SUMMARY
It is more than 25 years since the first case of AIDS was reported in the United Kingdom. In December 1981 a gay man was referred to a London hospital with opportunistic infections indicative of immunosuppression. National surveillance began the following year, in September 1982, with the notification of deaths and clinical reports of AIDS and Kaposi's sarcoma plus laboratory reports of opportunistic infections. Since then epidemiological surveillance systems have evolved, adapting to, and taking advantage of advances in treatments and laboratory techniques. The introduction of the HIV antibody test in 1984 led to the reporting of HIV-positive tests by laboratories and the establishment of an unlinked anonymous survey in 1990 measuring undiagnosed HIV infection among gay men attending sexual health clinics. The widespread use of highly active antiretroviral therapies (HAART) since 1996 has averted many deaths among HIV-positive gay men and has also resulted in a large reduction in AIDS cases. This led to a need for an enumeration of gay men with HIV accessing NHS treatment and care services (1995 onwards), more clinical information on HIV diagnoses for epidemiological surveillance (2000 onwards) and the routine monitoring of drug resistance (2001 onwards). Twenty-five years after the first case of AIDS was reported, gay and bisexual men remain the group at greatest risk of acquiring HIV in the United Kingdom. Latest estimates suggest that in 2004, 26 500 gay and bisexual men were living with HIV in the United Kingdom, a quarter of whom were undiagnosed. In this review, we examine how national surveillance systems have evolved over the past 25 years in response to the changing epidemiology of HIV/AIDS among gay and bisexual men in the United Kingdom as well as advances in laboratory techniques and medical treatments. We also reflect on how they will need to continue evolving to effectively inform health policy in the future.
INTRODUCTION
Twenty-five years have elapsed since AIDS was first reported in the United Kingdom. During that time 30 000 gay and bisexual men (referred to here as ‘gay men’) have been diagnosed with HIV, of whom 12 000 have progressed to AIDS and 10 000 have died [1]. Gay men remain the behavioural group at greatest risk of HIV in the United Kingdom, accounting for three-quarters of HIV infections diagnosed in 2004 that were probably acquired in the United Kingdom [2]. Cumulative figures mask temporal changes, whereby health promotion initiatives and the introduction of highly active antiretroviral therapies (HAART) have profoundly changed the epidemiology of HIV at different times. Nonetheless, HIV transmission continues among gay men in the United Kingdom, coupled in recent years with increases in gonorrhoea and syphilis and an outbreak of lymphogranuloma venereum (LGV) [2]. Behavioural surveillance also indicates increases in ‘high risk’ sexual behaviours [3–6].
Surveillance is the systematic collection, collation and analysis of data and its timely dissemination so that important trends and events may be detected, and necessary action can be taken to promote and protect public health [7]. The United Kingdom's HIV surveillance systems are some of the most comprehensive in the world, providing a wealth of national epidemiological data (Figs 1 and 2). In this review, we examine how these surveillance systems have evolved over time in response to the changing epidemiological patterns of HIV among gay men in the United Kingdom, and consider how they may need to adapt in the future.
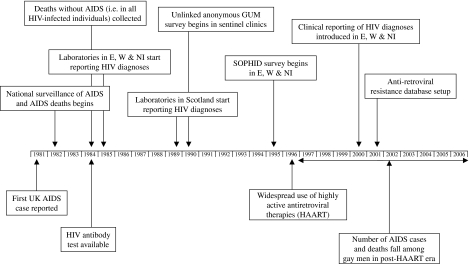
Fig. 1.
Timeline of HIV/AIDS surveillance in the United Kingdom. SOPHID, Survey of Prevalent HIV Infections Diagnosed.
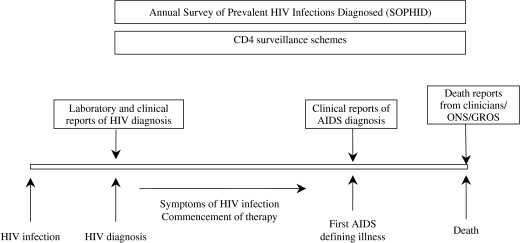
Fig. 2.
How HIV/AIDS surveillance works at an individual level in the United Kingdom. ONS, Office for National Statistics; GROS, General Register Office for Scotland.
Search strategy and selection criteria
Sources for this review were identified by searches of Medline and references from relevant articles; numerous articles were identified through searches of the authors' files. Search terms were ‘HIV infection’, ‘AIDS’, ‘homosexual’, ‘bisexual’, ‘gay’, ‘United Kingdom’, ‘surveillance’, ‘epidemiology’. English-language papers and reports were reviewed.
Early experiences: 1981–1983
The early picture of AIDS in the United Kingdom was a reflection of experience in the United States where, during 1981, Pneumocystis pneumonia (PCP) and Kaposi's sarcoma (KS) were reported among gay men in metropolitan areas [8, 9]. Described in December 1981, the first case of AIDS in the United Kingdom was in a 49-year-old gay man who regularly visited Florida and was referred with PCP and cytomegalovirus (CMV) infection to a London hospital [10].
In response to growing alarm and uncertainty surrounding the new syndrome, the Communicable Disease Surveillance Centre (CDSC) in collaboration with the Scottish Centre for Infection and Environmental Health (SCIEH) introduced national surveillance in September 1982 [11, 12]. Initially, surveillance consisted of death registrations mentioning PCP, KS and AIDS (provided weekly by the Office of Population Censuses and Surveys, now the Office for National Statistics, and the Registrar General's Office in Scotland), laboratory reports of opportunistic infections (with sexual orientation where possible) and clinical reports of PCP, KS and AIDS [11, 13, 14]. The American AIDS case definition was adopted at the outset, but by the early 1990s European and American case definitions had diverged, with Europe defining AIDS as having one of 28 illnesses and the United States, as having a CD4 cell count of <200 cells/mm3 or one of these 28 illnesses [15–17].
HIV antibody test: 1984 onwards
With the discovery of HIV as the causative agent for AIDS and the development of the HIV antibody test in 1984, surveillance was enhanced by asking microbiologists to report HIV-positive test results from 1985 [18, 19]. Prevalence studies using the HIV antibody test during 1984 revealed the extent of HIV infection among British gay men [20–24], and confirmed that AIDS cases were just the ‘tip of the iceberg’. Retrospective testing of stored residual samples, taken for routine hepatitis B infection tests, established that HIV had been present among gay men in the United Kingdom since 1980 and that prevalence was increasing [21]. By 1984–1985, prevalence among gay men attending some genito-urinary medicine (GUM) clinics was 35% in London and 11% outside [22, 23]. While the rise in HIV prevalence among GUM clinic attendees in London during the early 1980s was comparable to that observed in San Francisco, subsequent behavioural change (principally a reduction in partner numbers) at an earlier epidemic stage in the United Kingdom probably prevented the continued rapid rise in prevalence that was seen in the United States [25–28].
Unlinked anonymous serosurveys: 1990 onwards
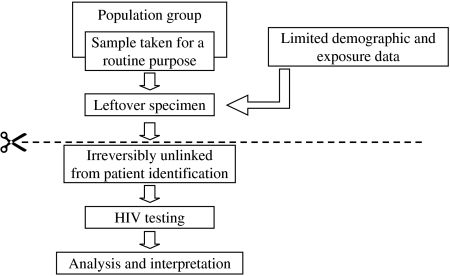
The above ad-hoc studies demonstrated the importance of measuring HIV prevalence, particularly the level of undiagnosed infections, and by the end of the 1980s there were calls for an ‘unlinked anonymous’ serosurveillance programme to accurately and routinely monitor prevalence within defined populations [29]. There would be no reliance on gay men seeking a named HIV test, therefore reducing participation bias. Such surveillance would improve the accuracy of future predictions about the size of the epidemic and the targeting of prevention campaigns. A range of unlinked serosurveys was proposed, including a GUM clinic survey incorporating ‘high-risk’ gay men where residual samples taken for routine syphilis serology would be irreversibly unlinked from any patient identifiable information and then tested for HIV (Fig. 3) [29]. Information on whether the person had been previously diagnosed would be retained, allowing a measure of undiagnosed HIV prevalence. There were lengthy debates about the individual's rights vs. the public health benefit, but by November 1988 the British government stated that it saw no legal or ethical objection to the surveys and implementation began early in 1990 [30].
Fig. 3.
Unlinked anonymous programme methodology. The unlinked anonymous programme measures HIV prevalence among defined populations in the United Kingdom. Gay men attending genito-urinary medicine (GUM) clinics having routine syphilis tests are one of these populations. After testing there is leftover specimen, which has limited demographic and exposure data attached to it, as well as sexual orientation and whether known to be HIV infected. Before testing the leftover specimen for HIV, all patient identifiable information (e.g. name, DOB) are irreversibly removed making it impossible to identify from whom the specimen came. The sample is then tested and results from all samples are analysed and interpreted at a population level.
The unlinked anonymous GUM survey revealed no significant changes in the seroprevalence of undiagnosed HIV infection among gay men attending GUM clinics with an acute STI between 1993 and 1998, indicating a high level of continuing transmission [1, 31]. This burden of undiagnosed HIV-1 infection in 1996 highlighted the need to extend the practice of routine voluntary, named testing for HIV in GUM clinics [32].
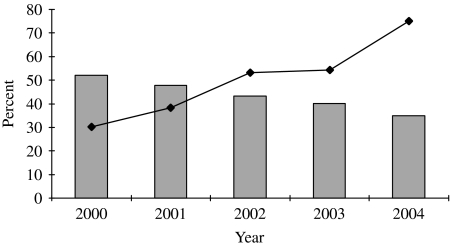
Promotion of HIV testing among gay men in GUM clinics was emphasized in both the 2001 English Department of Health's National Strategy for Sexual Health and HIV and the Chief Medical Officer's report, as well as in Making it Count, the 1998 health promotion strategy aimed at reducing HIV incidence among gay men in England [33–36]. The Chief Medical Officer's report recommended that all GUM attendees should be offered a HIV test on their first attendance, and that gay men should be offered HIV testing annually in all health-care settings [34]. Data from the unlinked anonymous GUM survey show that the uptake of HIV testing among gay men attending sentinel GUM clinics increased from 47% (3490/7378) in 1998 to 79% (6865/8774) in 2004 (Fig. 4) [2, 37]. Increased testing has made a considerable contribution to the rise in the number of HIV diagnoses among gay men in the United Kingdom since 1997, but there has also been continued transmission of HIV [38, 39].
Fig. 4.
Percentage of gay men attending 28 sentinel GUM clinics accepting a voluntary confidential HIV test (VCT) and percentage of HIV-infected gay men remaining undiagnosed after clinic visit, United Kingdom: 2000–2004.  , Percent of HIV-infected persons remaining undiagnosed after a clinic visit; –◆–, percent of all attendees that accept a VCT. (Source: Unlinked anonymous serosurveys. From: The UK Collaborative Group for HIV and STI Surveillance. Mapping the Issues. HIV and other Sexually Transmitted Infections in the United Kingdom, 2005. London: Health Protection Agency Centre for Infections. November 2005.)
, Percent of HIV-infected persons remaining undiagnosed after a clinic visit; –◆–, percent of all attendees that accept a VCT. (Source: Unlinked anonymous serosurveys. From: The UK Collaborative Group for HIV and STI Surveillance. Mapping the Issues. HIV and other Sexually Transmitted Infections in the United Kingdom, 2005. London: Health Protection Agency Centre for Infections. November 2005.)
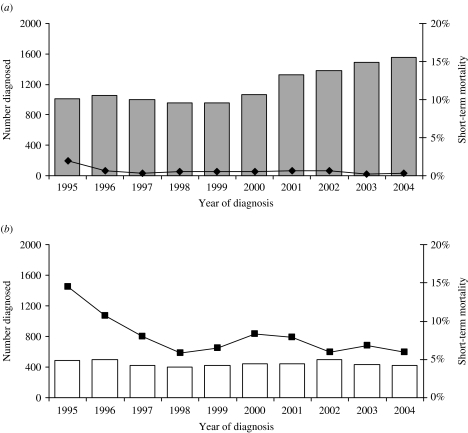
Despite the substantial increase in the uptake of HIV testing among gay men attending GUM clinics, one in ten of gay men continue to be diagnosed with HIV with a CD4 cell count <200 cells/mm3 (Fig. 5) [40, 41]. In addition, in 2004, more than 40% of gay men with undiagnosed HIV left sentinel GUM clinics remaining unaware of their HIV infection [2]. Late diagnosis of infection may lead to unnecessary morbidity and an increase in mortality within a short period of diagnosis [2]. One suggestion to reduce the number of men being diagnosed late during the course of infection has been the introduction of rapid HIV antibody testing in community settings. However, pilot schemes have reached different conclusions as to whether these services would be acceptable to users [42, 43]. Research also suggests that people are not diagnosed any earlier than in a standard GUM clinic [42]. Understanding why some men continue to present at a very late stage of infection and barriers to HIV testing, perhaps through qualitative research, will be important in reducing morbidity and mortality among HIV-infected gay men in the future. Surveillance will need to be able to monitor the uptake of testing among gay men in non-GUM settings if rapid testing becomes more widespread.
Fig. 5.
Pattern of diagnosis* and short-term mortality† among HIV-infected gay men, England, Wales and Scotland: 1995–2004. (a) Estimated number of gay men diagnosed ‘early’ ( ); short-term mortality of gay men diagnosed ‘early’ (–◆–). (b) Estimated number of gay men diagnosed late (–□–); short-term mortality of gay men diagnosed late (–■–). (* Late diagnosis: CD4 count <200 cells/mm3 within 30 days of diagnosis; earlier diagnosis ⩾200 cells/mm3. † Percentage of gay men known to have died within a year of diagnosis.) (Source: CD4 Surveillance Scheme. From: The UK Collaborative Group for HIV and STI Surveillance. Mapping the Issues. HIV and other Sexually Transmitted Infections in the United Kingdom, 2005. London: Health Protection Agency Centre for Infections. November 2005.)
); short-term mortality of gay men diagnosed ‘early’ (–◆–). (b) Estimated number of gay men diagnosed late (–□–); short-term mortality of gay men diagnosed late (–■–). (* Late diagnosis: CD4 count <200 cells/mm3 within 30 days of diagnosis; earlier diagnosis ⩾200 cells/mm3. † Percentage of gay men known to have died within a year of diagnosis.) (Source: CD4 Surveillance Scheme. From: The UK Collaborative Group for HIV and STI Surveillance. Mapping the Issues. HIV and other Sexually Transmitted Infections in the United Kingdom, 2005. London: Health Protection Agency Centre for Infections. November 2005.)
Estimates and projections: 1987 onwards
From the outset, it was clear that AIDS would have a major impact on health-care services. Surveillance data were used to predict the future burden [12, 25, 44]. Fortunately, early predictions were never realized. A Department of Health working group estimated that between 20 000 and 50 000 people were HIV-infected at the end of 1987, and that 16 000–40 000 people would develop AIDS over the next 10–15 years [25]. At the time it was still unclear as to whether all those infected with HIV would develop AIDS. A report published a year later revised the original estimates downwards predicting that 8750–17 500 gay men had been infected with HIV in England and Wales by the end of 1988 [44]. Some commentators argued that these were underestimates, with more than 60 000 people, possibly 100 000, having been infected [45].
Estimates of the number of people living with HIV in the United Kingdom have become more sophisticated over time. From 1995 onwards, the ‘direct method’ was used to calculate the number of people living with HIV in the United Kingdom by categorizing the population into a set of mutually exclusive risk groups of known size, and applying estimates of risk group-specific HIV prevalence to each group [46, 47]. In 2000, this method estimated that there were 17 000 HIV-infected gay men living in the United Kingdom of whom 19% were undiagnosed [48]. In 2005, however, a new method, multi-parameter evidence synthesis (MPES) was introduced [49]. Using MPES, an estimated 26 500 HIV-infected gay men were living in the United Kingdom during 2004, of whom 34% were undiagnosed [2]. The distinctive feature of the MPES method is the simultaneous incorporation of multiple sources of information on model parameters and functions [49]. However, the proportion of undiagnosed gay men seems to be high compared to earlier estimates using the direct method especially since the uptake of HIV testing has increased substantially over time without a dramatic rise in HIV incidence [2]. On the other hand, earlier reports of undiagnosed infections may have been substantial underestimates. The method continues to be refined.
Changing patterns and reporting of AIDS: 1996 onwards
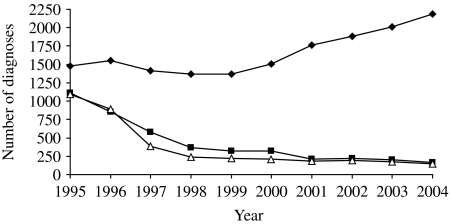
The use of antiretroviral therapy in the United Kingdom began in 1988 with clinical trials using zidovudine (AZT) as monotherapy and was superseded by dual therapy in the early 1990s [50]. However, it was only after the introduction and widespread use of HAART from 1996 onwards that the impact of treatment was seen at a population level. As in many other industrialized countries, HAART led to a large reduction in AIDS cases and deaths in HIV-infected gay men in the United Kingdom and a changing spectrum of AIDS-defining illnesses and causes of death, notably a decline in diseases caused by opportunistic infections (e.g. PCP and KS) and an increase in diseases such as non-Hodgkin's lymphoma (NHL) (Fig. 6) [2, 51–55].
Fig. 6.
HIV and AIDS diagnoses and deaths in HIV-infected gay men, United Kingdom: 1995–2004. –◆–, HIV diagnoses; –■–, AIDS diagnoses; –△–, deaths. (Source: Reports of HIV/AIDS and deaths in HIV-infected individuals. From: The UK Collaborative Group for HIV and STI Surveillance. Mapping the Issues. HIV and other Sexually Transmitted Infections in the United Kingdom: 2005. London: Health Protection Agency Centre for Infections. November 2005.)
In the context of HAART, AIDS was no longer an unbiased marker of irreversible end-stage disease progression, with fewer AIDS cases and even fewer AIDS reports with complete epidemiological information. To compensate for this information loss, clinicians in England, Wales and Northern Ireland were asked to report new HIV diagnoses as well as initial AIDS- defining illnesses from 2000, to supplement laboratory reporting [56]. The extra information collected on these reports has been used to examine the epidemiology of HIV among gay men in the United Kingdom in greater depth, including trends in ethnicity, migration and possible transmission via oral sex [57–59].
The introduction of the clinical HIV report and the widespread use of HAART had an unexpected effect on national surveillance, however, with a decline in the reporting of initial AIDS-defining illnesses. While simultaneous HIV and AIDS diagnoses are still reported, there has been a reduction in reports of AIDS occurring after HIV diagnosis. It is unclear whether this is because of ambiguity about reporting changes, or if reporters no longer consider an initial AIDS diagnosis after HIV diagnosis as clinically relevant in the HAART era. Clearly this change has implications for the interpretation of trends in AIDS diagnoses and inter-country comparisons, particularly as some countries (e.g. Spain) only collect information on AIDS at a national level for the present [60]. Work is ongoing to assess the extent of underreporting; to understand the need for AIDS reporting, and the meaning of AIDS in the post-HAART era; and ultimately to make recommendations about the future of AIDS reporting in the United Kingdom.
Mortality over 25 years – the impact of HAART
Information on deaths in patients with AIDS has been collected since the start of the epidemic. However, once the HIV antibody test became widely available in 1984, it became clear that deaths were occurring in HIV-infected individuals without AIDS [61, 62]. The AIDS report form was modified to include deaths in HIV-infected individuals who had not progressed to AIDS [61], and when electronic mortality records were made available from 1993 onwards, all deaths in those aged <60 years were routinely ‘matched’ to HIV/AIDS reports to capture ‘non-AIDS’ deaths and those where ‘HIV/AIDS’ was not stated on the death certificate perhaps because of the stigma attached to the infection and/or sexual risk behaviours [63]. With an ageing cohort of HIV-infected gay men – in 2004, 28% (5087) of gay men accessing HIV-related services were aged ⩾45 years [2] – the age cut-off of 60 years requires upward revision to ensure completeness of mortality data.
The impact of HIV on premature mortality among younger men and the impact of HAART has been clearly demonstrated using surveillance data, with the crude age-specific mortality rate for HIV rising from 0·9/100 000 men in 1985 to a peak of 10·3/100 000 in 1994 (accounting for 9·3% of deaths in men aged 15–44 years) [64]. In 1997, the ‘all cause’ mortality rate for MSM was 4·1/100 MSM, falling sharply to 1·0/100 MSM in 2003 [65]. While non-specific ‘pneumonia’ has been the most common cause of death in HIV-infected gay men in the pre- (35%) and post- (19%) HAART eras, of those who died during 2002–2004, the principal cause of death was cardiovascular disease in 12%, NHL in 10% and PCP in 9% [66]. There is little evidence as yet, of a significant increase in deaths from untreatable, multidrug-resistant HIV infections in the cohort of gay men who have been on HAART since the mid-1990s [67]. However, existing data collection may not be sensitive enough to identify these deaths at a national level as information on antiretroviral therapies or drug resistance at death is not routinely collected. Improvements in the collection of this information may therefore be beneficial in monitoring future mortality trends and clinicians should remain vigilant.
Gay men living with diagnosed HIV infection: 1996 onwards
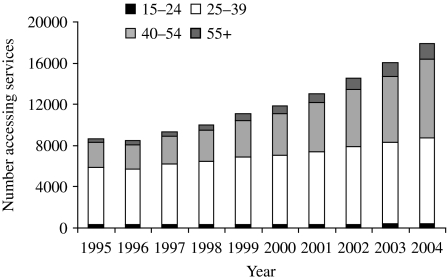
By the mid-1990s surveillance of HIV/AIDS in the United Kingdom was evolving to encompass improvements in treatment and care and to meet the growing data needs of HIV service providers and financiers. An annual Survey of Prevalent HIV Infections Diagnosed (SOPHID) was introduced in England, Wales and Northern Ireland in 1995 to monitor the number of people accessing treatment and care through the (national) health service [68]. In Scotland, equivalent information is collected through a surveillance system involving the collection of CD4 count data [69]. Improvements in survival and continued diagnoses among gay men have led to increasing numbers living with diagnosed HIV; 17 932 gay men were accessing services in 2004 compared with 11 846 in 2000 (Fig. 7) [2]. Surveillance data are used to allocate funding for HIV care, and at an estimated average cost of £15 000 per annum for each HIV-diagnosed person accessing HIV services, the implications for funding are evident as well as the advantage of accurate figures [52]. However, the use of surveillance data for allocating funds has its limitations since the data will not fully reflect the costs of treatment and levels of clinical activity as the system was not set up to capture this level of detail. Future estimates may be enhanced by supplementing surveillance data with data from other sources.
Fig. 7.
HIV-infected gay men accessing treatment and care services by age group, United Kingdom: 1995–2004. (Source: SOPHID and CD4 Surveillance Scheme. From: The UK Collaborative Group for HIV and STI Surveillance. Mapping the Issues. HIV and other Sexually Transmitted Infections in the United Kingdom, 2005. London: Health Protection Agency Centre for Infections. November 2005.)
STIs in HIV-infected gay men in the post-HAART era
Historically it has been purported that population level increases in other STIs, particularly gonorrhoea, were indicative of increased HIV transmission. While analyses were always subject to ecological fallacy, they are now further complicated by a high proportion of STI diagnoses occurring in diagnosed HIV-infected gay men post-HAART. Enhanced surveillance of outbreaks of syphilis and LGV collect information on HIV status, as does the sentinel Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) [2, 70–72]. Half (785/1583) of the gay men diagnosed with syphilis in London in 2004 were known to be HIV positive [2]. Similarly, 81% (119/147) of gay men diagnosed with LGV in the United Kingdom in 2004 and 32% (123/381) of those with gonorrhoea were HIV positive [2]. There have also been recent reports of outbreaks of shigella, hepatitis A and hepatitis C among known HIV-positive gay men [73–75]. Behavioural surveillance since 1998 shows that ‘serosorting’ – where men select partners on the basis of their HIV status – is occurring, with HIV-positive men seeking HIV-positive (i.e. seroconcordant) partners [5, 76–79]. In view of these behaviours, our interpretation of STI trends in relation to HIV transmission needs to be re-evaluated.
An additional impact of the acquisition of STIs by a significant number of diagnosed HIV-infected MSM is that diagnosed, and therefore total (diagnosed and undiagnosed), HIV prevalence can no longer be accurately measured by the unlinked anonymous GUM survey. This is because since the re-emergence of syphilis [80, 81], sexually active, diagnosed HIV-positive MSM have been encouraged to regularly test for syphilis. In the unlinked anonymous survey, residual blood from syphilis serology is irreversibly unlinked from patient identifiable information and then tested for HIV [29]. As a consequence, the diagnosed HIV prevalence as measured by the survey has become substantially inflated. To overcome this source of bias, only undiagnosed prevalence has been reported in recent years [2].
Continuing transmission of HIV in the post-HAART era: 1996–2004
Prior to HAART, back-calculation analyses from AIDS cases indicated that HIV incidence among gay men in the United Kingdom peaked in 1983 (when there were about 6000 infections), rapidly decreased and then remained stable [82]. Gonorrhoea incidence has declined dramatically [83]. There was no further reduction in HIV incidence during the late 1980s and mid-1990s [31, 84, 85]. Incidence was about 4/100 person-years among gay men attending four GUM clinics in London between 1988 and 1994, with a higher incidence (9/100 person-years) among younger (aged <30 years) men [84].
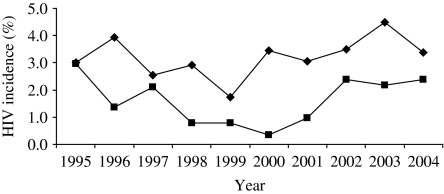
In the post-HAART era, gay men still remain the group most at risk of acquiring HIV within the United Kingdom, with substantial evidence for continuing HIV transmission [2]. The use of HAART meant the end of back-calculation methods using AIDS cases to determine HIV incidence, as the natural course of infection had been altered. Since 1998, a laboratory technique the Serological Testing Algorithm for Recent HIV Seroconversion (STARHS) has been developed and applied using the unlinked anonymous technique (including retrospectively) to routine syphilis serology specimens from gay men attending the 16 GUM clinics in the unlinked anonymous survey in England, Wales and Northern Ireland [86–88]. Currently, it is only validated to be used for subtype B HIV infections. Annual HIV incidence in this sample of gay men has remained constant, varying between 2 and 3·5/100 person-years between 1995 and 2004, with no statistically significant trends over time (Fig. 8) [87, 88]. In comparison to the early 1990s, however, incidence seems to be higher among older gay men (in 2004, highest at 4·5/100 person-years in men aged 35–44 years) [2]. This differential in HIV incidence between older and younger gay men has also been observed in The Netherlands [89]. There are future plans to test every newly diagnosed infection among gay men to see whether it is recent or not, and this will allow further analyses and monitoring of incidence in subtype B infections.
Fig. 8.
Estimated* HIV incidence† among gay men attending 16 sentinel GUM clinics, England, Wales and Northern Ireland: 1995–2004. –◆–, London; –■–, outside London [* estimated using the Serological Testing Algorithm for Recent HIV Seroconversion (STARHS); † trend not significant]. (Source: Unlinked anonymous serosurvey. From: The UK Collaborative Group for HIV and STI Surveillance. Mapping the Issues. HIV and other Sexually Transmitted Infections in the United Kingdom, 2005. London: Health Protection Agency Centre for Infections. November 2005.)
Monitoring transmitted drug resistance: 2001 onwards
The emergence of drug-resistant strains of HIV and transmission of these strains (transmitted resistance) has implications for the initiation of therapy, necessitating the use of resistance assays prior to treatment [90], and therapy options. The United Kingdom's HIV drug resistance database, established in 2001, collects information from resistance tests carried out during routine clinical care [90, 91]. Using these data it has been shown that up until 2002, the prevalence of transmitted resistance (all behavioural risk groups combined) increased over time, with the largest increase observed for non-nucleoside reverse transcriptase inhibitors, fitting in with UK prescribing practices [91]. Between 2002 and 2004, however, the prevalence of transmitted resistance declined [92].
CONCLUSION
Public health surveillance of HIV among gay men in the United Kingdom has come a long way over the last 25 years providing some of the most comprehensive surveillance data in the world. In this review, we have examined how national surveillance systems have evolved in response to the changing epidemiology of HIV/AIDS among gay men in the United Kingdom as well as advances in laboratory techniques and medical treatments.
There are several key challenges for HIV surveillance among gay men in the United Kingdom in the future. First, improvements in the monitoring of HIV incidence among gay men are needed to determine transmission patterns by age, geography and over time to better inform prevention services. This will involve expanding the coverage of incidence testing to all HIV diagnoses, linking demographic and clinical information to these samples and developing tests for non-B subtype incident infections. To further understand the behaviours that underlie the continuing transmission of HIV among gay men, behavioural research, such as the INSIGHT study, will need to investigate factors leading to seroconversion [93].
At the other end of the clinical spectrum, continued and further monitoring of the uptake of HIV testing and late-stage disease, including the role of AIDS surveillance in the post-HAART era will be important in reducing morbidity and mortality among HIV-infected gay men in the future. This should be supplemented by qualitative research to help us understand why some men continue to present at a very late stage of infection despite efforts to encourage routine HIV testing. Finally, the transmission of STIs among networks of HIV-positive gay men in the United Kingdom has created a need for enhanced surveillance systems collecting information on STI diagnoses and HIV status among gay men [94]. These have helped us understand the contribution of HIV-positive gay men to the recent increase in STIs but further work needs to be done to determine the implications for HIV transmission to HIV-negative gay men and the interpretation of STI surveillance data.
What is clear is that as the epidemiology of HIV and other STIs continues to evolve among gay men in the United Kingdom, our surveillance systems will need to further adapt, as they have done in the past, so that information can be effectively used to inform health policy in the years to come.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Health Protection Agency Centre for Infections, HIV and Sexually Transmitted Infections Department, Communicable Disease Surveillance Centre, and Scottish Centre for Infection & Environmental Health 2004. . Unpublished Quarterly Surveillance Tables No. 63, 04/2, July .
- 2.The UK Collaborative Group for HIV and STI Surveillance London: Health Protection Agency, Centre for Infections; 2005. . Mapping the Issues. HIV and other Sexually Transmitted Infections in the United Kingdom in 2005. . November . [Google Scholar]
- 3.Dodds JP, Mercey DE Centre for Sexual Health & HIV Research, Department of Primary Care & Population Sciences, Royal Free & University College Medical School; 2004. . Sexual Health Survey of Gay Men – London 2003. [Google Scholar]
- 4.Hickson F Sigma Research; 2003. Out and about: findings from the United Kingdom Gay Men's Sex Survey 2002. , December . [Google Scholar]
- 5.Elford J et al. Trends in sexual behaviour among London homosexual men 1998–2003: implications for HIV prevention and sexual health promotion. Sexually Transmitted Infections. 2004;80:451–454. doi: 10.1136/sti.2004.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williamson LM, Hart GJ. HIV optimism does not explain increases in high risk sexual behaviour among gay men in Scotland. AIDS. 2004;18:834–836. doi: 10.1097/00002030-200403260-00025. [DOI] [PubMed] [Google Scholar]
- 7.Langmuir AD. The surveillance of communicable diseases of national importance. New England Journal of Medicine. 1963;268:182–192. doi: 10.1056/NEJM196301242680405. [DOI] [PubMed] [Google Scholar]
- 8.Hymes K et al. Kaposi's sarcoma in homosexual men: a report of 8 cases. Lancet. 1981;ii:598. doi: 10.1016/s0140-6736(81)92740-9. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb MS et al. Pneumocystis pneumonia – Los Angeles. Morbidity and Mortality Weekly Report. 1981;30:250–252. [PubMed] [Google Scholar]
- 10.Du Bois RM et al. Primary pneumocystis carinii and cytomegalovirus infection. Lancet. 1981;ii:1339. doi: 10.1016/s0140-6736(81)91353-2. [DOI] [PubMed] [Google Scholar]
- 11.Public Health Laboratory Service. Surveillance of the Acquired Immunodeficiency Syndrome (AIDS) in the United Kingdom, January 1982–July 1983. Communicable Disease Report. 1983;83:3. [Google Scholar]
- 12.McEvoy M, Tillett HE. Some problems in the prediction of future numbers of cases of the acquired immunodeficiency syndrome in the UK. Lancet. 1985;ii:541–542. doi: 10.1016/s0140-6736(85)90472-6. [DOI] [PubMed] [Google Scholar]
- 13.Public Health Laboratory Service. The surveillance of Kaposi's sarcoma and opportunistic infections in homosexual males in England and Wales. Communicable Disease Report. 1982;82:3–4. [Google Scholar]
- 14.O'Connor BH, McEvoy MB, Galbraith NS. Kaposi's sarcoma/AIDS surveillance in the UK. Lancet. 1983;i:872. doi: 10.1016/s0140-6736(83)91405-8. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control. Update of Acquired Immune Deficiency Syndrome (AIDS) – United States. Morbidity and Mortality Weekly Report. 1982;31:507–508. , 513–514. [PubMed] [Google Scholar]
- 16.Ancelle-Park RA. European AIDS definition. Lancet. 1992;339:671. doi: 10.1016/0140-6736(92)90815-k. [DOI] [PubMed] [Google Scholar]
- 17.Public Health Laboratory Service Case definitions for AIDS – Europe and the United States part company Communicable Disease ReportCDR Weekly 199331. [PubMed] [Google Scholar]
- 18.Public Health Laboratory Service. Acquired Immune Deficiency Syndrome, United Kingdom: May 1985. Communicable Disease Report. 1985;85:3–4. [Google Scholar]
- 19.Goldberg DJ et al. collaborating microbiologists. A system for surveillance of voluntary HIV testing; results of the first two years, 1989–1990. AIDS. 1992;6:495–500. and . [PubMed] [Google Scholar]
- 20.Cheingsong-Popov R et al. Prevalence of antibody to Human T-lymphotropic virus type III in AIDS and AIDS-risk patients in Britain. Lancet. 1984;ii:477–480. doi: 10.1016/s0140-6736(84)92562-5. [DOI] [PubMed] [Google Scholar]
- 21.Mortimer PP et al. Prevalence of antibody to human T lymphotropic virus type III by risk group and area, United Kingdom 1978–1984. British Medical Journal. 1985;290:1176–1178. doi: 10.1136/bmj.290.6476.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jesson WJ et al. Prevalence of anti-HTLV-III in UK risk groups 1984/85. Lancet. 1986;i:155. doi: 10.1016/s0140-6736(86)92288-9. [DOI] [PubMed] [Google Scholar]
- 23.Carne CA et al. Rising prevalence of Human T-Lymphotropic Virus type III (HTLV-III) infection among homosexual men in London. Lancet. 1985;i:1261–1262. doi: 10.1016/s0140-6736(85)92324-4. [DOI] [PubMed] [Google Scholar]
- 24.Loveday C et al. Human immunodeficiency viruses in patients attending a sexually transmitted disease clinic in London, 1982–7. British Medical Journal. 1989;298:419–422. doi: 10.1136/bmj.298.6671.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Health, The Welsh Office. Short-term Prediction of HIV Infection and AIDS in England and Wales. London: HMSO; 1988. . Report of a Working Group. [Google Scholar]
- 26.Weller IVD, Hindley DJ, Adler MW. Gonorrhoea in homosexual men and media coverage of the acquired immunodeficiency syndrome in London 1982–3. British Medical Journal. 1984;289:1041. doi: 10.1136/bmj.289.6451.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber JN et al. Three-year prospective study of HTLV-III/LAV infection in homosexual men. Lancet. 1986;i:1179–1182. doi: 10.1016/s0140-6736(86)91160-8. [DOI] [PubMed] [Google Scholar]
- 28.Carne CA et al. Prevalence of antibodies to human immunodeficiency virus, gonorrhoea rates, and changed sexual behaviour in homosexual men in London. Lancet. 1987;i:656–658. doi: 10.1016/s0140-6736(87)90415-6. [DOI] [PubMed] [Google Scholar]
- 29.Gill ON, Alder MW, Day NE. Monitoring the prevalence of HIV: foundations for a programme of unlinked anonymous testing in England and Wales. British Medical Journal. 1989;299:1295–1298. [Google Scholar]
- 30.Department of Health 1988. . Press release: ‘Government announces new steps to monitor the spread of HIV infection’, 23 November .
- 31.Catchpole MA et al. Serosurveillance of prevalence of undiagnosed HIV-1 infection in homosexual men with acute sexually transmitted infection. British Medical Journal. 2000;321:1319–1320. doi: 10.1136/bmj.321.7272.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simms I et al. on behalf of the collaborative group. Trends in undiagnosed HIV-1 infection among attenders at genitourinary medicine clinics, England, Wales and Northern Ireland: 1990–6. Sexually Transmitted Infections. 1999;75:332–336. doi: 10.1136/sti.75.5.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Department of Health. The National Strategy for Sexual Health and HIV. London: Department of Health; 2001. [Google Scholar]
- 34.Chief Medical Officer http://www.publications.doh.gov.uk/cmo/annualreport2003/notime.htm. 2006. http://www.publications.doh.gov.uk/cmo/annualreport2003/notime.htm . No time to wait: the importance of early diagnosis of HIV. From Health Check, CMO annual report 2003 ( ). Accessed July .
- 35.Hickson F Sigma Research; 1998. . Community HIV & AIDS Prevention Strategy. Making it count: a theory, ethics and evidence based health promotion strategy to reduce the incidence of HIV infection through sex between men in England. , March . [Google Scholar]
- 36.Hickson F London: Sigma Research, Terence Higgins Trust; 2002. Making it count: a collaborative planning framework to reduce the incidence of HIV infection during sex between men. . Published on behalf of the CHAPS Partnership, 2nd edn, September . [Google Scholar]
- 37.The UK Collaborative Group for HIV and STI Surveillance London: Health Protection Agency, Centre for Infections; 2004. . Focus on Prevention. HIV and other Sexually Transmitted Infections in the United Kingdom in 2003. . November . [Google Scholar]
- 38.Macdonald N et al. Recent trends in diagnoses of HIV and other sexually transmitted infections in England and Wales amongst men who have sex with men. Sexually Transmitted Infections. 2004;80:492–497. doi: 10.1136/sti.2004.011197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dougan S et al. Does the recent increase in HIV diagnoses among MSM in the UK reflect a rise in HIV incidence or increased uptake of HIV testing? Sexually Transmitted Infections. 2007;83:120–125. doi: 10.1136/sti.2006.021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta SB et al. on behalf of the CD4 Surveillance Scheme Advisory Group. CD4 cell counts in adults with newly diagnosed HIV infection: results of surveillance in England and Wales, 1990–1998. AIDS. 2000;14:853–861. doi: 10.1097/00002030-200005050-00012. [DOI] [PubMed] [Google Scholar]
- 41.Chadborn TR et al. No time to wait: how many HIV-infected homosexual men are diagnosed late and consequently die? (England and Wales, 1993–2002. AIDS. 2005;19:513–520. doi: 10.1097/01.aids.0000162340.43310.08. [DOI] [PubMed] [Google Scholar]
- 42.Weatherburn Pet al. HIV testing for African people and MSM: comparing needs, access and effectiveness in clinic and community settings in a UK city. Annual Conference of the British HIV Association, Brighton, UK, 29 March–1 April 2006HIV Medicine20067 (Suppl. 1): 32
- 43.Prost A et al. ‘There is such a thing as asking for trouble’: taking rapid HIV testing to gay venues is fraught with challenges. Sexually Transmitted Infections 2007 doi: 10.1136/sti.2006.023341. . Published online: 17 January . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Day NEet al. Acquired Immune Deficiency Syndrome in England and Wales to end 1993: projections using data to end September 1989. Report of a working group convened by the Director of the Public Health Laboratory Service. Communicable Disease Report, January 1990 (Suppl. 1): 1–12.
- 45.Rees M. Short-term prediction of HIV infection and AIDS: a critique of the Working Group's Report to the Department of Health. Inernational Journal of STD & AIDS. 1990;1:10–17. doi: 10.1177/095646249000100104. [DOI] [PubMed] [Google Scholar]
- 46.Petruckevitch A et al. Direct estimates of prevalent HIV infection in adults in England and Wales for 1991 and 1993: an improved method. Genitourinary Medicine. 1997;73:348–354. doi: 10.1136/sti.73.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGarrigle CA et al. Estimating adult HIV prevalence in the UK in 2003. The direct method of estimation. Sexually Transmitted Infections. 2006;82:iii78–iii86. doi: 10.1136/sti.2006.020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Public Health Laboratory Service. Adult HIV prevalence in the United Kingdom, 2000: estimated using the ‘direct’ method. Communicable Disease Report (CDR) Weekly. 2002;12(5) [Google Scholar]
- 49.Goubar A London: Health Protection Agency Centre for Infections; 2006. Bayesian multi-parameter synthesis of HIV surveillance data in England and Wales, 2001. , Technical Report, March . [Google Scholar]
- 50.Imrie J Dealing with the HIV Pandemic in the 21st Century: Health Systems’ Responses Past, Present and Future. Oxford University Press; 2006. United Kingdom. [Google Scholar]
- 51.Aalen OO et al. New therapy explains the fall in AIDS incidence with a substantial rise in number of persons on treatment expected. AIDS. 1999;13:103–108. doi: 10.1097/00002030-199901140-00014. [DOI] [PubMed] [Google Scholar]
- 52.Mocroft A et al. AIDS across Europe, 1994–98: the EuroSIDA study. Lancet. 2000;356:291–296. doi: 10.1016/s0140-6736(00)02504-6. [DOI] [PubMed] [Google Scholar]
- 53.Mocroft A et al. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS. 2002;16:1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 54.Mocroft A et al. Changes in AIDS-defining illnesses in a London clinic, 1987–1998. Journal of Acquired Immune Deficiency Syndrome. 1999;21:401–407. [PubMed] [Google Scholar]
- 55.Ives NJ, Gazzard BG, Easterbrook PJ. The changing pattern of AIDS-defining illnesses with the introduction of highly active antiretroviral therapy (HAART) in a London clinic. Journal of Infection. 2001;42:134–139. doi: 10.1053/jinf.2001.0810. [DOI] [PubMed] [Google Scholar]
- 56.Public Health Laboratory Service, Communicable Disease Surveillance Centre. Enhancement of HIV infection reporting. Communicable Disease Report (CDR) Weekly. 2000;10:1–4. [Google Scholar]
- 57.Dougan S et al. The epidemiology of HIV among black and minority ethnic men who have sex with men in England and Wales. Sexually Transmitted Infections. 2005;81:345–350. doi: 10.1136/sti.2004.012328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dougan S et al. Men who have sex with men who are born abroad and diagnosed with HIV in England and Wales: an epidemiological perspective. International Journal of STD and AIDS. 2005;16:618–621. doi: 10.1258/0956462054944552. [DOI] [PubMed] [Google Scholar]
- 59.Gilbart VG, Evans BG, Dougan S. HIV transmission among men who have sex with men through oral sex [Letter] Sexually Transmitted Infections. 2004;80:324. doi: 10.1136/sti.2004.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.EuroHIV Saint-Maurice: Institut de Veille Sanitaire; 2006. . HIV/AIDS surveillance in Europe. Mid-year report 2005. . No. 72. [Google Scholar]
- 61.Public Health Laboratory Service. The surveillance of HIV-1 infection and AIDS in England and Wales. Communicable Disease Report. CDR Review. 1991;1(5):R51–56. [Google Scholar]
- 62.McCormick A. Unrecognised HIV related deaths. British Medical Journal. 1991;302:1365–1367. doi: 10.1136/bmj.302.6789.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCormick A. Trends in mortality statistics in England and Wales with particular reference to AIDS from 1984 to April 1987. British Medical Journal. 1998;296:1289–1292. doi: 10.1136/bmj.296.6632.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nylén G et al. Mortality in young adults in England and Wales: the impact of the HIV epidemic. AIDS. 1999;13:1535–1541. doi: 10.1097/00002030-199908200-00014. [DOI] [PubMed] [Google Scholar]
- 65.Josh Forde, Health Protection Agency Centre for Infections . Personal communication.
- 66.Ciancio BCet al. Trends in mortality and causes of death among HIV-infected individuals diagnosed in the UK: 1993–2004. XVI International AIDS Conference, Toronto, Canada, 13–18 August 2006
- 67.British HIV Association (BHIVA) 2006. www.bhiva.org www.bhiva.org . Mortality Audit 2006. BHIVA, October .
- 68.Molesworth A. Results from the 1995 survey of prevalent clinically diagnosed HIV infection in England, Wales, and Northern Ireland. Communicable Disease Report. CDR Review. 1997;7:R77–82. [PubMed] [Google Scholar]
- 69.Parpia T et al. The effect of combination therapy on immunological progression of human immunodeficiency virus disease at a population level. American Journal of Epidemiology. 2001;153:898–902. doi: 10.1093/aje/153.9.898. [DOI] [PubMed] [Google Scholar]
- 70.Redman C et al. Infectious syphilis in Scotland: the implementation of a new surveillance system. SCIEH Weekly Report. 2003;37:6–7. [Google Scholar]
- 71.Righarts AA et al. Syphilis surveillance and epidemiology in the United Kingdom. Eurosurveillance Monthly. 2004;9:21–25. [PubMed] [Google Scholar]
- 72.Health Protection Agency. Enhanced surveillance of lymphogranuloma venereum in England. Communicable Disease Report. 2004;14 [Google Scholar]
- 73.Browne R et al. Increased numbers of acute hepatitis C infections in HIV positive homosexual men; is sexual transmission feeding the increase? Sexually Transmitted Infections. 2004;80:326–327. doi: 10.1136/sti.2003.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Health Protection Agency. Outbreak of Shigella sonnei phage type Q in men who have sex with men (MSM) in London. Communicable Disease Report. 2004;14(43) [Google Scholar]
- 75.Health Protection Agency. Outbreak of Hepatitis A in men who have sex with men in south east London. Communicable Disease Report. 2004;14(45) [Google Scholar]
- 76.Dodds JP et al. Increasing risk behaviour and high levels of undiagnosed HIV infection in a community sample of homosexual men. Sexually Transmitted Infections. 2004;80:236–240. doi: 10.1136/sti.2003.007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reid D On the Move: Findings from the United Kingdom's Gay Men's Sex Survey 2003. Sigma Research; London: 2004. , December . [Google Scholar]
- 78.Hart GJ, Williamson LM. Increase in HIV sexual risk behaviour in gay men in Scotland, 1996–2002: Prevention failure. Sexually Transmitted Infections. 2005;81:367–372. doi: 10.1136/sti.2004.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elford J et al. High-risk sexual behaviour among London gay men: no longer increasing. AIDS. 2005;19:2171–2174. doi: 10.1097/01.aids.0000194133.28135.03. [DOI] [PubMed] [Google Scholar]
- 80.Wallace LA. Syphilis in Scotland 2003. SCIEH Weekly Report. 2004;38:38–39. [Google Scholar]
- 81.Simms I et al. The re-emergence of syphilis in the UK: the new epidemic phases. Sexually Transmitted Disease. 2005;32:220–226. doi: 10.1097/01.olq.0000149848.03733.c1. [DOI] [PubMed] [Google Scholar]
- 82.Nicoll A et al. Assessing the impact of national anti-HIV sexual health campaigns: trends in the transmission of HIV and other sexually transmitted infections in England. Sexually Transmitted Infections. 2001;77:242–247. doi: 10.1136/sti.77.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gellan MCA, Ison CA. Declining incidence of gonorrhoea in London: a response to fear of AIDS? Lancet. 1986;i:656–658. doi: 10.1016/s0140-6736(86)90440-x. [DOI] [PubMed] [Google Scholar]
- 84.Miller E et al. Incidence of HIV infection in homosexual men in London, 1988–1994. British Medical Journal. 1995;311:545. doi: 10.1136/bmj.311.7004.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldberg D et al. HIV infection among homosexual/bisexual males attending genito-urinary medicine clinics in Scotland. Sexually Transmitted Infections. 1998;74:185–188. doi: 10.1136/sti.74.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Janssen RS et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. Journal of the American Medical Association. 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 87.Murphy G et al. HIV incidence appears constant in men who have sex with men despite widespread use of effective antiretroviral therapy. AIDS. 2004;18:265–272. doi: 10.1097/00002030-200401230-00016. [DOI] [PubMed] [Google Scholar]
- 88.Murphy G et al. Is HIV incidence increasing in homo/bisexual men attending STI clinics in England, Wales and Northern Ireland? Commuicable Disease and Public Health. 2004;7:11–14. [PubMed] [Google Scholar]
- 89.Dukers NTM et al. HIV incidence on the increase among homosexual men attending an Amsterdam sexually transmitted disease clinic: using a novel approach for detecting recent infections. AIDS. 2002;16:F19–F24. doi: 10.1097/00002030-200207050-00001. [DOI] [PubMed] [Google Scholar]
- 90.UK Collaborative Group on Transmission of HIV Resistance in Primary Infections. Analysis of prevalence of HIV-1 drug resistance in primary infections in the United Kingdom. British Medical Journal. 2001;322:1087–1088. doi: 10.1136/bmj.322.7294.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.UK Group on Transmitted HIV Drug Resistance. Time trends in primary resistance to HIV drugs in the United Kingdom: multicentre observational study. British Medical Journal. 2005;331:1368. doi: 10.1136/bmj.38665.534595.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Health Protection Agency. HIV Drug Resistance in the United Kingdom: data to end of 2004. Communicable Disease Report. 2006;16(4) [Google Scholar]
- 93.Elam G et al. Intentional and unintentional UAI among gay men who HIV test in the UK: qualitative results from an investigation into risk factors for seroconversion among gay men who HIV test (INSIGHT) HIV Medical Journal. 2006 (Suppl. 1), Abstract 027, [Google Scholar]
- 94.Dougan S, Evans BG, Elford J. Sexually transmitted infections in Western Europe among HIV positive men who have sex with men. Sexually Transmitted Disease. 2007 doi: 10.1097/01.olq.0000260919.34598.5b. (in press). [DOI] [PubMed] [Google Scholar]