SUMMARY
The aim of the study was to estimate the prevalence and risk factors associated with infection by high-risk human papillomavirus (HR-HPV) in cervix and squamous intra-epithelial lesions (SIL) in imprisoned women. This was done by a cross-sectional study of imprisoned women attending the gynaecological clinic in Foncalent prison in Alicante, Spain. The study period was from May 2003 to December 2005. HR-HPV infection was determined through Digene HPV Test, Hybrid Capture II (HC-II). HPV typing was determined by multiplex nested PCR assay combining degenerate E6/E7 consensus primers. Multiple logistic regression modelling was used for the analysis of associations between variables where some were considered possible confounders after checking for interactions. A total of 219 women were studied. HR-HPV prevalence was 27·4% and prevalence of SIL was 13·3%. HIV prevalence was 18%, higher in Spaniards than in migrant women (24·6% vs. 14·3%, P<0·05). In multivariate analyses, risk factors for HPV infection were younger age (P for trend=0·001) and tobacco use (OR 2·62, 95% CI 1·01–6·73). HPV infection (OR 4·8, 95% CI 1·7–13·8) and HIV infection were associated with SIL (OR 4·8, 95% CI 1·6–14·1). The commonest HPV types were HPV16 (29·4%), HPV18 (17·6%), HPV39 (17·6%) and HPV68 (17·6%). The prevalence of both HR-HPV infection and SIL in imprisoned women found in this study is high. Determinants for each of the outcomes studied were different. HPV infection is the most important determinant for SIL. A strong effect of HIV co-infection on the prevalence of SIL has been detected. Our findings reinforce the need to support gynaecological clinics in the prison setting.
INTRODUCTION
Cervical screening programmes have proven to be an effective strategy for cervical cancer (CC) prevention. In Spain, where incidence and mortality of CC is low in the general population [1], there is little information about the risk for CC in vulnerable populations.
Imprisoned women have multiple risk factors for human papillomavirus (HPV) infection and progression to CC. Several publications from different countries have described a higher risk of CC in these women together with a lower coverage of cervical screening programmes [2, 3]. There are few studies, however, that have estimated the prevalence of HPV infection in this setting. Bickel et al. [4] described an HPV prevalence of 35% in 114 women from a prison in New York City in 1991 and Lopes et al. [5] a prevalence of 16% in 262 imprisoned women in Sao Paulo. In 1996, in Spain, de Sanjosé et al. reported an HPV prevalence of 46% among 157 women in Barcelona prison, of which 56% were HIV co-infected [6]. No further studies have been conducted since then. The objective of this work is to estimate the prevalence and risk factors associated with both the infection by high-risk HPV (HR-HPV) and squamous intra-epithelial lesions (SIL) in imprisoned women.
SUBJECTS AND METHODS
A cross-sectional study of imprisoned women attending the gynaecological clinic in Foncalent prison, Alicante, Spain, was done. The study period was from May 2003 to December 2005.
The gynaecological clinic in Foncalent prison of was created in 1985 to offer voluntary check-ups that include breast examination and echography, cervical smear, full blood counts and other blood tests if required, as well as appropriate referral services. The same female gynaecologist has run the clinic over all these years. All the women who voluntarily attended the gynaecological clinic during the study period were informed and invited to participate in the study. None of the women refused.
A structured questionnaire designed especially for this study was administered by the gynaecologist. The questionnaire included information on sociodemographic characteristics (age, educational level, marital status, occupation, country of birth), sexual practices (age of first sexual intercourse, number of lifetime sexual partners), reproductive health (number and dates of pregnancies, children, TOPs (voluntary termination of pregnancy), use of contraceptives methods), general health (smoking habits, weight and height, history of major illness), gynaecological history (previous cervical smears and results, past history of STI). Smokers were classified according to having ever smoked or not and whether they had smoked in the last year. HIV, hepatitis B virus (HBV), hepatitis C virus (HCV) and syphilis serology were also recorded.
The outcome variables were HR-HPV infection and SIL. All women had a cervical specimen taken for Papanicolaou cytology and HPV testing. Smears were read blinded to all study variables in the reference Pathology service.
Laboratory methods
HR-HPV infection was determined through the HPV DNA test Hybrid Capture II (HC-II; Digene Corporation, Gaithersburg, MD, USA) using the oncogenic or probe B cocktail (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), in accordance with the manufacturer's instructions. The samples with a relative light unit/cut-off ratio >1·0 were considered positive for one or more HPV genotypes included in the probe B cocktail and the specimens with a relative light unit/cut-off ratio of between 1·0 and 2·5 were retested to confirm positive results. Sensitivity limit to this assay is 100 000 copies/ml of sample. In all HC-II-positive samples, HPV typing was determined by a multiplex nested PCR assay that combines degenerate E6/E7 consensus primers and type-specific primers. This PCR system identifies all HR-HPV types included in the high-risk probe of HC-II test (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68), and the additional HPV types 66, −6, −11, −42, 43 and −44. The DNA was extracted from cells recovered from a 200 μ aliquot of the original samples, using an automatic BioRobot M48 (Qiagen, Inc., Valencia, CA, USA). The quality of each DNA sample was verified with the amplification of a fragment of β-globin gene using the PC04/GH20 primer set. HPV type assignation was done by the analyses of PCR products' electrophoresis mobility in agarose gels [7].
Cervical smears
Cervical smears were performed with an Ayre's wooden spatula and an endocervical brush. Samples were fixed with Labofix and classified according to Bethesda 2001.
Statistical methods
Univariate analysis of demographic and clinical differences were analysed using the two-sample t test for normally distributed continuous data, the Mann–Whitney test for non-parametric data, the χ2 test for frequencies and Fisher's test and the χ2 test for trend. Multiple logistic regression was used to study the relationships between HPV infection (HPV-positive vs. HPV-negative) and SIL (low- and high-grade SIL vs. normal smear) and the explanatory variables looking for confounding and effect modification. Model building was done using forward procedures. Only variables that retained statistically significant associations with the outcome variables were left in multivariate analyses. Data analysis was performed on microcomputer using stata version 8 software (Stata Corp, College Station, TX, USA).
RESULTS
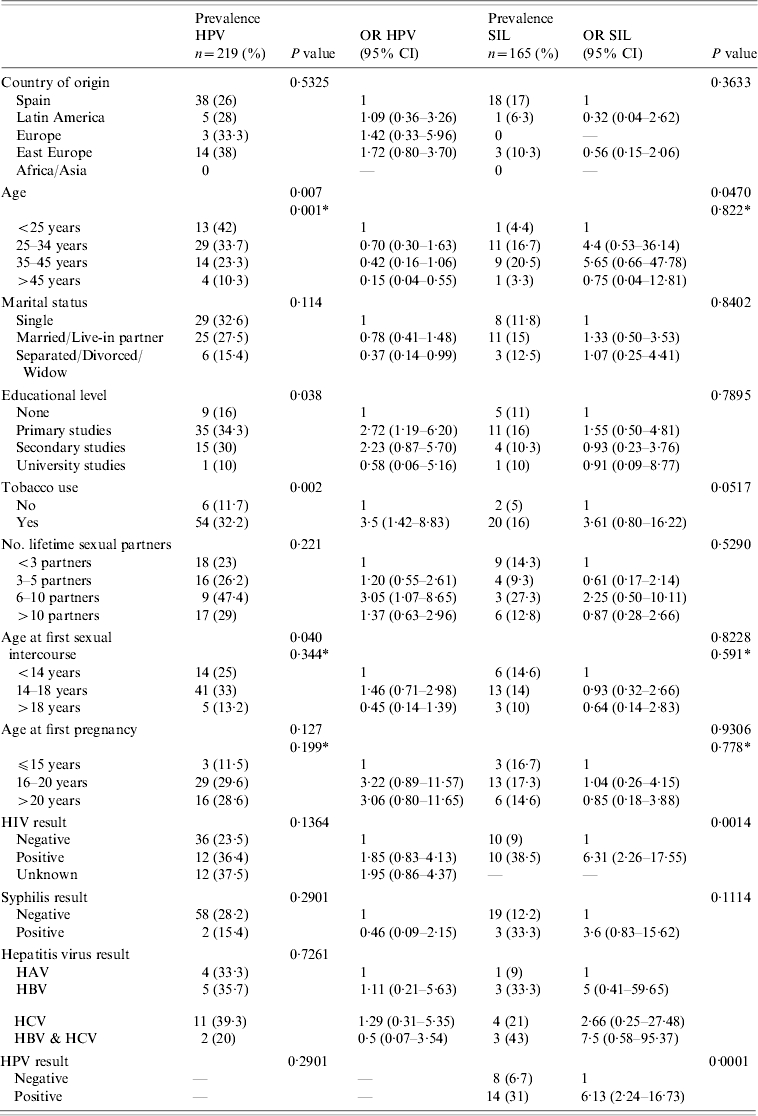
In total, 219 women were included in the study. Their sociodemographic characteristics are presented in Table 1. Overall, 67% were Spanish, median age was 34 years (range 20–66) and 26% had no formal education. Over three quarters (77%) of the women had ever smoked. Median age at first sexual intercourse was 18 years (range 11–28) and 46% had had at least one TOP. HIV prevalence was 17·7% (95% CI 12·53–24) and was higher in Spaniards (24·6%, 95% CI 17·48–32·93) than in women from other countries (14·3%, 95% CI 0·36–57·87).
Table 1.
Sociodemographic characteristics of imprisoned women in Foncalent prison, Alicante

TOP, Termination of pregnancy; HIV, human immunodeficiency virus; HCV, hepatitis C virus.
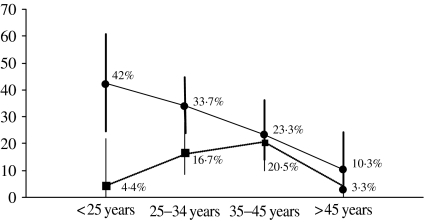
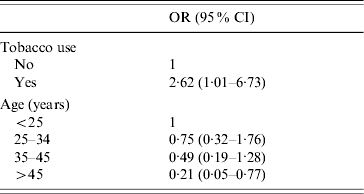
HR-HPV prevalence estimated by HPV DNA test HC-II was 27·4% (95% CI 21·6–33·8) and as seen in the Figure, a descending trend is observed with increasing age (P for trend 0·001). Age, educational level, smoking habit and age at first sexual intercourse yielded statistically significant associations with HPV infection in univariate analyses (Table 2). In multivariate analyses, only age and having smoked remained statistically associated with HPV infection (Table 3).
Fig.
HPV prevalence (●) and IL prevalence (■) by age group.
Table 2.
Prevalence and univariate odds ratios (OR) for HPV infection and squamous intraepithelial lesion (SIL) in imprisoned women in Foncalent prison, Alicante
HAV, Hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus.
P for trend.
Table 3.
Multivariate analyse of variables associated with HPV infection in women of Foncalent prison, Alicante
All the women (n=219) had a cervical smear done of which 8·2% had low-grade SIL and 2% high-grade SIL (Table 4). HPV prevalence was higher in women with SIL (Table 4). Prevalence of SIL was 10% (22/219) (95% CI 6·40–14·81). For the univariate and multivariate analyses, we only used data from 165 women (75·3%); we excluded women with ASCUS (atypical squamous cells of undetermined significance) or invalid results.
Table 4.
Prevalence of cytological abnormalities according to the Bethesda classification and HPV prevalence for each lesion
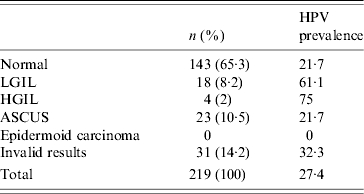
LGIL, Low-grade intraepithelial lesion; HGIL, high-grade intraepithelial lesion; ASCUS, atypical squamous cells of undetermined significance.
In univariate analyses, the risk of having a SIL was higher in women aged 25–45 years compared to <25 years, women who had ever smoked, those who tested HIV-positive and in women infected by HR-HPV (OR 6·32, 95% CI 2·43–16·43) (Table 2). The age pattern, as can be seen in the Figure, was very different from the HPV infection descending trend, and a peak of prevalence was observed in women aged 35–45 years. In multivariate analyses, HPV infection (OR 4·8, 95% CI 1·7–13·8) and HIV co-infection were associated with SIL (OR 4·8, 95% CI 1·6–14·1).
The commonest HPV types in the 34 samples which were typed were HPV16 (29·4%), HPV18 (17·6%), HPV39 (17·6%), HPV68 (17·6%) followed by HPV31 (8·8%), HPV59 (8·8%), HPV35 (8·8%), HPV45 (8·8%) and HPV52 (8·8%).
DISCUSSION
The prevalence of both HR-HPV infection (HPV DNA test HC-II), and SIL in imprisoned women found in this study is high, 27% and 10% respectively. However, determinants for each of the outcomes studied were different. Risk factors for HPV infection were age and tobacco use. HPV infection, together with HIV infection were the most important determinants for SIL.
The prevalence of HPV infection found in these women is high but similar to that found in the prison setting and other groups of socially excluded women [6, 8–10]. In the same Spanish city, HPV prevalence in female sex workers has been reported to be 31% [9] while prevalence in women from the general population has been estimated to be 10% [11]. In Barcelona, also in a prison setting, de Sanjosé et al. published a HPV prevalence of 46% in a sample of 157 women of which 56% were HIV-positive. HIV–infected women had a fivefold increase in the risk of being HPV infected as did those women who had used drugs for more than 10 years. The commonest HPV type detected in these women was HPV16, followed by HPV18, −39 and −61.
Age was a strong determinant of HPV infection, a finding that has been well documented in groups of women with high HPV prevalence rates. The decreasing trend with age has been associated with acquired immunity to HPV [10–12]. We identified smoking history as a risk factor for HPV infection in both univariate and multivariate analyses. As for SIL, having smoked was a risk factor in univariate analyses and, although the risk was doubled in multivariate analyses, differences were no longer statistically significant (data not shown). Smoking HPV infection associations are inconclusive [15, 16]. Smoking has been reported as a risk factor for HPV persistence and progression to disease but other studies have shown no association [12, 13]. As well as the biological effect of smoking on cervical cells, smoking history may also identify a group of women with poorer health habits and higher frequency of sexual risk exposures.
Infection by HIV was associated with non-statistically significant higher prevalence of HPV infection which disappeared in multivariate analyses. In the previously mentioned study of imprisoned women in Barcelona, HIV-infected women had a fivefold increase in the prevalence of HPV infection compared to HIV-negative women [6]. We have, however, detected a strong effect of HIV on the prevalence of SIL. HIV prevalence in the women in this study is very high (18%), and was higher in Spaniards compared to migrant women. HIV prevalence in Spanish prisons is very high, and has been associated with injecting drug use, while in a recently published study AIDS was the commonest cause of death among prison inmates in Spain from 1994 to 2005 [14]. Little is known about the determinants for HPV infection but immune response seems to be crucial [12, 15–20]. In this respect, infection by HIV has been reported to be associated with faster clinical progression rates, especially in women with a low CD4 count [21, 22].
This study contributes to the body of knowledge about HPV infection in socially excluded women. Moreover, very few studies have been done on imprisoned women, a group with important health needs. Additional diagnostic tests, such as the use of HPV detection, have allowed the identification of high prevalences of HR-HPV infection which would have otherwise been undetected. The women who participated in this study were in prison for minor theft and their time in prison was short. In this sense, it has been well described how the prison health services may act as one of the few contacts with the health system that is available to socially vulnerable groups. Our findings reinforce the need to support gynaecological clinics in the prison setting and support the use of molecular diagnostic methods for HR-HPV infection.
CONCLUSIONS
The prevalence of both HPV infection and SIL found in imprisoned women in Alicante in this study is high, 27% and 13% respectively.
Determinants for each of the outcomes studied were different. Risk factors for HPV infection were age and tobacco use. HPV infection and HIV infection were the most important determinants for SIL.
HIV prevalence in this study is very high, 18%, and was higher in Spaniards compared to migrant women. HIV prevalence in Spanish prisons is very high, and has been associated with injecting drug use.
ACKNOWLEDGEMENTS
This study has been supported financially with grants RCESP C03/09 and Red de SIDA (RD06/006), MPI 1117/03.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Globocan 2002 www.dep.iarc.fr/dataava/infodata.htm. 2006. www.dep.iarc.fr/dataava/infodata.htm . Cancer incidence, mortality and prevalence worldwide ( ). Accessed April .
- 2.Smith PF et al. HIV infection among women entering the New York State correctional system. American Journal of Public Health. 1991;81:35–40. doi: 10.2105/ajph.81.suppl.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso-Sanz M et al. Microbiological study of some microorganisms implicated in sexually transmitted diseases among the female prison population. Enfermedades Infecciosas y Microbiologia Clinica. 1996;14:474–478. [PubMed] [Google Scholar]
- 4.Bickell NA et al. Human papillomavirus, gonorrhea, syphilis, and cervical dysplasia in jailed women. American Journal of Public Health. 1991;81:1318–1320. doi: 10.2105/ajph.81.10.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopes F et al. HIV, HPV, and syphilis prevalence in a women's penitentiary in the city of Sao Paulo, 1997–1998. Cadernos de Saúde Pública. 2001;17:1473–1480. doi: 10.1590/s0102-311x2001000600018. [DOI] [PubMed] [Google Scholar]
- 6.De Sanjosé S et al. Human papillomavirus and human immunodeficiency virus infections as risk factors for cervix cancer in women prisoners. Medicina Clinica (Barcelona) 2000;115:81–84. doi: 10.1016/s0025-7753(00)71472-2. [DOI] [PubMed] [Google Scholar]
- 7.Sotlar K et al. Detection and typing of human papillomavirus by E6 nested multiplex PCR. Journal of Clinical Microbiology. 2004;42:3176–3184. doi: 10.1128/JCM.42.7.3176-3184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassen E et al. Cervical human papillomavirus infection in Tunisian women. Infection. 2003;31:143–148. doi: 10.1007/s15010-003-3112-7. [DOI] [PubMed] [Google Scholar]
- 9.Del Amo J Prevalence and determinants of high risk human papillomavirus in migrant and Spanish female sex workers. XXIV Scientific Meeting of the Spanish Society of Epidemiology; Logroño, Spain: 2006. , 3–6 October . . Abstract No. 147. [Google Scholar]
- 10.Del Amo J et al. Influence of age and geographical origin in the prevalence of high risk human papillomavirus in migrant female sex workers in Spain. Sexually Transmitted Infections. 2005;81:79–84. doi: 10.1136/sti.2003.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.González C et al. Higher prevalence of human papillomavirus infection in migrant women from Latin America in Spain. Sexually Transmitted Infections. 2006;82:260–262. doi: 10.1136/sti.2005.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho GY et al. Risk of genital human papillomavirus infection in women with human immunodeficiency virus-induced immunosuppression. International Journal of Cancer. 1994;56:788–792. doi: 10.1002/ijc.2910560605. [DOI] [PubMed] [Google Scholar]
- 13.Sellors JW et al. Incidence, clearance and predictors of human papillomavirus infection in women. Canadian Medical Association Journal. 2002;167:871–873. [PMC free article] [PubMed] [Google Scholar]
- 14.Remartinez EJ et al. Trends in mortality in a Spanish prison from 1994–2004. Revista Española de Salud Pública. 2005;79:673–682. [PubMed] [Google Scholar]
- 15.Palefsky JM et al. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. Journal of the National Cancer Institute. 1999;91:226–236. doi: 10.1093/jnci/91.3.226. [DOI] [PubMed] [Google Scholar]
- 16.Minkoff H et al. A longitudinal study of human papillomavirus carriage in human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. American Journal of Obstetrics and Gynecology. 1998;178:982–986. doi: 10.1016/s0002-9378(98)70535-6. [DOI] [PubMed] [Google Scholar]
- 17.Ahdieh L et al. Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus seropositive and seronegative women. American Journal of Epidemiology. 2000;151:1148–1157. doi: 10.1093/oxfordjournals.aje.a010165. [DOI] [PubMed] [Google Scholar]
- 18.Cu-Uvin S et al. Prevalence of lower genital tract infections among human immunodeficiency virus (HIV)-seropositive and high-risk HIV-seronegative women. HIV Epidemiology Research Study Group. Clinical Infectious Diseases. 1999;29:1145–1150. doi: 10.1086/313434. [DOI] [PubMed] [Google Scholar]
- 19.Sun XW et al. Human papillomavirus infection in women infected with the human immunodeficiency virus. New England Journal of Medicine. 1997;337:1343–1349. doi: 10.1056/NEJM199711063371903. [DOI] [PubMed] [Google Scholar]
- 20.Seck AC et al. Cervical intraepithelial neoplasia and human papillomavirus infection among Senegalese women seropositive for HIV-1 or HIV-2 or seronegative for HIV. International Journal of STD & AIDS. 1994;5:189–193. doi: 10.1177/095646249400500307. [DOI] [PubMed] [Google Scholar]
- 21.Fruchter R et al. Multiple recurrentes of cervical intravenous intraepithelial neoplasia in women with the HIV. American Journal of Obstetrics and Gynecology. 1996;87:338–344. doi: 10.1016/0029-7844(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 22.Six C et al. Comparative prevalence, incidence, and short-term prognosis of cervical squamous intraepithelial lesion amongst HIV-infected and HIV-uninfected women. AIDS. 1998;12:1047–1056. [PubMed] [Google Scholar]