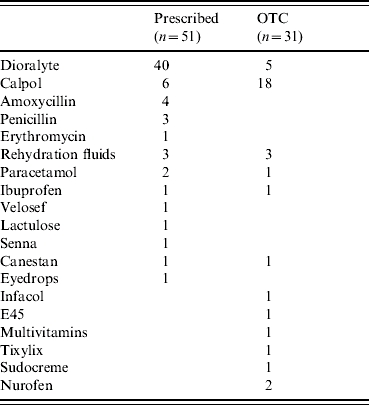
Few statistically significant differences are evident when comparing the resource use by rotavirus-positive children with those without rotavirus. Notably, parents of children who had rotavirus made significantly more telephone calls to NHS Direct.
Costs
Table 5 presents the direct health-care sector cost of illness, these are just the costs incurred by the health service, not those incurred directly by parents (e.g. out-of-pocket medical expenses), or indirectly as a result of the child's illness (e.g. lost income), or by society (e.g. lost productivity).
Table 5.
Direct health-care costs (£ sterling, 2001/2002 prices)

This is the P value of a Mann–Whitney U test (due to a non-normal distribution) of the difference in means between rotavirus and not rotavirus.
It can be seen in Table 5 that the direct medical cost for the average child presenting to a GP with symptoms of gastroenteritis was £60; there is little variation in this cost, with regard to whether the gastroenteritis was the result of rotavirus, or some other cause. The cost of NHS Direct contact in the rotavirus sample is significantly different compared to those with other causes.
Table 6 presents the cost incurred by parents, where these can be valued or quantified, of their child's illness. Parents whose children did not have rotavirus spent significantly more on over-the-counter medicines than those whose children had rotavirus. Notably, these children were prescribed fewer medicines by their GPs (see Table 3).
Table 6.
Indirect and non-health-care costs (£ sterling, 2001/2002 prices)
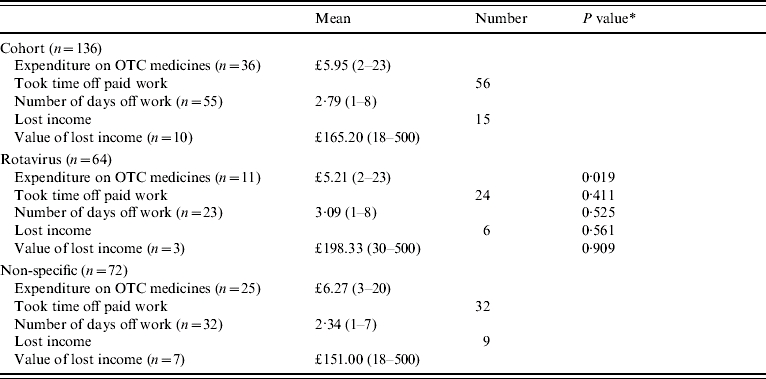
OTC, Over-the-counter.
This is the P value of a Mann–Whitney U test (due to a non-normal distribution) of the difference in means or a χ2 test of the difference in proportions between rotavirus and not rotavirus.
A presentation of these parental costs (lost income and expenditure on over-the-counter medicines) as averages for each sample as a whole is given in Table 7. The summation of these costs, that is total parental costs per episode of gastroenteritis, is also reported. Table 7 further presents estimates of the value of work time lost, that is the value of lost productivity. It is evident in Table 7, that those parents whose children had gastroenteritis that was not caused by rotavirus, on average spent more on over-the-counter medicines, lost more income (although this is difference is not statistically significant) and as a result experienced greater parental costs than those parents of children with rotavirus (£17 compared with £10, P value 0·018).
Table 7.
Parental and societal costs and losses (£ sterling, 2001/2002 prices)
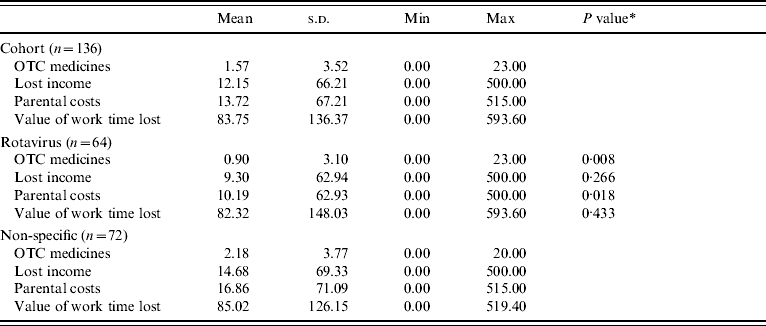
OTC, Over-the-counter.
This is the P value of a Mann–Whitney U test (due to a non-normal distribution) of the difference in means between rotavirus and no viruses, and other viruses and no viruses, respectively.
Finally, Table 8 presents the total cost-of-illness estimates. Three total costs are presented, each representing different perspectives. The first is simply that of the health-care sector, and includes only those costs incurred by the NHS, that is the direct medical costs of gastroenteritis. The second total cost adds parental (indirect and direct) costs to the direct medical costs per episode, giving a (narrow) societal perspective of the cost of illness; while the third total cost gives a much wider societal perspective and includes direct medical costs, parental costs and societal costs, but is net of lost income, in order to avoid double counting of lost productivity.
Table 8.
Total cost of illness per episode of gastroenteritis (£ sterling, 2001/2002 prices)

This is the P value of a Mann–Whitney U test (due to a non-normal distribution) of the difference in means between rotavirus and other causes.
This is a summation of the direct costs of medical care to the health service and to the parents (e.g. over-the-counter medicines) and the costs to society of lost production.
It was found that the mean cost of illness for an episode of acute gastroenteritis for the health sector was £60; however, it was £176 for society. When this illness is caused by rotavirus the cost of an episode was £59 to the health sector and £143 to society, and when it is the result of other causes the cost of an episode was £61 to the health sector and £148 to society (notably much of this higher cost is driven by a lengthy hospital stay by one child who was eventually diagnosed with Campylobacter spp.). These costs were, however, not significantly different between groups.
Burden of disease
Parashar et al. [5] estimated that some 88–92% of episodes of rotavirus are cared for at home, 8–10% of episodes are seen by a physician and 0·2–1·5% are hospitalized. Applying these estimates to countries in the European Union, Sorriano et al. [19] calculated that there are some 102 293 physician visits for rotavirus gastroenteritis each year in the United Kingdom. A similar study [20] which also attempts to estimate the burden of rotavirus in Europe (although specifically for hospitalizations) reports that four prospective observational studies in paediatricians' offices in Austria, Switzerland, and Germany found incidence rates of 0·84, 1·8, and 4·1/100 children aged <5 years, respectively [21–24]. Applying these incidence rates to the 3·10 million children in England and Wales aged <5 years [25], implies that the number of physician visits for rotavirus in England and Wales could range from 26 000 to 127 000 annually. We assume a conservative (albeit gross) estimate of 80 000 rotavirus episodes of rotavirus present to a GP annually, the result of 167 000 episodes of gastroenteritis presenting to a GP (given that 48% of all gastroenteritis episodes are caused by rotavirus).
From the cost per episode estimates presented earlier, and the assumed number of GP consultations, the societal burden of disease for gastroenteritis in the community in England and Wales is estimated to be £29.4 million, of which £2.3 million is the cost incurred by parents and family and £10.0 million is the cost incurred by the health service. Similar calculations specifically for rotavirus suggest that the societal burden of disease is £11.4 million; of which £0.8 million is incurred by parents and £4.7 million is incurred by the health service.
The cost of laboratory investigations can also be added to this estimated burden. As discussed earlier, while every sample was tested in this structured surveillance it is not routine for GPs to send stool samples from gastroenteritis sufferers for testing; the infectious intestinal disease (IID) study [26] found that 1·5 samples were sent for testing for every 35 children with rotavirus that presented to their GP. Given this proportion and the number of presentations for gastroenteritis and rotavirus annually, it is estimated that some 7200 laboratory investigations of samples from gastroenteritis sufferers would be undertaken each year, 3400 of these would be from rotavirus-specific gastroenteritis sufferers. The cost of routine enteric investigations vary across laboratories, but one laboratory in the East Anglia region reports a cost of £15.08 per GP sample (M. Sillis, personal communication). Given this and the estimated number of investigations, the annual cost of laboratory testing of stool samples is £109 000 and £51 000 for gastroenteritis and rotavirus, respectively. Therefore, the cost of investigations adds an additional 0·37% to the total disease burden, such that the estimated total burden is £29.5 million for gastroenteritis and £11.5 million for rotavirus-specific gastroenteritis in the community.
DISCUSSION
The results of the structured surveillance we have undertaken suggest that 48% of all gastroenteritis cases presenting to GPs are caused by rotavirus. There have been few other studies which have focused on the prevalence in the community, but those that have report lower rates. Isaacs et al. [27] found, in a very early community surveillance study conducted in five Oxfordshire general practices, that 20% of stool samples from children (aged <15 years) had rotavirus pathogens present. Another study undertaken in Canada found that around 20% of children presenting at paediatric practices and child-care centres with diarrhoea had rotavirus [28]; and a further study in the United States reported that 18% of all diarrhoea-associated outpatient visits were caused by rotavirus [29]. While an Austrian study of community-acquired gastroenteritis found 34% of children aged ⩽48 months with diarrhoea, who consulted a paediatrician, were rotavirus positive [30]. We believe the higher incidence of disease in this current study is a consequence of a firmer case definition of what constitutes an episode of gastroenteritis and the use of highly sensitive molecular methods to identify pathogens [10].
It was estimated that gastroenteritis costs society £176 per episode and rotavirus gastroenteritis £143 per episode. In general, this is similar to other estimates of the cost of an episode of rotavirus-caused diarrhoea, although not necessarily comparable due to methodology differences. A recent study [31] gathered clinical and cost information from GPs and parents of children attending Oxfordshire nurseries and presenting with diarrhoea at GP practices in the Midlands. This was used to estimate the incidence and burden of disease, which included both direct health-care costs and parental time off work costs. It was estimated that the mean cost per rotavirus episode was £173 in nurseries and £167 in general practice. Another UK study also reports a similar cost per episode. In a prospective study of various infectious intestinal diseases (IID study) the average cost per case of rotavirus was £164 [32]. However, this estimate includes those who did not seek medical attention (something the structured surveillance design did not allow for) and it also includes adult infections, and thus greater indirect costs. Studies conducted abroad also report similar estimates. In Austria, a cost-of-illness study, conducted alongside a prospective epidemiological study, estimated that the societal cost of rotavirus was €250 per child (about £180) [30]. These estimates include the cost of diagnostic tests, and use the human capital method to calculate lost productivity. Lower estimates have, however, been reported. A US study found that the median cost of diarrhoea- and rotavirus-associated diarrhoea outpatient visits was $47 and $57, respectively (about £26 and £32) [29]. This estimate is, however, derived from medial insurance claims, and thus does not include any indirect costs or other health system costs, just the cost incurred by the third-party payer. It is important to note that although the cost-of-illness estimates produced in this paper and those discussed above, are estimated with great rigour, a degree of caution should be exercised, and they should be considered indicative rather than definitive. This is because averages are presented, and these can be heavily influenced by single events (for example the high hospitalization costs of the child with campylobacteriosis).
Many researchers (including those discussed above) have estimated the burden of illness for rotavirus gastroenteritis, but because the total burden is a function of the population at risk, it is only valid to compare the estimates reported with those found by others in the United Kingdom. Only three such estimates exist, two estimate the total cost of providing hospital treatment only and the other includes adult illness, therefore, again they are not strictly comparable, but are discussed for completeness. From a retrospective analysis of a paediatric hospital's records, it was estimated that the (direct medical) cost of hospital treatment for rotavirus was £6.3 million per year in England and Wales [33]. While analyses which combined national admissions and laboratory surveillance data, estimated that the cost of hospitalization in 1997 in Scotland for rotavirus gastroenteritis ranged from £275 000 to £835 000 [34]. A further study, the IID study, estimated the total cost of rotavirus infection in children and adults in England in 1994 to be £18.2 million [32]. However, this did not include any hospitalization costs.
A unique feature of this study is the inclusion of NHS Direct as a health-care resource. This national telephone helpline was introduced in 2001 as a means of increasing access to health care and diverting some demand away from traditional primary-care providers. The study found that a number of the parents called NHS Direct, but subsequently took their children to the GP, and thus were recruited to the study. However, it is highly likely that other parents treated their children at home on the advice of the NHS Direct. This is one potential reason for the poor recruitment, and could also imply that the incidence of illness in the community has been underestimated. Further, as it was introduced after the first year of recruitment, it may also explain the lower rates of recruitment in the second and third seasons. This is supported by a recent analysis of NHS Direct calls [35], which found that 10·3% of all calls were for diarrhoea and vomiting, a large proportion of which were for children aged <5 years. Furthermore, local NHS Direct data on the number of times that the treatment algorithm for diarrhoea and vomiting was used within the Norfolk and Cambridgeshire Primary Care Trusts (PCTs) (M. Streather, personal communication) shows a steady increase each year, from an average of 273 calls in the winter months of 2001 in Cambridge to 337 calls in the winter months of 2004.
The estimated economic burden of rotavirus gastroenteritis is considerable and suggests that there is a need to alleviate some of it. Concurrent research, using a decision model, has been undertaken to evaluate the cost effectiveness of a vaccination programme for rotavirus [36]. The results of which are useful in deciding whether it is worthwhile for the NHS to introduce a vaccine, or if alternative approaches are necessary.
ACKNOWLEDGEMENTS
The authors acknowledge the help of the GPs and their practices who recruited patients and samples; the parents and children who participated in the research; and Amanda Howe, Christopher Hand, Chris Abell who provided advice during the analysis. Funding for the project was obtained from NHS R&D Eastern Region HSR/1199/06.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Snyder JD, Merson MH. The magnitude of the global problem of diarrhoeal disease: a review of active surveillance data. Bulletin of the World Health Organization. 1982;60:605–613. [PMC free article] [PubMed] [Google Scholar]
- 2.Bern C et al. The magnitude of the global problem of diarrhoeal disease: a ten year update. Bulletin of the World Health Organization. 1992;70:705–714. [PMC free article] [PubMed] [Google Scholar]
- 3.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bulletin of the World Health Organization. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 4.Murray C The Global Burden of Disease 2000 Project: Aims, Methods, and Data Sources. Geneva: World Health Organization; 2001. [Google Scholar]
- 5.Parashar UD et al. Global illness and deaths caused by rotavirus disease in children. Emerging Infectious Disease. 2003;9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruuska T, Vesikari T. A prospective study of acute diarrhoea in Finnish children from birth to 2½ years of age. Acta Paediatrica Scandanvia. 1991;80:500–507. doi: 10.1111/j.1651-2227.1991.tb11893.x. [DOI] [PubMed] [Google Scholar]
- 7.Pang XL et al. Human caliciviruses in acute gastroenteritis of young children in the community. Journal of Infectious Diseases. 2000;181:288–294. doi: 10.1086/315590. (Suppl. 2): [DOI] [PubMed] [Google Scholar]
- 8.Vesikari T, Giaquinto C, Huppertz HI. Clinical trials of rotavirus vaccines in Europe. Pediatric Infectious Disease Journal. 2006;25:S42–S47. doi: 10.1097/01.inf.0000197565.45345.4e. [DOI] [PubMed] [Google Scholar]
- 9.Drummond M Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 1997. [Google Scholar]
- 10.Iturriza Gomara M et al. Structured surveillance of infantile gastroenteritis in East Anglia, UK: incidence of infection with common viral gastroenteric pathogens. Epidemiology and Infection. 2007 doi: 10.1017/S0950268807008059. . doi:10.1017/S0950268807008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice DP. Estimating the cost of illness. American Journal of Public Health and the Nation's Health. 1967;57:424–440. doi: 10.2105/ajph.57.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgson TA, Meiners MR. Cost-of-illness methodology: a guide to current practices and procedures. Milbank Memorial Fund Quarterly Health and Society. 1982;60:429–462. [PubMed] [Google Scholar]
- 13.Rice DP, Hodgson TA, Kopstein AN. The economic costs of illness: a replication and update. Health Care Finance Review. 1985;7:61–80. [PMC free article] [PubMed] [Google Scholar]
- 14.Akobundu E et al. Cost-of-illness studies: a review of current methods. Pharmacoeconomics. 2006;24:869–890. doi: 10.2165/00019053-200624090-00005. [DOI] [PubMed] [Google Scholar]
- 15.Netten A, Rees T, Harrison G. Unit Costs of Health and Social Care 2001. PSSRU University of Kent at Canterbury; 2001. [Google Scholar]
- 16.The Comptroller and Auditor General London: 2002. . NHS Direct in England. [Google Scholar]
- 17.British Medical Association & Royal Pharmaceutical Society of Great Britain. British National Formulary. London: BMJ; 2002. [Google Scholar]
- 18.Office for National Statistics London: 2002. . The New Earnings Survey. [Google Scholar]
- 19.Soriano-Gabarró M et al. Burden of rotavirus disease in European Union countries. Pediatric Infectious Disease Journal. 2006;25:S7–S11. doi: 10.1097/01.inf.0000197622.98559.01. [DOI] [PubMed] [Google Scholar]
- 20.PROTECT Advisory Board. The paediatric burden of rotavirus disease in Europe. Epidemiology and Infection. 2006;134:908–916. doi: 10.1017/S0950268806006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehlken B et al. Prospective population-based study on rotavirus disease in Germany. Acta Paediatrica. 2002;91:769–775. doi: 10.1080/08035250213227. [DOI] [PubMed] [Google Scholar]
- 22.Frühwirth M et al. A prospective evaluation of community acquired gastroenteritis in paediatric practices: impact and disease burden of rotavirus infection. Archives of Disease in Childhood. 2001;84:393–397. doi: 10.1136/adc.84.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frühwirth M et al. International variation in disease burden of rotavirus gastroenteritis in children with community- and nosocomially acquired infection. Pediatric Infectious Disease Journal. 2001;20:784–791. doi: 10.1097/00006454-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Laubereau B et al. Rotavirus gastroenteritis in infants and children Schweizerische Medizinische Wochenschrift. 1999;129:1822–1830. . Results of a prospective study in the area of Geneva and Basel 1997/1998 (RoMoS). RoMoS Study Group [in German]. [PubMed] [Google Scholar]
- 25.Office for National Statistics . Age Structure of England and Wales 1961–2074. ONS Population Estimateshttp://www.statistics.gov.uk/populationestimates/svg_pyramid/default.htm). Accessed 29 December 2006.
- 26.Food Standards Agency Norwich: 2000. . A report of the study of infectious intestinal disease in England. [Google Scholar]
- 27.Isaacs D, Day D, Crook S. Childhood gastroenteritis: a population study. British Medical Journal. 1986;293:545–546. doi: 10.1136/bmj.293.6546.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waters V et al. Etiology of community-acquired pediatric viral diarrhea: a prospective longitudinal study in hospitals, emergency departments, pediatric practices and child care centers during the winter rotavirus outbreak, 1997 to 1998 Pediatric Infectious Disease Journal. 2000;19:843. doi: 10.1097/00006454-200009000-00007. . The Pediatric Rotavirus Epidemiology Study for Immunization Study Group. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman CM et al. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatric Infectious Disease Journal. 2001;20:14–19. doi: 10.1097/00006454-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Frühwirth M et al. Economic impact of community- and nosocomially acquired rotavirus gastroenteritis in Austria. Pediatric Infectious Disease Journal. 2001;20:184–188. doi: 10.1097/00006454-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Buttery J et al. Clinical and cost impact of rotavirus diarrhoea in Oxfordshire Nurseries and primary care patients. Archives of Disease in Childhood. 2001;84:A61. [Google Scholar]
- 32.Roberts JA et al. The study of infectious intestinal disease in England: socio-economic impact. Epidemiology and Infection. 2003;130:1–11. doi: 10.1017/s0950268802007690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noel JS et al. Impact of rotavirus infection on a paediatric hospital in the east end of London. Journal of Clinical Pathology. 1994;47:67–70. doi: 10.1136/jcp.47.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowden JM. An estimate of the costs of cases of rotavirus infection admitted to hospital in Scotland, 1997. Health Bulletin (Edinburgh) 2001;59:188–192. [PubMed] [Google Scholar]
- 35.Cooper DL et al. What can analysis of calls to NHS direct tell us about the epidemiology of gastrointestinal infections in the community? Journal of Infection. 2003;46:101–105. doi: 10.1053/jinf.2002.1090. [DOI] [PubMed] [Google Scholar]
- 36.Lorgelly PK et al. Exploring the cost effectiveness of an immunization programme for rotavirus gastroenteritis in the UK. Epidemiology and Infection. 2007 doi: 10.1017/S0950268807008151. . doi:10.1017/S0950268807008151. [DOI] [PMC free article] [PubMed] [Google Scholar]


