SUMMARY
Prospective surveillance was conducted in three remote Aboriginal communities with high rates of rheumatic heart disease in order to investigate the epidemiology of group A β-haemolytic streptococci (GAS). At each household visit, participants were asked about sore throat. Swabs were taken from all throats and any skin sores. GAS isolates were emm sequence and pattern-typed using standard laboratory methods. There were 531 household visits; 43 different emm types and subtypes (emmST) were recovered. Four epidemiological patterns were observed. Multiple emmST were present in the population at any one time and household acquisition rates were high. Household acquisition was most commonly via 5- to 9-year-olds. Following acquisition, there was a 1 in 5 chance of secondary detection in the household. Throat detection of emmST was brief, usually <2 months. The epidemiology of GAS in these remote Aboriginal communities is a highly dynamic process characterized by emmST diversity and turnover.
INTRODUCTION
Remote tropical Aboriginal communities of northern Australia have among the highest reported rates of acute rheumatic fever (ARF) and rheumatic heart disease (RHD) in the world; yet, the epidemiology of streptococcal infection in these settings appears to differ from that seen in temperate climates [1]. Specifically, symptomatic group A streptococcal (GAS) pharyngitis is rare whereas GAS pyoderma is common.
Much of what we know about the epidemiology of GAS comes from studies conducted in temperate climates [2–4], although there are data from tropical and subtropical settings [5, 6]. One of the most comprehensive studies, with relevance to remote Aboriginal populations, was that done in the 1960s and 1970s at the Red Lake Reservation [7]. Using M protein serotyping, investigators demonstrated the introduction, dissemination and waning of successive serotypes in schoolchildren of an indigenous US community. The mean number of new strain acquisitions per child was 4·7 (range 1–9) per 24 months. Some serotypes lingered in the community for extended periods whilst others moved rapidly through the population. The phenomenon of rapid strain replacement has also been described in a US farming community [8].
One of the limitations of M serotyping is the proportion of strains that are non-typable, particularly in Australian Aboriginal communities [9]. In the 1990s, Beall and others developed emm sequence typing based on the nucleotide sequence of the variable 5′-end of the emm gene encoding for M protein. M serotypes and emm types appeared to be concordant [10] and a comprehensive online emm database is now available [11]. Bessen and others have also used a polymerase chain reaction (PCR)-based mapping technique to identify different arrangements of emm and related genes within the mga regulon, or emm pattern typing. Patterns A–C are associated with GAS strains recovered from the throat, pattern D from the skin and pattern E from both throat and skin [12]. With few exceptions, GAS with a given emm sequence type have a predictable emm pattern [13].
A previous longitudinal study of GAS epidemiology in a remote Aboriginal island community also used a typing method based on the mga regulon (vir typing) [14]. This showed high strain diversity and turnover. It also demonstrated simultaneous colonization with multiple vir types and used a model to estimate that each child carried a specific vir type for an average of 4 months. We now report a longitudinal study, using emm sequence typing, of three mainland Aboriginal communities with exceptionally high rates of ARF and RHD. One of the aims of the study was to collect throat swabs and pyoderma swabs from children in households at high risk for ARF. If anyone subsequently developed ARF, it could be determined whether GAS pyoderma or GAS pharyngitis was the antecedent infection. We also aimed to examine the household and community epidemiology of pharyngitis and pyoderma and determine whether there were features unique to the study population, including household and personal acquisition, length of carriage, secondary transmission and the effect of age.
METHODS
Study sites and consultation
The study was conducted in three communities, separated by at least 400 km, located in the Top End of the Northern Territory (NT), Australia. At the request of community elders, the community names have not been disclosed. Each has an all-age prevalence of RHD >25/1000 population compared to 0·26/1000 in the non-Aboriginal population. A prolonged period of community consultation preceded approval of the study protocol by the regional ethics committee. Household and individual consent was obtained and surveillance was commenced in communities 1 and 2 in August 2003. Researchers encountered logistic problems in community 2 and the study was transferred to community 3 in June 2004. Surveillance was completed in July 2005.
Surveillance
Researchers endeavoured to visit each study household on a monthly basis. Each person present was asked about sore throat and skin sores in the local language. All throats were examined and swabbed for culture. Limbs and exposed areas were also examined and any pyoderma lesions swabbed according to a standard protocol. Each individual episode was defined as a ‘consultation’. People with pyoderma, symptomatic pharyngitis or other medical conditions of concern were transported to the Community Health Centre and provided with treatment. Specimens were transported by air in a cold container to a central laboratory.
Household units and definitions
It was not feasible to follow individuals over an extended period because of population mobility; similarly, a school-based study was impractical because attendance was <50%. Households corresponded to family groupings and were regarded as the primary epidemiological units. In order to increase chances of encountering further cases of ARF, study households were selected because they had members with a known history of ARF or RHD. A household was defined as a single family group that lived in one house or two adjacent houses. Participants were considered to belong to a household if they said they belonged at enrolment and were present on at least two subsequent visits. The number of people who were seen only once in any household, as a ratio of household size, was used as a measure of household turnover. Because of the month-to-month variation in the number of people present at each visit and variation in the number of successful visits (when data and specimens were obtained) for each household, the median number of visits and the consultations per visit was calculated by household and by community.
For the purposes of this study, we have used emmST to denote an emm type and emm subtype. A household emmST acquisition was defined as the initial recovery of an emmST from any member of a household. A concurrent acquisition was the finding of more than one person with the same emmST in the household at the time of acquisition. Household persistence was defined as the length of time (months) before an emmST was no longer detected in a household and did not reappear for the remainder of the study period. A secondary event was the recovery of an acquired emmST from another household member at a subsequent visit, acknowledging that this may be due to secondary transmission, re-introduction or previously undetected concurrent acquisition. Community persistence was defined as the time (in months) from first detection of an emmST in study households until no detection for ⩾6 months, or until the end of the observation period.
Laboratory methods
The methods used for culture and identification of β-haemolytic streptococci have been detailed in a previous report [15]. The procedure for emm sequence typing followed that recommended by the Centers for Disease Control and Prevention (CDC) [11] with minor modifications. We used InstaGeneTM matrix (Bio-Rad, Hercules, CA, USA) for DNA sample preparation; 20 colonies were harvested directly from culture plates and resuspended in 200 μl of the InstaGene matrix suspension. All other steps were according to the manufacturer's instructions. PCR was conducted in 50 μl reaction volumes with 5 μl of DNA sample, 5 μl dNTPs (2 mm), 1 μl each of primers 1 and 2 (25 μm), 2·5 U Taq DNA polymerase, 5 μl of 10× Taq buffer (Qiagen, Valencia, CA, USA), sdH2O to 50 μl. PCR clean-up was done using QIAquick 96 PCR purification kit (Qiagen) according to the manufacturer's instructions, eluting in 60 μl sdH2O.
For sequencing reactions we used the BigDye® Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) in a reaction volume of 10 μl (2 μl purified PCR product, 0·5 μl primer emmseq2, 4 μl premix and 3·5 μl sdH2O). Clean-up was achieved by adding 10 μl sdH2O and using DyeEx96 (Qiagen). The product was then desiccated and sent to a commercial sequencing facility for separation. For sequence analysis, electropherograms were viewed with the Lasergene© SeqMan software v.7.0.0 (DNAstar, Madison, WI, USA) and sequences were blasted against the Streptococcus pyogenes emm sequence database on the CDC website [11]. New (novel) emm sequences were submitted to the administrator for assignment. emm pattern typing of GAS isolates was done according to the method of Bessen and co-workers [16].
Data analysis
Epidemiological data were analysed using stata 8 (Stata Corporation, College Station, TX, USA). The Pearson product-moment correlation coefficient was used to assess correlations. Confidence intervals were calculated using standard methods. Because of differences in surveillance time for the three communities, the variability of household visits and the number of people seen at each household visit, rates were reported per household visit and per consultations when comparing households, and per 100 community consultations when comparing communities.
RESULTS
Household surveillance
Forty-nine households and 1173 people were enrolled. There were 531 household visits and 4842 consultations. Household crowding was extreme in communities 1 and 3 (Table 1). There were limited data from community 2. The median household turnover was 18% [interquartile range (IQR) 10–25] in community 1 and 31% (IQR 25–37) in community 3, although the periods of surveillance differed. Local ceremonial events restricted surveillance in August 2004 and March 2005. Overall prevalence rates for pharyngitis, GAS throat carriage and pyoderma, by community and by age, have been reported elsewhere [1].
Table 1.
Comparative population and GAS isolate data from the three study communities
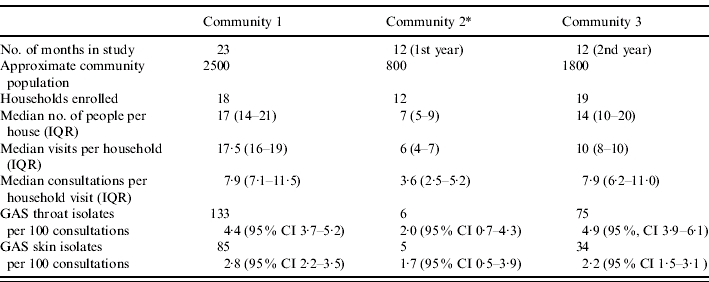
IQR, Interquartile range; CI, confidence interval.
Note that there were relatively few GAS isolates from community 2.
Community dynamics of GAS
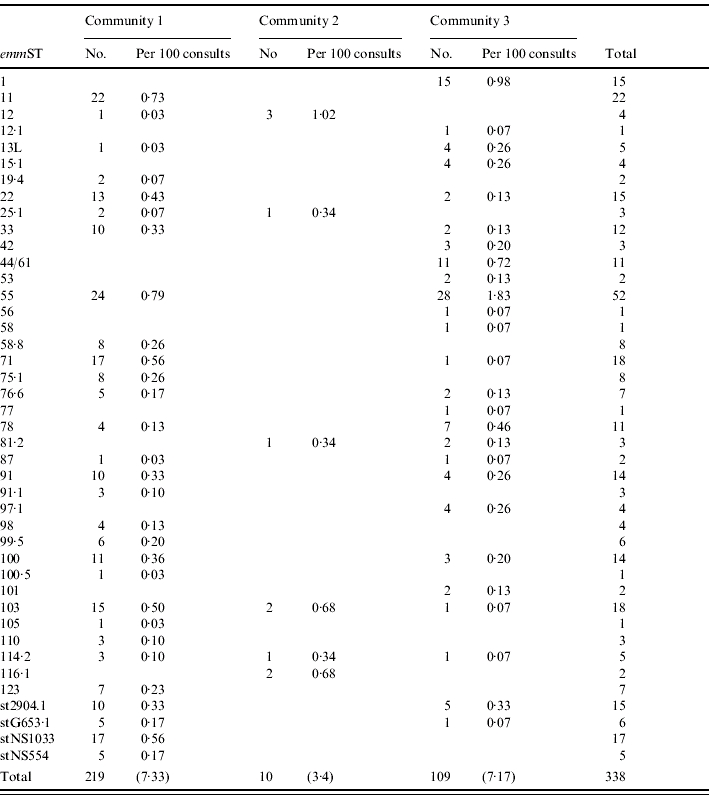
In total, 214 throat GAS isolates and 124 skin isolates were emm sequence-typed and 43 emmST identified (Table 2). Four novel emm subtypes were found, emm58·8, emm91·1, emm99·5 and emm100·5. Twelve of 28 emmST (43%) from community 1 were not found in the other communities, as were 1/6 emmST in community 2, and 12/26 (46%) in community 3 (Table 2). Thirteen emmST (emm 22, 25, 42, 44/61, 58, 78, 81, 97, 100, 101, 110, 114, stNS544) had been reported from the same region in the mid-1990s [17].
Table 2.
The different emm types and subtypes identified in study households of the three communities
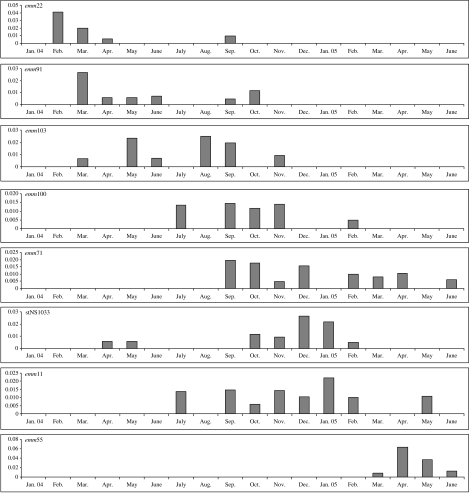
The length of time each emmST was detected in the study population varied greatly. A third of emmST (14/43) appeared, increased in prevalence, peaked and then departed from study households over a period of ⩾6 months. Figure 1 shows this pattern for community 1. Nine emmST (21%) persisted in the community for ⩾10 months. By comparison, 18 emmST (42%) were seen at just one community visit, often in several different households, without detectable transmission within study households or to other households. They were not encountered again. The outbreak strain, emm55, arrived suddenly, disseminated widely and peaked rapidly. It arrived in community 3 households in February 2005 and was first detected in community 1 households a month later. At both sites, outbreaks of acute post-streptococcal glomerulonephritis (APSGN) followed in its wake [18].
Fig. 1.
Composite of the eight most common emm sequence types for community 1 households. The x axis indicates the month and the y axis the number of GAS isolates as a proportion of the household consultations for that month.
The median time an emmST remained detectable in study households at each study site was 5 months (IQR 1–9). For the last 12 months of the study, when communities 1 and 3 could be directly compared, the median number of emmST found in the study population at any one time was 6·5 (IQR 4·8–8·3) in community 1 and 4 (IQR 3–7·5) in community 3.
Household dynamics of GAS
Household acquisition varied widely with a median of 5·5 (IQR 3·7–6·0) new emmST per household per year in community 1 and 4·0 (IQR 3·6–6·8) in community 3. These crude rates are based on a median of 97 (IQR 86–140) consultations per house per year in community 1 and 89 consultations (IQR 69–131) in community 3. When rates for individual households were adjusted for the number of visits and the number of consultations, the median household acquisition rate was 4·9 per household-year (IQR 4·2–9·1) for community 1 and 3·6 (IQR 1·9–7·9) for community 3.
There was strong correlation between emmST acquisition per household year and household size (Pearson correlation coefficient=0·88, P<0·001 for community 1, and 0·68, P<0·001 for community 3), but not between acquisition and household turnover. Six emmST were recovered just once (one individual on one occasion), whilst seven emmST were recovered from ⩾10 households over the study period and emm55 was recovered from 22 households.
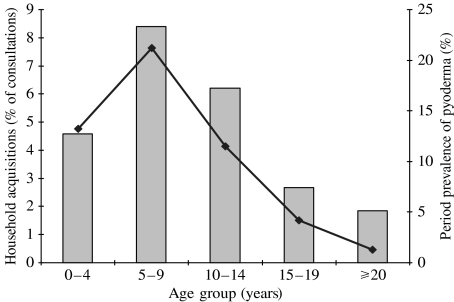
We documented 216 household emmST acquisitions, 141 via the throat, 72 via the skin and three from both sites. However, these are acquisitions of throat carriage compared to acquisitions of skin infection; moreover, every study consultation included a throat swab whereas skin swabs were only taken in the presence of pyoderma. Figure 2 shows that the individuals most likely to be responsible for a household acquisition were in the 5–9 years age group. On 20/216 occasions (9·3%, 95% CI 5·8–14) there was concurrent household acquisition and on 41/216 occasions (19%, 95% CI 13–25) the same emmST was recovered from one or more other household members at a subsequent visit (secondary event).
Fig. 2.
Age group of the individuals responsible for household introduction of new GAS emmST into the household as a proportion of consultations in that age group ( ) compared to the reported period prevalence of pyoderma in those age groups (◆—◆) [1].
) compared to the reported period prevalence of pyoderma in those age groups (◆—◆) [1].
If the first detected acquisition in a household is regarded as the index case, there was no significant difference in secondary events depending on whether initial GAS isolates had come from skin sores or throat. There were 40 instances where we had obtained serial swabs; an individual had an initial negative swab (or no skin swab in the case of pyoderma) but was positive the following month and was followed for consecutive months until the swabs were again negative. On 34/40 occasions (85%) the emmST was not recovered from the throat swab after a month, in four people (10%), the emmST was detected for 2 months. One person had emm55 isolated for 3 months and another for 4 months. Otherwise, we were not able to demonstrate prolonged carriage of specific emmST. We found no examples of an emmST moving from skin to throat and only one example of an emmST moving from throat to skin.
There were seven confirmed cases of ARF in study households during the study period and in each case we failed to recover GAS from the throat or skin of that person in the 2 months prior to presentation of the illness. In four cases this was because the person was absent from the household at the critical visits. We recovered 29 isolates of GAS from other household members in the 2 months prior to the person presenting with ARF, the month of presentation and 2 months following presentation (Table 3). They represented 15 different emmST.
Table 3.
GAS emmST recovered from other household members of the seven people in the study who had ARF; emmST recovered during the month of presentation and 2 months either side
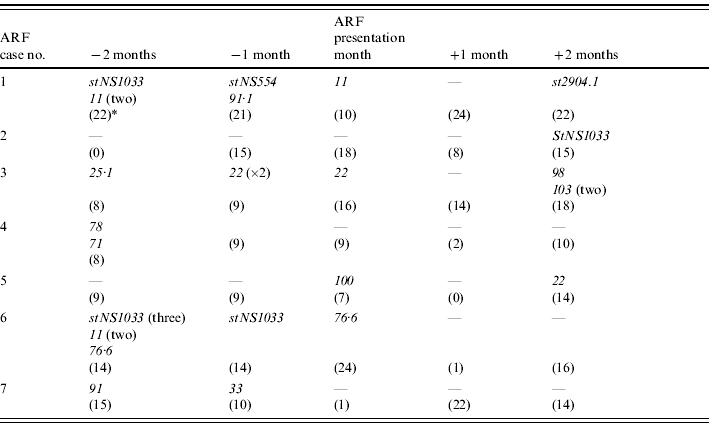
The number of household consultations at each visit is shown in parentheses.
Age and emmST and emm pattern-type distribution
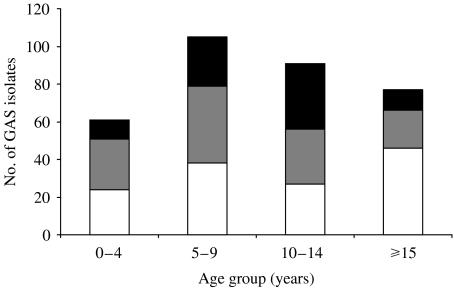
There were no detectable differences in rates of common emmST with age. The proportion of pattern type D decreased with age, from 44% of isolates in the 0–4 years age group to 25% in those aged ⩾15 years, presumably in relation to the declining prevalence of pyoderma (Fig. 3). Pattern types A–C were more common in the early teenage years. The outbreak of emm55 (pattern type A–C) mainly affected the 10–14 years age group, involving 10·3% of that population, compared to 3·4% of 0–4 years age group, 7·4% of the 5–9 years age group and 1% of people aged ⩾15 years.
Fig. 3.
The relationship between emm pattern type and age: emm pattern-type D is relatively more common in the younger age groups and decreases with age whilst patterns A–C were more common in the early teenage years. These data include the outbreak strain emm55 that made up 63% of all throat types (emm pattern types A–C). ■, Throat tropic A–C; , skin tropic D; □, skin and throat tropic E.
, skin tropic D; □, skin and throat tropic E.
DISCUSSION
A key finding of this study was the diversity of emmST within households and across the region. There were 43 different emmST overall with multiple emmST being present in the study population at any one time. In the second year of the study alone, 38 separate emmST were detected in a total study population of just over 1000 people. Except for 15 isolates of emm1 in community 3, classic rheumatogenic emm types were few. In this regard, the status of emm11 is unclear; it was detected in the household of the first case of ARF reported in this study (Table 3). This emmST has emm pattern type E and is serum opacity factor (SOF) positive, characteristics unlike other rheumatogenic types. Yet M serotype 11 was recovered from the throats of a number of people with ARF in Trinidad in the 1970s [5]. Subsequently, it found its way onto at least one published list of rheumatogenic GAS serotypes [19] but has not been universally accepted as such [20].
Marked emmST diversity was found in a previous study of an Aboriginal island community [17] and has been reported from populations in Ethiopia, Brazil, Thailand and India where rates of ARF and/or RHD are relatively high [21–24]. Moderate diversity has also been reported from Japan and Italy where the rates of RHD are low [25, 26]. Contrasting reports from temperate climate regions include a large study of Pittsburgh schoolchildren that detected only 13 different emmST over a 4-year period [4] and only 22 emmST were identified in a 2-year study of 202 families in Melbourne [27]. The number of different emmST seen in any one year in Salt Lake City (population 180 000), where there has been enhanced surveillance, is usually <20 [28].
It would be of no surprise to find that genetic diversity is greatest where the community burden of GAS is high and co-infection with multiple M types or emmST is common. Horizontal gene transfer appears to be the primary mechanism [29]. GAS are polylysogenic – they contain multiple prophage elements – accounting for most of the variability between strains, perhaps including factors that determine rheumatogenicity. Type-specific immune responses to epitopes of M protein put emm genes under selective pressure. Thus, diversity is likely to be greatest, with new types emerging more rapidly, where the burden of colonization and infection is highest. This has ominous implications for multivalent vaccine development, particularly for those based on M protein determinants; only 103/338 (30%) of GAS isolates, and 11/43 (26%) of the emmST in this study would have been covered by the proposed 26-valent vaccine [30]. This also includes 72/214 (34%) of GAS throat isolates.
Another finding of this study was the presence of 13 emmST that were previously reported in the Top End more than a decade ago, none known to be a classic rheumatogenic type. The persistently high rates of skin infection in the region suggest that skin-tropic strains are continuously cycling through these communities. Shulman and others have proposed that the decline in the incidence of ARF in the United States was related to the replacement of rheumatogenic strains by non-rheumatogenic strains [31]. Yet in the study communities with sustained high rates of ARF, persistence of recognized throat-tropic emmST or rheumatogenic types has not been demonstrated, and ARF outbreaks have never been reported. The incidence of ARF does not even vary with season [14]. This raises the possibility that rheumatogenic determinants, some perhaps independent of M protein, could circulate in settings highly conducive to phage-mediated lateral gene transfer between streptococcal strains [29].
We distinguished four basic epidemiological patterns although there was a degree of overlap. They included a group of emmST that appeared to move sequentially through the community over a 3- to 6-month period, emmST that persisted for a long period of time, often more than a year, and emmST that made brief single-month cameo appearances. The fourth pattern was an outbreak. It is difficult to determine the extent to which these patterns are due to properties of the organisms, the environment or the host response.
Throat acquisition of an M type or emmST, and its persistence, is not necessarily determined by type-specific antibodies to M protein [32–34], yet type-specific immunity does protect against disease [35]. Type-specific immunity is also highly variable from individual to individual, both in strength of response and duration, and is not necessarily determined by the severity of the original infection [36]. As distinct from reports elsewhere [4, 7], detection of each emmST in the throat was almost always brief, a paradoxical finding in view of the rates of ARF. This finding could be due to limitations of data collection. It could also be hypothesized that type-specific immunity leads to low rates of persistence in individuals with greater persistence in the community due to constant replenishment of the non-immune pool through population mobility. Similarly, a persistently high community turnover of emmST could modify the risk of an ARF outbreak. Additional facets of the host response that limit carriage could include non-type-specific immunity, such as to C5a peptidase and the hyaluronic acid capsule.
The pattern of sequential M type (or emmST) replacement (shown in Fig. 1), has been demonstrated elsewhere [4, 7, 14]. Similarly, strains that make a brief appearance and vanish again are well known; their contribution to disease burden is unclear, although they contribute to genetic diversity. Some GAS strains have an enhanced ability to disseminate and cause disease, as demonstrated by the dramatic arrival and dissemination of emm55. This pattern has previously been reported in the Top End and other parts of the world [37].
The household holds the key to the epidemiology of GAS. This was demonstrated in two classic studies of the 1960s [38, 39]. Levine and co-workers cultured the throats of all people who presented with a respiratory illness on an airforce base in Maine, USA; if a β-haemolytic streptococcus was recovered, patients were divided into those with GAS and those who were GAS-negative (some presumably having GCS/GGS) [39]. They then cultured the throats of family contacts living on the base and found that 48% of 244 child contacts (aged 5–9 years) of elementary schoolchildren with GAS also harboured GAS compared to 2% of 115 child contacts of GAS-negative children (P<0·002). On 72% of occasions (range 47–80%), the GAS-positive patients and their culture-positive contacts shared the same M serotype. The percentage of positive family contacts was also higher in larger families. The authors concluded that transmission of GAS ‘requires a fairly direct and close degree of contact between carriers and potential susceptibles, and that in our society the major locus of this combination of persons and the environment is the home’.
In our study, household acquisition and spread was highly variable; nevertheless, we could not conclude that GAS from pyoderma were more likely to be transmitted than those from the throat. However, if an individual introduced an emmST to a household, there was a 1 in 5 chance of a secondary event at a subsequent visit. In contrast, in a study of GAS pharyngitis in Melbourne families, a primary case was followed by at least one secondary case in 43% of families [27] and the mean time to detection of a secondary case was 8 days. In our study, household visits were usually a month apart; as such we may have missed a substantial proportion of secondary transmissions. In their landmark study of GAS carriage and pharyngitis in Cleveland families, Dingle and co-workers found that household acquisition of an M serotype most commonly came through a child aged <6 years [38]. This corresponds to the findings of our study, and also corresponds with the peak age group (5–9 years) of pyoderma in our study population. In the Cleveland study, if the index case had pharyngitis, then 25% of other household members acquired the same organism, with illness being more likely in younger children. If the index case had no illness, the secondary carrier rate was 9%. In some households the emmST persisted for many months, sometimes years.
It has been proposed that the distribution of emmST is determined by age, reflecting exposure to, and acquisition of, immunity to common emmST [40]. Whilst we did not find a clear age-dependent relationship with individual emmST, we did demonstrate a decline in the proportion of skin types with age that probably reflects rates of pyoderma. It is not clear how much of that decline is due to acquisition of immunity or a reduction in other predisposing factors such as scabies and local trauma.
This study shows that the epidemiology of GAS in remote Aboriginal communities is a remarkably dynamic process with great emmST diversity, a multiplicity of emmST present in the community at any one time, high household acquisition and secondary event rates and a relatively short detection time. Four different epidemiological patterns were recognized at the community level. Classic rheumatogenic GAS types were largely absent and symptomatic pharyngitis was rare. The hypothesis that skin infection plays a role in the pathogenesis of ARF in remote Aboriginal communities has yet to be proven. In the meantime, inadequate health awareness, crowded household conditions and population mobility are likely to be some of the driving forces behind GAS dissemination and diversity within the region.
ACKNOWLEDGEMENTS
The authors are grateful for the advice and support of Dr Edward Kaplan (University of Minnesota, Minneapolis) and Dr Bernard Beall (Centers for Diseases Control and Prevention, Atlanta). We also wish to thank Doreen Jing-Garrarbirra, Lena Djabibba, Ethelrida Dartinga, Hamish Cameron, Melita McKinnon, Palave Dasari, the study families and community health centre staff for their contributions. Murin Air (NT) and Western Pathology provided assistance with transportation of specimens. The study was supported by grants from the National Heart Foundation of Australia (no. PB 02M 0996), the National Health and Medical Research Council (no. ID 251690) and the Cooperative Research Centre for Aboriginal Health.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.McDonald M et al. Low rates of streptococcal pharyngitis and high rates of pyoderma in communities where rheumatic fever is hyperendemic. Clinical Infectious Diseases. 2006;43:683–689. doi: 10.1086/506938. [DOI] [PubMed] [Google Scholar]
- 2.Wannamaker L, McCarty M. Streptococcal Infections. New York: Columbia University Press; 1954. The epidemiology of streptococcal infections; pp. 157–175. , pp. [Google Scholar]
- 3.Quinn R, Lowry P, van der Zwaag R. Significance of hemolytic streptococci for Nashville school children: clinical and serologic observations. Southern Medical Journal. 1978;71:242–246. doi: 10.1097/00007611-197803000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Martin JM et al. Group A streptococci among school-aged children: clinical characteristics and the carrier state. Pediatrics. 2004;114:1212–1219. doi: 10.1542/peds.2004-0133. [DOI] [PubMed] [Google Scholar]
- 5.Potter E et al. Tropical acute rheumatic fever and associated streptococcal infections compared with concurrent acute glomerulonephritis. Journal of Pediatrics. 1978;92:325–333. doi: 10.1016/s0022-3476(78)80036-5. [DOI] [PubMed] [Google Scholar]
- 6.Majeed H et al. Group A streptococcal strains in Kuwait: a nine-year prospective study of prevalence and associations. Pediatric Infectious Diseases Journal. 1992;11:295–300. [PubMed] [Google Scholar]
- 7.Anthony B et al. The dynamics of streptococcal infections in a defined population of children: serotypes associated with skin and respiratory infections. American Journal of Epidemiology. 1976;104:652–666. doi: 10.1093/oxfordjournals.aje.a112344. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan EL, Wotton JT, Johnson DR. Dynamic epidemiology of group A streptococcal serotypes associated with pharyngitis. Lancet. 2001;358:1334–1337. doi: 10.1016/S0140-6736(01)06415-7. [DOI] [PubMed] [Google Scholar]
- 9.Hartas J et al. Characterisation of group A streptococcal isolates from tropical Australia with high prevalence of rheumatic fever: probing for signature sequences to identify members of the family of serotype 5. Microbial Pathogenesis. 1995;18:345–354. doi: 10.1006/mpat.1995.0031. [DOI] [PubMed] [Google Scholar]
- 10.Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. Journal of Clinical Microbiology. 1996;34:953–958. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Diseases Control and Prevention (CDC) Streptococcus laboratory; Atlanta, Georgia, USA: ). Accessed 2 January 2007. [Google Scholar]
- 12.Bessen D et al. Genetic correlates of throat and skin isolates of group A streptococci. Journal of Infectious Diseases. 1996;173:896–900. doi: 10.1093/infdis/173.4.896. [DOI] [PubMed] [Google Scholar]
- 13.McGregor KF et al. Group A streptococci from a remote community have novel multilocus genotypes but share emm-types and housekeeping alleles with isolates from worldwide sources. Journal of Infectious Diseases. 2004;189:717–723. doi: 10.1086/381452. [DOI] [PubMed] [Google Scholar]
- 14.Carapetis JR Sydney: University of Sydney; 1998. . Ending the heartache: the epidemiology and control of acute rheumatic fever and rheumatic heart disease in the Top End of the Northern Territory [Ph.D. Thesis]. [Google Scholar]
- 15.McDonald M et al. Recovering streptococci from the throat in remote tropical communities: a practical alternative to direct plating. Journal of Clinical Microbiology. 2006;44:547–551. doi: 10.1128/JCM.44.2.547-552.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bessen DE et al. Contrasting molecular epidemiology of group A streptococci causing tropical and nontropical infections of the skin and throat. Journal of Infectious Diseases. 2000;182:1109–1116. doi: 10.1086/315842. [DOI] [PubMed] [Google Scholar]
- 17.McGregor KF et al. Multilocus sequence typing of Streptococcus pyogenes representing most known emm types and distinctions among subpopulation genetic structures. Journal of Bacteriology. 2004;186:4285–4294. doi: 10.1128/JB.186.13.4285-4294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams S, Markey P. Acute post streptococcal glomerulonephritis in a remote community. Northern Territory Diseases Control Bulletin. 2005;12:16–18. [Google Scholar]
- 19.Massell B. Rheumatic Fever and Streptococcal Infection. Boston: Countway; 1997. p. 193. , p. [Google Scholar]
- 20.Bisno A. Group A streptococcal infections and acute rheumatic fever. New England Journal of Medicine. 1991;325:783–793. doi: 10.1056/NEJM199109123251106. [DOI] [PubMed] [Google Scholar]
- 21.Abdissa A et al. High diversity of group A streptococcal emm types among healthy schoolchildren in Ethiopia. Clinical Infectious Diseases. 2006;42:1362–1367. doi: 10.1086/503422. [DOI] [PubMed] [Google Scholar]
- 22.Teixeira LM et al. Genetic and phenotypic features of Streptococcus pyogenes strains isolated in Brazil that harbor new emm sequences. Journal of Clinical Microbiology. 2001;39:3290–3295. doi: 10.1128/JCM.39.9.3290-3295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruksakorn S et al. Epidemiological analysis of non-M-typeable group A Streptococcus isolates from a Thai population in northern Thailand. Journal of Clinical Microbiology. 2000;38:1250–1254. doi: 10.1128/jcm.38.3.1250-1254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma M, Sriprakash KS Streptococci – New Insights into an Old Enemy. Amsterdam: Elsevier; 2006. Heterogeneity of community based paediatric GAS isolates from India: challenges to the multivalent vaccine; pp. 49–53. , pp. [Google Scholar]
- 25.Tanaka D et al. emm typing of group A streptococcus clinical isolates: identification of dominant types for throat and skin isolates. Microbiology and Immunology. 2002;46:419–423. doi: 10.1111/j.1348-0421.2002.tb02715.x. [DOI] [PubMed] [Google Scholar]
- 26.Dicuonzo G et al. Group A streptococcal genotypes from pediatric throat isolates in Rome, Italy. Journal of Clinical Microbiology. 2001;39:1687–1690. doi: 10.1128/JCM.39.5.1687-1690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danchin MH Melbourne: University of Melbourne; 2006. . Disease burden and management of group A streptococcal pharyngitis in primary care in Australia. Department of Paediatrics [Ph.D. Thesis]. [Google Scholar]
- 28.Miner LJ et al. Molecular characterization of Streptococcus pyogenes isolates collected during periods of increased acute rheumatic fever activity in Utah. Pediatric Infectious Diseases Journal. 2004;23:56–61. doi: 10.1097/01.inf.0000105180.76624.33. [DOI] [PubMed] [Google Scholar]
- 29.Davies MR, Sriprakash KS Streptococci – New Insights into an Old Enemy. Amsterdam: Elsevier; 2006. Evidence for increased horizontal gene transfers among streptococci from group A streptococcal endemic regions; pp. 188–192. , pp. [Google Scholar]
- 30.Hu MC et al. Immunogenicity of a 26-valent group A streptococcal vaccine. Infection and Immunity. 2002;70:2171–2177. doi: 10.1128/IAI.70.4.2171-2177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shulman S et al. Temporal changes in streptococcal M protein types and near-disappearance of acute rheumatic fever in the United States. Clinical Infectious Diseases. 2006;42:441–447. doi: 10.1086/499812. [DOI] [PubMed] [Google Scholar]
- 32.Dunlap M, Harvey H. The carrier state and type-specific immunity in streptococcal disease. American Journal of Diseases of Children. 1967;114:229–243. doi: 10.1001/archpedi.1967.02090240043001. [DOI] [PubMed] [Google Scholar]
- 33.Guirguis N et al. Type-specific immunity and pharyngeal acquisition of group A streptococcus. American Journal of Epidemiology. 1982;116:933–939. doi: 10.1093/oxfordjournals.aje.a113495. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan Eet al. Two year persistence of group A streptococci (GAS) in the throat accompanied by falling streptococcal antibody titers: the upper respiratory carrier state confirmed and microbiologically/immunologically examined. Proceedings of the XVIth Lancefield International Symposium on Streptococci and Streptococcal DiseasesPalm Cove; Queensland, Australia: 2005 [Google Scholar]
- 35.Wannamaker L et al. Studies on immunity to streptococcal infections in man. American Journal of Diseases of Children. 1953;86:347–348. [PubMed] [Google Scholar]
- 36.Lancefield R. Persistence of type-specific antibodies in man following infection with group A streptococci. Journal of Experimental Medicine. 1959;110:271–292. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dillon H. Post-streptococcal glomerulonephritis following pyoderma. Reviews of Infectious Diseases. 1979;1:935–945. doi: 10.1093/clinids/1.6.935. [DOI] [PubMed] [Google Scholar]
- 38.Dingle J, Badger G, Jordan W. Illness in the Home: A Study of 25,000 Illnesses in a Group of Cleveland Families. Cleveland: Press of Western Reserve University; 1964. Streptococcal infections; pp. 97–117. , pp. [Google Scholar]
- 39.Levine J et al. Studies on the transmission within families of group A hemolytic streptococci. Journal of Laboratory and Clinical Medicine. 1966;67:483–494. [PubMed] [Google Scholar]
- 40.Jaggi P et al. Age influences the emm type distribution of pediatric group A streptococcal pharyngeal isolates. Pediatric Infectious Diseases Journal. 2005;24:1089–1092. doi: 10.1097/01.inf.0000190023.89759.96. [DOI] [PubMed] [Google Scholar]