SUMMARY
We conducted a 31-month retrospective review of all laboratory-confirmed Streptococcus suis infections admitted to public hospitals in Hong Kong. Strain identification, serotyping and antibiotic susceptibility tests were conducted on S. suis isolates. Twenty-one sporadic cases were identified, comprising 18 (86%) males and 3 (14%) females. About half were patients aged ⩾65 years. More cases occurred during summer. Occupational exposure was documented in five (24%) cases. The estimated annual incidence was 0·09/100 000 in the general population and 32/100 000 in people with occupational exposure to pigs and raw pork. The primary clinical manifestations were meningitis (48%), septicaemia (38%) and endocarditis (14%). The case-fatality rate was 5%. All available isolates from 15 patients were serotype 2, sensitive to penicillin, ampicillin, ceftriaxone, but resistant to tetracycline. Injury prevention and proper handling of pigs or raw pork should be advocated to both at-risk occupational groups and the general population.
INTRODUCTION
Streptococcus suis is a Gram-positive facultative anaerobic coccus under the genus Streptococcus. Thirty-five serotypes of S. suis have been identified and serotype 2 is the main agent of zoonoses [1]. The main reservoir is pigs and the bacterium is occasionally found in wild boars, horses, dogs, cats and birds [2]. S. suis is widespread in pig populations and asymptomatic tonsillar carriage can be found in up to 80% [3]. Humans become infected mainly through cutaneous contact with infected pigs or raw pork, especially when skin cuts and abrasions are present. The first human case of S. suis meningitis was reported in 1968 in Denmark [4]. Over 150 cases have been reported worldwide including the United Kingdom [5], France [6], Germany [7–10], The Netherlands [11], Sweden [12], New Zealand [13], Thailand [14], Singapore [15], Taiwan [16] and Hong Kong [17–19]. In July 2005, a large outbreak affecting over 200 people in Sichuan, China attracted international attention [20].
The epidemiology of S. suis in Hong Kong is not clearly understood because of the rarity of the infection and the paucity of published reports. Its incidence has not been well estimated since it was not a notifiable disease until August 2005. Previously published local case series of S. suis in Hong Kong were restricted to patients seen in different regional hospitals and did not encompass the whole territory. The recent outbreak in Sichuan, China calls for a pressing need to better define the risk and characteristics of human S. suis infection in Hong Kong in order to inform effective public health interventions. We aim to describe the epidemiological, clinical and laboratory findings of recent human S. suis infection in Hong Kong and to identify potential risk factors for the infection.
METHODS
We conducted a retrospective review of human S. suis cases admitted to all public hospitals in Hong Kong from 1 January 2003 to 31 July 2005 inclusive. A case was defined as a patient who had a positive culture of S. suis in a clinical specimen taken from any body site. Cases were identified by searching through records held in all microbiology laboratories under the Hospital Authority (HA) showing a positive laboratory result for S. suis during the study period. For each of these cases, additional clinical information was collected through the electronic database of the HA. HA hospitals accounted for over 80% of all in-patients in Hong Kong [21]. We also examined the clinical records of each case identified through the electronic clinical management system. We recorded data on clinical features and laboratory findings, the latter including haematological and biochemical investigations, blood and cerebrospinal fluid (CSF) cultures, and antibiotic susceptibility. Each case was interviewed by trained nurses using a standardized questionnaire, which collected data on basic demographics, onset date, symptoms, past medical illness, occupation and relevant exposure history (e.g. whether handled raw pork, visited a pig farm or slaughterhouse, kept pets at home), history of injury and travel. We analysed the characteristics of the cases as well as potential risk factors associated with infection. We calculated the annual incidence rate among the general population and occupational groups at risk using the denominator populations from the Census and Statistics Department, Hong Kong Special Administrative Region (HKSAR), and other relevant authorities.
Bacterial isolates from the respective hospitals were obtained and tested in the Public Health Laboratory Centre for serotyping and further strain characterization. API 20 Strep/rapid ID 32 Strep (bioMérieux China Ltd) were used for biochemical tests. Serotyping was performed by a co-agglutination test (monovalent co-agglutination reagents supplied by the Agriculture, Fisheries and Conservation Department, HKSAR). Antimicrobial susceptibility tests were performed using standardized disk diffusion methods as recommended by the Clinical and Laboratory Standards Institute, HKSAR.
RESULTS
Epidemiological findings
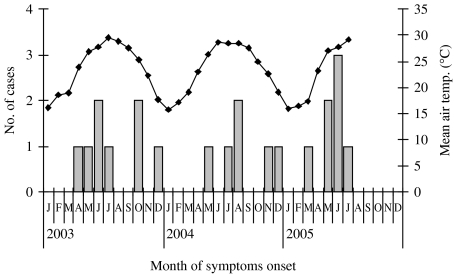
During the 31-month study period from 1 January 2003 to 31 July 2005, we identified 21 laboratory-confirmed S. suis infections among patients admitted to public hospitals, of which one (5%) was fatal. Eighteen (86%) cases were male and three (14%) were female (Tables 1 and 2). The median age was 62 years (range 26–89 years), and 48% were aged ⩾65 years. Twelve (57%) of 21 cases had disease onset in May, June, July or August (Fig.). They lived in different districts in Hong Kong and there was no geographic clustering. Among the 18 residential districts in Hong Kong, the residential areas with three or more cases included Kwun Tong (5), Wong Tai Sin (4), Shatin (3) and Yuen Long (3). None of the cases reported travelling to Sichuan during the incubation period.
Table 1.
Epidemiological and clinical features of the 21 patients
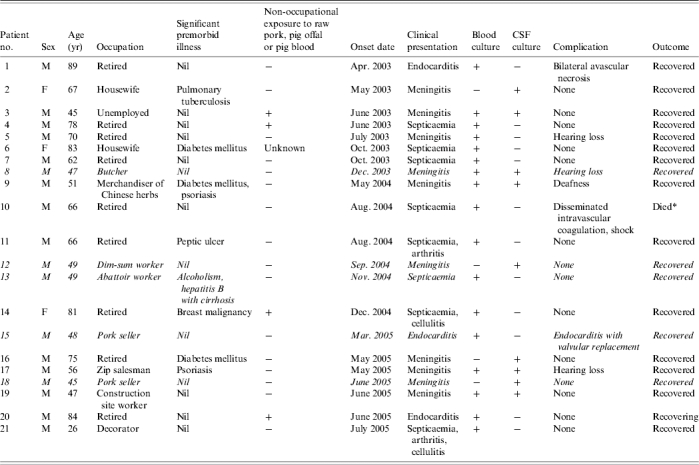
Coroner's report for cause of death pending.
Table 2.
Summary of S. suis cases in Hong Kong
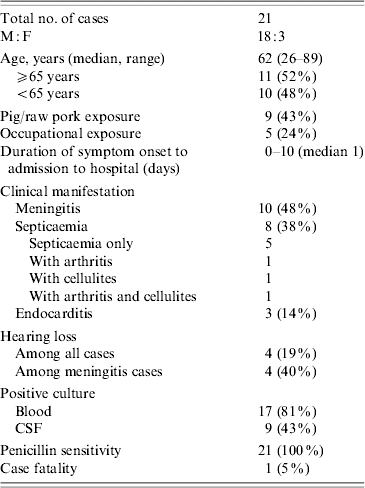
Fig.
Temporal distribution of Streptococcus suis infection ( ) and mean air temperature (–◆–) in Hong Kong, January 2003 to July 2005. [Mean air temperature (°C): mean of the daily temperature (°C) in a particular month recorded by the Hong Kong Observatory.]
) and mean air temperature (–◆–) in Hong Kong, January 2003 to July 2005. [Mean air temperature (°C): mean of the daily temperature (°C) in a particular month recorded by the Hong Kong Observatory.]
Six cases (29%) had underlying medical illnesses which might predispose to immunosuppression. These included diabetes mellitus (3), alcoholism (1), pulmonary tuberculosis (1) and breast carcinoma (1).
Five cases (24%) had occupational exposure to pigs or raw pork. Their occupations were butcher (3), abattoir worker (1) and dim-sum worker (1) (Table 1). The other 16 cases (76%) were not associated with occupations considered at risk of S. suis infection. Four of these 16 cases reported a history of purchasing raw pork from wet markets and handling raw pork in preparing meals before the onset of disease. No specific exposure was identified for the remaining 12 cases. No patient recalled consuming undercooked pork or pig offal or pig blood. A clear history of skin cuts and abrasions prior to disease was only reported in two cases. The dim-sum worker recalled injury on the left middle finger 1 day before the onset of symptoms. None of the home contacts had similar symptoms.
Using the mid-year population of Hong Kong [22], the annual incidence of S. suis infection among the general population was calculated as 0·09/100 000 (95% CI 0·06–0·14/100 000; i.e. 16 non-occupational related cases/6·88 millions per 2·6 years). It was estimated that about 6000 people in Hong Kong were pig farmers, abattoir workers, porters, health inspectors and other workers whose occupations involved frequent contact with pigs or raw pork (Agriculture, Fisheries and Conservation Department, and Food and Environmental Hygiene Department, HKSAR, unpublished data). Among this occupational group, the annual incidence of S. suis infection was calculated to be 32/100 000 (95% CI 21–49/100 000; i.e. five occupational related cases/6000 per 2·6 years), which was more than 350 times that of the general population.
Clinical presentation
The chief clinical manifestations were, in descending order of frequency, meningitis (10 cases, 48%), septicaemia (eight cases, 38%) and endocarditis (three cases, 14%). Among the eight patients presenting with septicaemia, one also had cellulitis, one had arthritis and one had both cellulitis and arthritis. The most common presenting symptoms were fever (95%), headache (38%), joint pain (38%), vomiting (24%) and hearing loss (19%). The median duration between symptom onset and hospital admission was 1 day (range 0–10 days).
Sixteen (76%) of the cases were found to have a peripheral blood leucocytosis (range 11·3–26·4×109 l). Liver functions were mildly altered with raised alkaline phosphatase (range 135–176 IU/l) or alanine transferase (range 67–325 IU/l) in nine cases (43%), while creatinine was slightly raised in five cases (range 112–145 μmol/l). Seventeen (81%) cases had a positive blood culture for S. suis, nine (43%) cases had positive CSF cultures and five (24%) cases had both. Among cases presenting as meningitis, 67% and 90% had positive cultures for blood and CSF respectively. Among cases presenting as septicaemia, the corresponding proportions were 100% and 0% respectively.
Cases with meningitis stayed significantly longer in hospital than those with non-meningitic manifestations (median difference 3·5 days, P=0·045, Mann–Whitney U test). In addition, 40% (4/10) of the patients with meningitis had hearing loss. Out of the three patients with endocarditis, one required valvular replacement. The only fatal case was a 66-year-old male with septicaemia complicated by disseminated intravascular coagulation.
Laboratory findings
Non-duplicate isolates from 15 patients were available for characterization. All isolates were identified as S. suis serotype 2 by both biochemical and serological tests. Strains were sensitive to penicillin, ampicillin, ceftriaxone, vancomycin and resistant to tetracycline. Resistance to macrolides (clindamycin and erythromycin) was found in 20% (3/15) of the isolates.
DISCUSSION
The case series in our study provided the first territorial estimates of the annual incidence of S. suis infection in Hong Kong, namely 0·09/100 000 (95% CI 0·06–0·14/100 000) for the general population and 32/100 000 (95% CI 21–49/100 000) for groups with occupational exposures. There have been three published local cases series of S. suis infections conducted over different catchment populations of some hospitals during the past three decades: eight patients from Queen Mary Hospital, 1978–1981 [17]; 30 patients from Queen Elizabeth Hospital, 1981–1984 [18]; and 25 patients from the Prince of Wales Hospital and the United Christian Hospital, 1984–1993 [19]. The previous series had a different mix of populations and occupational subgroups. In comparison, our study included all public hospitals in Hong Kong accounting for over 80% of total in-patient days [21]. Thus, the incidence estimates in our study are likely to be more representative of the territory. Our incidence estimate was not much different from a previous local series [19] which gave an annual risk of 0·17/100 000 population. Unfortunately, no breakdown of incidence estimates by occupational groups was given in the previous case series.
It is noteworthy that S. suis infections which did not result in hospitalization in a public hospital would not be picked up by our study. Seroprevalence studies would be required to estimate the prevalence of mild or subclinical infections, especially among groups with occupational exposures. S. suis is an uncommon human infection worldwide with a cumulative total of around 150 case reports in the literature [23]. Due to the scarcity of published data, international comparisons of incidence rates are difficult to make. Nonetheless, the incidence rate in Hong Kong appears substantially higher than that in The Netherlands (0·002/100 000 in the general population and 1·2–3·5/100 000 in high-risk occupation groups) [11]. The reasons for this difference are unclear, but the intensity and sensitivity of surveillance is probably an important factor. Moreover, the lack of epidemiological linkage between the cases suggests that S. suis infection in Hong Kong has been occurring sporadically during the past 2 years and was not part of a large outbreak of common origin.
Our study found more cases occurring in summer, which is consistent with findings from previous local case series. Some studies from mainland China also recorded large outbreaks of S. suis during summer [24]. It has been postulated that moving and mixing of young pigs when they are weaned facilitates the transmission of the infection among the pigs [25]. Transportation during the hot and humid weather might pose additional stress to pigs and render them more prone to S. suis infection [17]. Hot and humid temperature might also facilitate multiplication of S. suis.
Another interesting finding in our study concerns the epidemiological profile of the cases. Contrary to common belief, S. suis infections with occupational exposures accounted for only a minority (24%) of cases, although such occupational groups have a much higher risk of infection. Our study results contrast with a previous local series (1978–1981) in which all cases had occupational exposures identified.
Another notable feature is that older persons aged ⩾65 years accounted for almost half of the cases. Elderly people may be more vulnerable, due to the presence of pre-existing medical conditions which suppress the immune system. However, asplenism was not a common predisposing factor among Hong Kong patients, as reported for other countries [11, 23]. Among persons aged <65 years, occupational exposures to pigs and raw pork became more important, being present in about half (45%) of such cases. The absence of cases among children may reflect absence of exposure, surveillance bias, or other factors. The male predominance (81%) in non-occupational cases remains difficult to explain.
A major uncertainty is how a sizable proportion (57%) of cases became infected in the absence of clear exposures to pigs and raw pork. Non-occupational exposures to raw pork are difficult to ascertain accurately in a retrospective review due to recall bias, since we were asking about exposures that occurred some months previously. A similar problem was encountered while ascertaining minor cuts or abrasions before onset of symptoms. Our series found two out of 14 (14%) who recalled a clear history of skin cuts or abrasions, which is comparable in magnitude (10–29%) with other studies in Asia and in Europe [19]. A case-control study would be needed to better define sources of exposure and thereby improve the incubation period estimate. In the present situation the wearing of gloves while handling raw pork seems a sensible precaution, whether or not one has a visible wound.
Our study showed that meningitis was the most common clinical presentation of S. suis infection in Hong Kong. However, compared with previous local series, our study found a higher proportion of cases with septicaemia without central nervous system involvement (38% vs. 0–16%). In overseas studies, about 12% of the cases were reported as presenting as septicaemia [19]. Deafness is an important clue for clinical diagnosis of S. suis infection; it occurred in nearly half of the meningitis cases in our study. Hearing loss appears within 1–12 days prior to admission [19] and is often permanent. Mono- or polyarthritis and endocarditis are also often found in S. suis infections in Hong Kong. In our series, arthritis associated with S. suis affected mainly knee, hip and wrist joints and occasionally shoulder and ankle. One previous case series reported one patient presenting with acute polyarthritis and S. suis was isolated from the knee aspirates [19]. Four endocarditis cases associated with S. suis have been documented in the four case series [17–19] and one patient in our study required valvular replacement. Unlike some studies which reported diarrhoea being present in a significant proportion (15%) of cases [19], only one case (5%) among our 21 patients reported this symptom. This makes it unlikely that a gastrointestinal route of transmission was involved.
Ours series shows a case-fatality rate of 5% which is almost the same as the previous local series [19] but lower than another study (23%) [18]. Case-fatality rates reported elsewhere ranged from 7% to 13% [5, 11, 14]. Low case-fatality rate may be partly attributable to early hospital admission and administration of appropriate antibiotics. The only death in our series was associated with intravascular coagulation, which was also reported in two of the other local series [18, 19]. Similar to other studies worldwide [14, 15, 19, 26], all S. suis isolates in our study were sensitive to penicillin. Penicillin-class antibiotics are thus antibiotics of first choice in treating S. suis infections in Hong Kong.
In bacteriological diagnosis of S. suis infection, blood cultures had a sensitivity of about 85% for all cases, while CSF cultures detected 90% of cases presenting with meningitis. However, due care should be exercised when interpreting laboratory results from commercial kits that may not have been thoroughly validated for uncommon or emerging bacterial pathogens. Where circumstances permit, it is always advisable to use a second test kit to verify the identity of such pathogens and to resort to a reference laboratory service when equivocal results are obtained. For the 15 patient isolates available in this study, rapid ID 32 Strep was able to identify all 15 (100%) whereas API 20 Strep could only identify 12 (80%) of the isolates as being S. suis. Both kits suggested an identity of serotype 1 or 2 S. suis with very high confidence. From our experience with these strains, the serotype suggestion may not always be accurate. A useful differentiating test for serotype 1 or 2 isolates is to supplement the kits with a conventional raffinose fermentation test. All isolates in this study were able to ferment raffinose broth within 24 h at 37°C. In terms of serotyping, serotype 2 is the predominant strain causing human infections in Hong Kong and remains the most commonly documented S. suis infection in humans [27].
In the absence of an effective vaccine for humans the main preventive measures against S. suis infection rely on injury prevention and cautious handling of pigs or raw pork. This should apply not only to workers with occupational exposures, but also to the general public who account for the majority of cases. Since the S. suis bacteria are readily killed at 60°C for 10 min [28], well cooked pork should not pose a hazard. It is particularly important not to neglect cross-contamination between cooked and raw pork at home and in stalls selling pork.
ACKNOWLEDGEMENTS
We thank the health professionals in Hospital Authority for retrieving the case records and laboratory results; all staff in Public Health Laboratory Centre for doing the laboratory tests for S. suis; staff in the Agriculture, Fisheries and Conservation Department, and Food and Environmental Hygiene Department, HKSAR, for providing the relevant data; all medical and nursing staff in the Epidemiology Section, Surveillance and Epidemiology Branch, Centre for Health, HKSAR, for investigating the cases of this series.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Costa AT et al. Serotyping and evaluation of the virulence in mice of Streptococcus suis strains isolated from diseased pigs. Revista do Instituto de Medicina Tropical de São Paulo. 2005;47:113–115. doi: 10.1590/s0036-46652005000200012. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization 2005. http://www.wpro.who.int/media_centre/fact_sheets/fs_20050802.htm. http://www.wpro.who.int/media_centre/fact_sheets/fs_20050802.htm . Fact sheets on Streptococcus suis (2 August . ). Accessed 2 January 2006.
- 3.Clifton-Hadley FA et al. Further studies on the subclinical carrier state of Streptococcus suis type 2 in pigs. Veterinary Record. 1984;114:513–518. doi: 10.1136/vr.114.21.513. [DOI] [PubMed] [Google Scholar]
- 4.Perch B, Kristjansen P, Skadhange JN. Group R Streptococci pathogenic for man. Acta Pathologica et Microbiologica Scandinavica. Section B, Microbiology. 1968;74:69–76. [PubMed] [Google Scholar]
- 5.Walsh B, Williams AE, Satangi J. Streptococcus suis type 2: pathogenesis and clinical disease. Reviews in Medical Microbiology. 1992;3:65–71. [Google Scholar]
- 6.Dupas D, Vignon M, Geraut C. Streptococcus suis meningitis: a severe noncompensated occupational disease. Journal of Occupational Medicine. 1992;34:1102–1105. [PubMed] [Google Scholar]
- 7.Lütticken R et al. Meningitis caused by Steptococcus suis: case report and review of the literature. Infection. 1986;14:181–185. doi: 10.1007/BF01645260. [DOI] [PubMed] [Google Scholar]
- 8.Büngener W, Bialek R. Fatal Steptococcus suis septicaemia in an abattoir worker. European Journal of Clinical Microbiology & Infectious Diseases. 1989;8:306–308. doi: 10.1007/BF01963457. [DOI] [PubMed] [Google Scholar]
- 9.Köhler W et al. Steptococcus suis type 2 (R-Steptokokken) als Erreger von Berufskrankheiten: Bericht über eine Erkrankung und Literaturübersicht. Zeitschrift für die gesamte innere Medizin und ihre Grenzgebiete. 1989;44:144–148. [PubMed] [Google Scholar]
- 10.Berlit P, Heene DL. Meningitis durch Streptococcus suis: eine berufsbedingte Erkrankung bei Metzgern. Intensivbehandlung. 1989;14:101–103. [Google Scholar]
- 11.Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Reviews of Infectious Diseases. 1988;10:131–137. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- 12.Atterholm I et al. Streptococcus suis-meningit: en ny yrkessjukdom I Sverige? Läkartidningen. 1985;82:119. [PubMed] [Google Scholar]
- 13.Robertson D, Blackmore DK. Occupational exposure to Streptococcus suis type 2. Epidemiology and Infection. 1989;103:157–164. doi: 10.1017/s0950268800030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suankratay C et al. Streptococcus suis meningitis in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health. 2004;35:868–876. [PubMed] [Google Scholar]
- 15.Tambyah PA et al. Streptococcus suis infection complicated by purpura fulminans and rhabdomyolysis: case report and review. Clinical Infectious Diseases. 1997;24:710–712. doi: 10.1093/clind/24.4.710. [DOI] [PubMed] [Google Scholar]
- 16.Yen MY et al. Streptococcus suis meningitis complicated with permanent perceptive deafness: report of a case. Journal of the Formosan Medical Association. 1994;93:349–351. [PubMed] [Google Scholar]
- 17.Chau PY, Huang CY, Kay R. Streptococcus suis meningitis: an important underdiagnosed disease in Hong Kong. Medical Journal of Australia. 1983;1:414–417. [PubMed] [Google Scholar]
- 18.Khin Thi OO, Chan J. The epidemic of Group-R Streptococcal (Streptococcus suis) meningitis and septicaemia in Hong Kong. Journal of the Hong Kong Medical Association. 1985;37:134–136. [Google Scholar]
- 19.Kay R, Cheng AF, Tse CY. Streptococcus suis infection in Hong Kong. Quarterly Journal of Medicine. 1995;88:39–47. [PubMed] [Google Scholar]
- 20.World Health Organization . Outbreak associated with Streptococcus suis in pigs in China (http://www.who.int/csr/don/2005_08_03/en/index.html). Accessed 2 January 2006
- 21.Health and Medical Development Advisory Committee, HKSAR 2005. http://www.hwfb.gov.hk/hmdac/english/dis_papers/files/hmdac_paper.pdf. p. 16.http://www.hwfb.gov.hk/hmdac/english/dis_papers/files/hmdac_paper.pdf . Building a healthy tomorrow – discussion paper on the future service delivery (July . ), p. ). Accessed 2 January 2006.
- 22.Census and Statistics Department, HKSAR http://www.info.gov.hk/censtatd/eng/hkstat/index2.html. 2006. http://www.info.gov.hk/censtatd/eng/hkstat/index2.html . Population and vital events, Hong Kong in figures ( ). Accessed 5 January .
- 23.Health Protection Agency, United Kingdom Streptococcus suishttp://www.hpa.org.uk/infections/topics_az/zoonoses/strep_suis/menu.htm). Accessed 5 January 2006
- 24.Windsor ER, Elliot SD. Streptococcal infection in young pigs, an outbreak of streptococcal meningitis in weaned pigs. Journal of Hygiene. 1975;75:69–78. doi: 10.1017/s0022172400047070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clifton-Hadley FA, Enright MR, Alexander TJL. Survival of Streptococcus suis type 2 in pig carcasses. Veterinary Research. 1986;118:275. doi: 10.1136/vr.118.10.275. [DOI] [PubMed] [Google Scholar]
- 26.Marie J et al. Antimicrobial susceptibility of Streptococcus suis isolated from swine in France and from humans in different countries between 1996 and 2000. Journal of Antimicrobial Chemotherapy. 2002;50:201–209. doi: 10.1093/jac/dkf099. [DOI] [PubMed] [Google Scholar]
- 27.Staats JJ et al. Streptococcus suis: past and present. Veterinary Research Communications. 1997;21:381–407. doi: 10.1023/a:1005870317757. [DOI] [PubMed] [Google Scholar]
- 28.Clifton-Hadley FA, Enright MR. Factors affecting the survival of Streptococcus suis type 2. Veterinary Record. 1984;114:584–586. doi: 10.1136/vr.114.24.584. [DOI] [PubMed] [Google Scholar]