Abstract
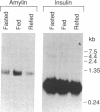
Amyloid deposits in the islets of Langerhans of the pancreas are a common finding in non-insulin-dependent diabetes mellitus. The main protein constituent of these deposits is a 37-amino acid peptide known as amylin that resembles calcitonin gene-related peptide, a neuropeptide. We have isolated cDNA clones corresponding to the rat amylin precursor from an islet cDNA library and we show that this peptide is encoded in a 0.9-kilobase mRNA that is translated to yield a 93-amino acid precursor. The amylin peptide is bordered by dibasic residues, suggesting that it is proteolyzed like calcitonin gene-related peptide. The peptide sequences flanking the amylin sequence do not resemble the calcitonin gene-related peptide flanking sequences. RNA hybridization studies show that amylin mRNA is abundant in the islets of Langerhans but is not present in the brain or seven other tissues examined. Dietary changes, such as fasting or fasting and refeeding, have little effect on amylin mRNA expression. This tissue specificity suggests that amylin is involved in specific signaling pathways related to islet function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahronheim J. H. The Nature of the Hyaline Material in the Pancreatic Islands in Diabetes Mellitus. Am J Pathol. 1943 Sep;19(5):873–882. [PMC free article] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A., Cooper G. J., Lewis C. E., Morris J. F., Willis A. C., Reid K. B., Turner R. C. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987 Aug 1;2(8553):231–234. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- Clark A., Holman R. R., Matthews D. R., Hockaday T. D., Turner R. C. Non-uniform distribution of islet amyloid in the pancreas of 'maturity-onset' diabetic patients. Diabetologia. 1984 Nov;27(5):527–528. doi: 10.1007/BF00290389. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Leighton B., Dimitriadis G. D., Parry-Billings M., Kowalchuk J. M., Howland K., Rothbard J. B., Willis A. C., Reid K. B. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7763–7766. doi: 10.1073/pnas.85.20.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRLICH J. C., RATNER I. M. Amyloidosis of the islets of Langerhans. A restudy of islet hyalin in diabetic and non-diabetic individuals. Am J Pathol. 1961 Jan;38:49–59. [PMC free article] [PubMed] [Google Scholar]
- Ghiso J., Jensson O., Frangione B. Amyloid fibrils in hereditary cerebral hemorrhage with amyloidosis of Icelandic type is a variant of gamma-trace basic protein (cystatin C). Proc Natl Acad Sci U S A. 1986 May;83(9):2974–2978. doi: 10.1073/pnas.83.9.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Eanes E. D., Wiley C. A. Amyloid fibrils formed from a segment of the pancreatic islet amyloid protein. Biochem Biophys Res Commun. 1988 Sep 15;155(2):608–614. doi: 10.1016/s0006-291x(88)80538-2. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Hayden D. W., Jordan K., Ghobrial H. K., Mahoney W. C., Westermark P. Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic beta cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques. Am J Pathol. 1988 Jan;130(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Leighton B., Cooper G. J. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature. 1988 Oct 13;335(6191):632–635. doi: 10.1038/335632a0. [DOI] [PubMed] [Google Scholar]
- Nakazato M., Sasaki H., Furuya H., Sakaki Y., Kurihara T., Matsukura S., Kangawa K., Matsuo H. Biochemical and genetic characterization of type I familial amyloidotic polyneuropathy. Ann Neurol. 1987 Jun;21(6):596–598. doi: 10.1002/ana.410210612. [DOI] [PubMed] [Google Scholar]
- Palmert M. R., Golde T. E., Cohen M. L., Kovacs D. M., Tanzi R. E., Gusella J. F., Usiak M. F., Younkin L. H., Younkin S. G. Amyloid protein precursor messenger RNAs: differential expression in Alzheimer's disease. Science. 1988 Aug 26;241(4869):1080–1084. doi: 10.1126/science.2457949. [DOI] [PubMed] [Google Scholar]
- Pettersson M., Ahrén B., Böttcher G., Sundler F. Calcitonin gene-related peptide: occurrence in pancreatic islets in the mouse and the rat and inhibition of insulin secretion in the mouse. Endocrinology. 1986 Aug;119(2):865–869. doi: 10.1210/endo-119-2-865. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Amara S. G., Evans R. M. Alternative RNA processing: determining neuronal phenotype. Science. 1984 Sep 21;225(4668):1315–1320. doi: 10.1126/science.6089345. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanke T., Bell G. I., Sample C., Rubenstein A. H., Steiner D. F. An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J Biol Chem. 1988 Nov 25;263(33):17243–17246. [PubMed] [Google Scholar]
- Seifert H., Sawchenko P., Chesnut J., Rivier J., Vale W., Pandol S. J. Receptor for calcitonin gene-related peptide: binding to exocrine pancreas mediates biological actions. Am J Physiol. 1985 Jul;249(1 Pt 1):G147–G151. doi: 10.1152/ajpgi.1985.249.1.G147. [DOI] [PubMed] [Google Scholar]
- Terazono K., Yamamoto H., Takasawa S., Shiga K., Yonemura Y., Tochino Y., Okamoto H. A novel gene activated in regenerating islets. J Biol Chem. 1988 Feb 15;263(5):2111–2114. [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., O'Brien T. D., Hayden D. W., Johnson K. H. Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am J Pathol. 1987 Jun;127(3):414–417. [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986 Nov 14;140(3):827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wilander E., Westermark G. T., Johnson K. H. Islet amyloid polypeptide-like immunoreactivity in the islet B cells of type 2 (non-insulin-dependent) diabetic and non-diabetic individuals. Diabetologia. 1987 Nov;30(11):887–892. doi: 10.1007/BF00274799. [DOI] [PubMed] [Google Scholar]