SUMMARY
We report a retrospective analysis of 1933 Brucella strains isolated from humans and animals in Latin American countries between 1968 and 1991 and in Argentina between 1994 and 2006. During the first period 50% of strains were from humans, mainly from Argentina, Mexico and Peru but, while B. suis was the main cause of infection in Argentina, B. melitensis was responsible for most infections in the other countries. In Argentina in the later years, B. melitensis and B. suis were observed more frequently than in the first period while isolation of B. abortus decreased. Of 145 B. melitensis human isolates, eight gave susceptibility patterns to dyes and penicillin and two were B. melitensis biovar 3, which has never been reported in animals. Forty-six percent of B. suis isolated were resistant to dyes which is an atypical feature in this species.
INTRODUCTION
While in some countries the incidence of brucellosis is declining as a result of control measures in cattle, goats and sheep, the disease persists in the Mediterranean basin, the Middle East, the Arabian Gulf and some Latin American countries [1–3]. Brucellosis has been reported in Latin America since the first decade of the 20th century and remains to this day a major zoonosis despite campaigns for its control. Its persistence and wide distribution in different animal hosts is facilitated by the peculiar geographic, climatic and economic conditions of the area. Some determining factors which have not been sufficiently defined are the methods of cattle husbandry, nomadic or semi-nomadic goat herding and the incidence of porcine brucellosis. Control programmes are sometimes ineffective due to the lack of sustainable funding over time [4–6]. Isolation of a Brucella sp. in humans and animals provides irrefutable evidence of the infection [1, 7, 8] but in this region statutory diagnosis is achieved mainly by serological tests, and isolation of Brucella spp. from clinical specimens often relies on epidemiological investigations or look-back exercises.
At present the genus Brucella comprises six species which are classified by reactions in biochemical tests and preferred animal hosts [9]. However, the genus is highly homogeneous with all members showing >95% homology in DNA–DNA pairing studies [10, 11]. Recently, distinctive Brucella strains have been isolated from marine animals and a new species, named B. maris, has been proposed [12, 13]. Currently, B. abortus, B. melitensis, B. suis, B. canis and B. maris are known to be pathogenic for humans and may give rise to systemic infection involving any organ system and present with non-specific symptoms. Because of this protean clinical picture, the diagnosis of brucellosis may be confused with other infectious or non-infectious diseases leading to delay in treatment [14, 15].
Our laboratory has been a national centre for human brucellosis since 1994 and performs serological and bacteriological diagnosis of infection in patients with symptoms and/or history compatible with this disease. We report here a retrospective analysis of 1933 Brucella strains isolated from humans and animals in several Latin American countries from 1968 to 1991 and in Argentina from 1994 to 2006.
MATERIALS AND METHODS
Geographic distribution of the strains
From 1968 to 1991, 1377 strains isolated from humans and animals in Latin America were confirmed as Brucella spp. at the Pan American Zoonosis Centre (CEPANZO) in Buenos Aires, Argentina and stored at the National Laboratories and Institutes of Health Administration (ANLIS). The animal strains were isolated from domestic cattle, goats, pigs, sheep, dogs and horses, and wild animals such as the buffalo (Bubalus bubalis), fox (Dusicyon gymnocercus antiquus), grey weasel (Didelphis marsupialis), capybara (Hydrochoerus hydroachaeris) and ferret (Galictis furax huranox). Their origins were Argentina, Brazil, Chile, Colombia, Cuba, Dominican Republic, Ecuador, El Salvador, Honduras, Mexico, Nicaragua, Paraguay, Peru, Uruguay and Venezuela (Table 1). Most of the strains were from Argentina (32%), Peru (25%) and Mexico (18%), and were mostly recovered from humans, but those from Brazil, Chile, Colombia, and Cuba were predominantly from cattle.
Table 1.
Geographical origin and species of origin of 1377 Brucella strains isolated during 1968–1991 in Latin America
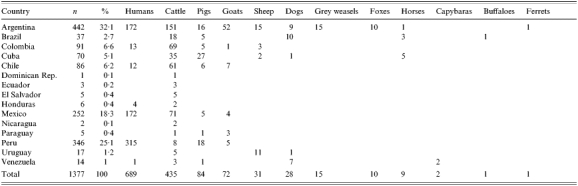
Argentina has five geographical regions characterized by diversities in livestock and agriculture. Bovine and porcine species are mainly concentrated in the humid Pampa (HP); bovine and caprine in the Northwest (NW); bovine and ovine in the Northeast (NE); bovine, ovine and caprine in Cuyo (CU); and ovine and caprine in Patagonia (PAT) [16].
From 1994 to 2006, 367 strains from humans were collected at ANLIS, the headquarters of the newly implemented National Human Brucellosis Network (NHBN), a project aiming at standardizing serological and bacteriological diagnosis and the rapid treatment of patients throughout the country. Primary isolation from clinical specimens was performed in clinical diagnostic laboratories mainly from HP (66·7%), followed by NW (14·4%), CU (13·9%) and NE (3·8%) and sent to NHBN headquarters for typing. The NHBN project also isolates and characterizes Brucella spp. from animals for epidemiological purposes, and examined 189 strains from dogs between 1996 and 2006.
Type strains B. abortus 544, B. melitensis 16M, B. suis 1330, B. canis RM 6-66 and B. ovis 63/290, and vaccine strains B. abortus S19 and B. melitensis Rev. 1 were used as controls.
Culture samples
Animal strains were isolated from milk, vaginal swabs, semen, aborted foetuses, mammary, retropharyngeal or internal iliac lymph nodes, abscesses in reproductive organs, testes or epididymes, spleen and liver. Blood cultures were taken mainly from dogs and wild animals. Commercial dehydrated media or laboratory-prepared selective media were used for the isolation of Brucella from domestic and wild animals according to standard practice [17]. Human strains were isolated from blood cultures, bone marrow, CSF, liver tissue, abscesses, joint and prostatic fluid, knee cyst and lymph nodes. Routine isolation techniques such as monophasic and biphasic blood culture, lysis centrifugation [18] and automated blood culture systems (Bactec or BactAlert) were used [19, 20]. For monophasic blood culture, a commercial liquid medium Hemo Brucella (Britania SA, Buenos Aires, Argentina) or an in-house medium [21] were used, and for the biphasic medium, Hemoline (bioMérieux, Marcy l'Etoile, France) was employed.
Identification of Brucella
Isolates were identified as Brucella spp. in the clinical diagnostic laboratories on the basis of morphology, motility at 20°C and 37°C, reactions in triple sugar iron (TSI) agar, lactose fermentation on MacConkey agar, acid production from glucose, haemolysis on blood agar, production of catalase, oxidase, urease (Christensen method), citrate utilization, and nitrate reduction. At the reference laboratory colonies were tested for agglutination in acriflavine 1:1000 in distilled water to distinguish between smooth and rough forms. The strains submitted were usually in the smooth form except for B. ovis and B. canis. Cultures exhibiting rough colonies cannot be typed with monospecific sera or with smooth Brucella phages; in these cases smooth colony variants were selected for further typing [7, 17].
Typing of strains
Brucella species and biovars were determined by standard methods [8, 22] and included serum and CO2 requirement, H2S production, growth in the presence of Thionin (20 μg/ml) and Basic fuchsin (20 μg/ml), urease test (Bauer's method) and agglutination with polyclonal monospecific anti-A, -M, and -R antisera. They were also tested for susceptibility to Brucella BK, RC, Wb and Iz phages at routine test dilution (RTD) and Tb phage at RTD and 10 000 RTD [23]. B. abortus biovar (bv.) 1 was differentiated from S19 vaccine strains by its ability to grow on thionin blue (2 μg/ml), erythritol (1 mg/ml) and penicillin (5 IU/ml) and B. melitensis bv. 1 was distinguished from vaccine Rev. 1 strains by growth patterns on Thionin, Basic fuchsin, penicillin and streptomycin (2·5 μg/ml); growth patterns on Safranin O (100 μg/ml) and Malachite green (2 μg/ml) were also investigated [7].
The oxidative metabolic patterns of the strains from the first period of sampling were determined at CEPANZO, using the Warburg apparatus, Braun V 85 model, series B [17]. PCR–RFLP analysis of the omp31 gene using AvaII and SalI restriction enzymes was also performed to confirm the identification of B. canis [24, 25]. After typing, the strains were maintained lyophilized at 4°C or in cryo-preservation medium at −70°C.
RESULTS
Period 1968–1991
Of the 1377 strains of Brucella spp. isolated in Latin America during the period 1968–1991 from humans and animals, B. melitensis was the most frequently isolated species from humans, followed by B. suis and B. abortus (Table 2). B. abortus was the main cause of infection in cattle, B. melitensis in goats, B. suis in pigs and B. ovis in sheep; B. canis and B. suis were both isolated from dogs. B. abortus was found in buffaloes, foxes, capybaras and ferrets, while B. abortus and B. suis were isolated from horses and grey weasels.
Table 2.
Sources and species of 1377 Brucella strains isolated in Latin American countries (1968–1991)
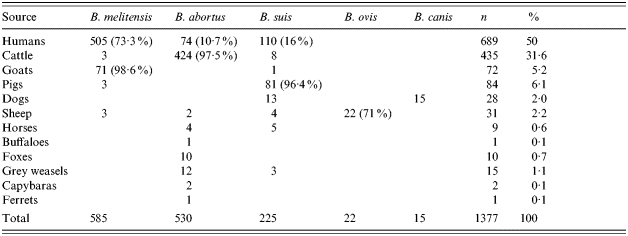
Table 3 shows that B. melitensis bv. 1, B. abortus bv. 1 and bv. 4, and B. suis bv. 1 were the main biovars isolated. Thirty isolates were confirmed as B. abortus S19 and 85 isolates of B. suis bv. 1 were resistant to dyes in a manner atypical for this species (B. suis 1a). These strains grew on Thionin, but also on Basic fuchsin, Safranin O, Thionin blue, Malachite green and penicillin [4, 26, 27].
Table 3.
Brucella species and biovars isolated in Latin America (1968–1991)
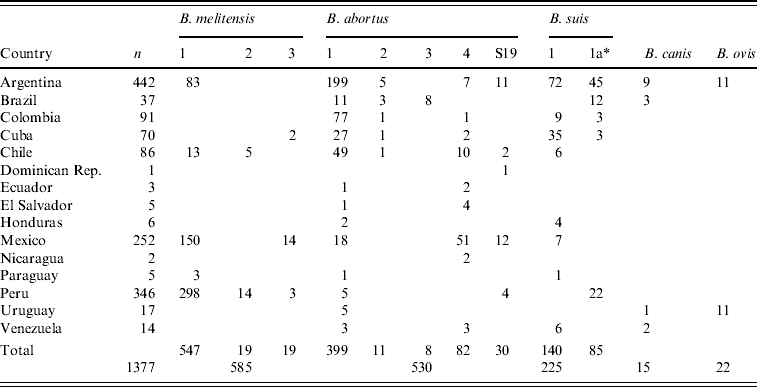
Resistant to dyes.
During this first period 172 Brucella spp. were isolated from humans in Argentina (data not shown) mainly from geographical regions HP and NW. Of these, 55 were B. abortus (50 bv. 1, three bv. 4, one bv. 2 and one S19), 90 B. suis (47 bv. 1 and 43 bv. 1a) and 27 B. melitensis bv. 1. One strain (classified as B. melitensis 1a) grew slowly, produced small colonies and was susceptible to penicillin and dyes, similar to the B. melitensis Rev. 1 vaccine, but it was inhibited by 2·5 μg/ml streptomycin [28].
Period 1994–2006
Among the 367 strains isolated from humans in Argentina during this period (Table 4), B. abortus was isolated from 75 cases country-wide, the majority being biovar 1 (86·6%). Almost all of the 145 B. melitensis isolated were biovar 1 (93·1%), followed by biovar 1a (4·8%), biovar 3 (1·4%) and biovar 2a (0·7%), based on differences in the quantitative distribution of the ‘A’ and ‘M’ antigens. In contrast, the 144 B. suis strains almost equally divided into biovars 1 and 1a, 54·2% and 45·8% respectively. All but four of the 189 B. canis strains were from mongrel dogs in Buenos Aires.
Table 4.
Brucella strains isolated from humans in Argentina 1994–2006
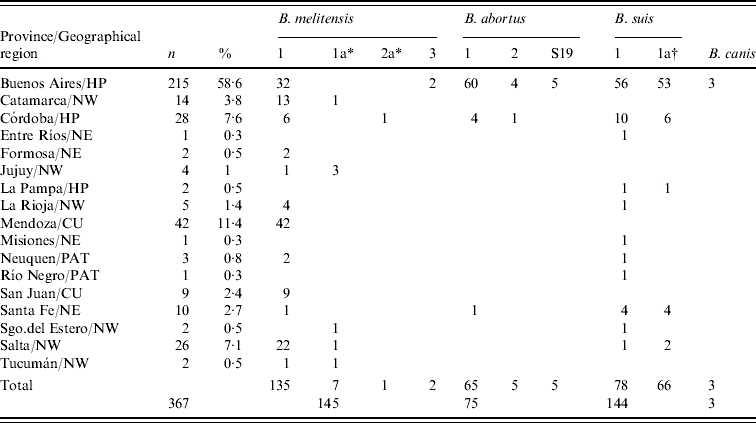
NW, Northwest, NE, Northeast, CU, Cuyo, HP, Humid Pampa; PAT, Patagonia.
Sensitive to dyes, penicillin and streptomycin.
Resistant to dyes.
DISCUSSION
In Latin America, brucellosis is considered to be one of the most important zoonotic infections because of its impact on public health and the economy. Its distribution is closely related to the concentration of livestock [4–6]. The animal population in the region is mainly cattle followed by sheep, goats and pigs, but it appears that as a result of control programmes the incidence of bovine brucellosis has been reduced [29–33]. The main objective of this report was to present the geographical origins and sources of 1933 strains (isolated from humans and/or animals in both sampling periods, and 189 dogs) in 15 Latin American countries, especially Argentina. A reason for the low number of isolates recovered from domestic animals given the high prevalence of the disease in this group could be that although bacteriological diagnosis is recommended as a confirmatory test, it is inadequate for detection of the disease in large numbers of animals. The lack of laboratories with facilities for bacteriological diagnosis will also impact on this. The prevalence of bovine brucellosis varies considerably from one country to another with rates ranging from 0·5% to 10%, the incidence of brucellosis in pigs is not known with certainty and there are few countries reporting data on caprine and ovine brucellosis [4–6, 29–33].
Of the 1377 strains isolated in 1968–1991, 31·6% were from cattle (mainly from Argentina) most of them being B. abortus and, as a result of vaccination or excretion of the strains by vaccinated animals, B. abortus S19 strain was recovered from cattle, humans and a grey weasel. As expected pigs and goats were infected mainly with B. suis and B. melitensis respectively and sheep with B. ovis. However, B. suis, B. melitensis and B. abortus were also found in sheep, probably because of cross-contamination between animal species due to farming practices where continuous contact among the herds exists. B. ovis strains were recovered only from Argentina and Uruguay where epididymitis in rams is a major problem. In dogs B. canis and B. suis were identified and the high number of B. suis might be explained by contact with infected farm animals or their ingestion of contaminated food. The need to consider the potential carrier role of this animal when kept in close proximity to other infected animals has been highlighted [1, 4]. Furthermore, the data show that biovar 1 of each species predominated over other biovars (B. melitensis 93·5%, B. abortus 75·3% and B. suis 62·2%).
During the first period, half of all Brucella spp. from human sources were from Argentina (90 B. suis, 55 B. abortus and 27 B. melitensis), Mexico and Peru, but, in the two latter countries B. melitensis was the main cause of infection. Currently the control strategy of goat brucellosis in Mexico has been redesigned in high-risk areas and the implementation of massive vaccination with Rev. 1 vaccine has resulted in a reduction of human cases [30].
In Argentina, cattle are the largest livestock population followed by sheep, goats and pigs. According to official reports the estimated prevalence of brucellosis in cattle ranged from 10% to 13% for farms with an individual rate of 4–5%. In other species, surveys found that in Buenos Aires 28·6% of sheep were not recommended for breeding owing to brucellosis. The individual prevalence in goats was 0·5–0·8% in the Northwest of the country and studies in pigs between 1960–1980 found a regional prevalence of 14·2–25% [4, 16]. However, there is no organized programme for monitoring porcine brucellosis but some industrial breeders screen animals by serological testing and slaughter to control the disease [16]. The use of Rev. 1 vaccine to control goat infection was authorized at the end of 2006 [34].
B. suis was isolated more frequently from humans during the first period (1968–1991). B. suis 1a, observed since 1980 [4], and also isolated in Brazil, Colombia, Cuba and Peru, represented almost half of B. suis in humans in both sampling periods. These strains were mainly from Buenos Aires, where cattle production is by far the most relevant followed by sheep farming [16]. Although B. abortus was the second most frequently isolated during the first period, only 69 cases were found in Buenos Aires and 75 cases in the entire country in 1994–2006. This epidemiological change was probably due to the success of cattle vaccination with vaccine strain B. abortus S19. In Argentina new legislation for the control and prevention of bovine brucellosis was introduced in 1993 resulting in a significant increase in vaccinated animals across the country [16]. This vaccine strain was isolated from only a single human case during the first period and from five cases during the second period, confirming its potential pathogenicity for humans.
B. melitensis was more frequently isolated in the second period, mostly in CU and the HP region. Of 145 B. melitensis strains recovered, eight from northwest provinces had atypical phenotypic characteristics and based on a difference in the quantitative distribution of the ‘A’ and ‘M’ antigens were classified into two biovar subtypes [28]. The two B. melitensis bv. 3 strains [35, 36] were the first to be isolated from humans in Argentina but it is the main biovar in Southern Europe and the Middle East. Almost all 189 B. canis strains isolated from dogs in 1996–2006 were from Buenos Aires (97·8%), with a peak in 1999–2000 and 2005–2006 coinciding with outbreaks in breeding kennels. This suggests that sanitary control in some kennels is not sufficiently effective [37]. Human infection caused by B. canis has been reported and currently tests are performed on patients for serum antibodies to rough Brucella strains [e.g. rapid slide agglutination test (RSAT) and IELISA using B. canis antigen], when brucellosis is suspected [25, 38].
In conclusion, although the true incidence of human brucellosis is unknown in Latin American countries, B. melitensis remains the principal cause of infection, while B. suis causes substantial morbidity in Argentina. The isolation of Brucella from humans reflects its presence in the animal population. Human infection is assumed to be frequently under-diagnosed because clinical symptoms may be confused and isolation procedures are not routinely applied, therefore care should be taken when considering the geographic distribution of the pathogen and its species. These data provide epidemiological information as guidelines for future control programmes, as well as informing of a new B. melitensis variant and a B. suis strain resistant to dyes in a manner atypical for this species.
ACKNOWLEDGEMENTS
We are very grateful to Deborah B. Hasan for helpful assistance with the typing of the strains and to Dr Klaus Nielsen at the Canadian Food Inspection Agency, Animal Research Institute, Ontario, Canada, for critically reading the manuscript.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Corbel MJ, Young EJ, Corbel MJ. Brucellosis: Clinical and Laboratory Aspects. Boca Raton, FL: CRC; 1989. Brucellosis: epidemiology and prevalence worldwide; pp. 25–40. , pp. [Google Scholar]
- 2.Corbel MJ. Brucellosis: an overview. Emerging Infectious Diseases. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office International des Epizooties, World Animal Health Organization 1996. http://www.oie.int/hs2/report.asp http://www.oie.int/hs2/report.asp . Annual reports.
- 4.García Carrillo C. Animal and Human Brucellosis in the Americas. Paris: Office International des Epizooties; 1990. p. 296. , pp. [Google Scholar]
- 5.López-Merino A, Young EJ, Corbel MJ. Brucellosis: Clinical and Laboratory Aspects. Boca Raton, FL: CDR; 1989. Brucellosis in Latin America; pp. 151–161. , pp. [Google Scholar]
- 6.Moreno E. Brucellosis in Central America. Veterinary Microbiology. 2002;90:31–38. doi: 10.1016/s0378-1135(02)00242-0. [DOI] [PubMed] [Google Scholar]
- 7.Alton GG Techniques for the Brucellosis Laboratory. Paris, France: Institut National de la Recherche Agronomique; 1988. Bacteriological methods; pp. 13–61. , pp. [Google Scholar]
- 8.OIE. Manual of Standards for Diagnostic Tests and Vaccines. 5th edn. Paris: Office International des Epizooties; 2004. Identification of the agent. , chapters 2.3.1 and 2.6.2. [Google Scholar]
- 9.Osterman B, Moriyon I. International committee on systematics of procaryotes, subcommittee on the taxonomy of Brucella. Minutes of the meeting, 17 September 2003, Pamplona, Spain. International Journal of Systematic and Evolutionary Microbiology. 2006;56:1173–1175. [Google Scholar]
- 10.Verger JM et al. Brucella, a monospecific genus as shown by deoxyribonucleic acid hybridisation. International Journal of Systematic Bacteriology. 1985;35:292–295. [Google Scholar]
- 11.Verger JM et al. Taxonomy of the genus Brucella. Annales de l'Institut Pasteur. 1987;138:235–240. doi: 10.1016/0769-2609(87)90199-2. [DOI] [PubMed] [Google Scholar]
- 12.Cloeckaert A et al. Classification of Brucella spp. isolated from marine mammals by DNA polymorphism at the omp2 locus. Microbes and Infection. 2001;3:729–738. doi: 10.1016/s1286-4579(01)01427-7. [DOI] [PubMed] [Google Scholar]
- 13.Verger JM et al. Classification of Brucella strains isolated from mammals using DNA-DNA hybridisation and ribotyping. Research in Microbiology. 2000;151:797–799. doi: 10.1016/s0923-2508(00)01145-1. [DOI] [PubMed] [Google Scholar]
- 14.Solera J, Martinez-Alfaro E, Espinosa A. Recognition and optimum treatment of brucellosis. Drugs. 1997;53:245–246. doi: 10.2165/00003495-199753020-00005. [DOI] [PubMed] [Google Scholar]
- 15.Young EJ. An overview of human brucellosis. Clinical Infectious Disease. 1995;21:283–290. doi: 10.1093/clinids/21.2.283. [DOI] [PubMed] [Google Scholar]
- 16.Samartino LE. Brucellosis in Argentina. Veterinary Microbiology. 2002;90:71–80. doi: 10.1016/s0378-1135(02)00247-x. [DOI] [PubMed] [Google Scholar]
- 17.Alton GG, Jones LM, Pietz DE. Laboratory Techniques in Brucellosis. Geneva, Switzerland: World Health Organization; 1975. Bacteriological methods; pp. 11–67. . Monograph series 55. , pp. [PubMed] [Google Scholar]
- 18.Etemadi H et al. Isolation of Brucella spp. from clinical specimens. Journal of Clinical Microbiology. 1984;20:586. doi: 10.1128/jcm.20.3.586-.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagupsky P. Detection of Brucellae in blood cultures. Journal of Clinical Microbiology. 1999;37:3437–3442. doi: 10.1128/jcm.37.11.3437-3442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagupsky P, Nechama Peled, Press J. Use of Bactec 9240 blood culture system for detection of Brucella melitensis in synovial fluid. Journal of Clinical Microbiology. 2001;39:738–739. doi: 10.1128/JCM.39.2.738-739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia Carrillo C, Lucero NE, De Diego AI. Brucelosis Bovina. Buenos Aires, Argentina: Hemisferio Sur; 1993. Diagnóstico bacteriológico; pp. 97–109. , pp. [Google Scholar]
- 22.Corbel MJ, Brinley Morgan WJ, Krieg NR, Holt JG. Bergey's Manual of Systematic Bacteriology. Baltimore, MD: Williams & Wilkins Co.; 1984. Genus Brucella, Meyer and Shaw 1920; pp. 377–388. , pp. [Google Scholar]
- 23.Corbel MJ, Thomas EL. Use of phage for the identification of Brucella canis and Brucella ovis cultures. Research in Veterinary Science. 1985;35:35–40. [PubMed] [Google Scholar]
- 24.Vizcaino N et al. DNA polymorphism at the omp-31 locus of Brucella spp.: evidence for a large deletion in Brucella abortus, and other species-specific markers. Microbiology. 1997;143:2913–2921. doi: 10.1099/00221287-143-9-2913. [DOI] [PubMed] [Google Scholar]
- 25.Lucero NE et al. Unusual clinical presentation of brucellosis caused by Brucella canis. Journal of Medical Microbiology. 2005;54:505–508. doi: 10.1099/jmm.0.45928-0. [DOI] [PubMed] [Google Scholar]
- 26.Corbel MJ, Thomas EL, Garcia Carrillo C. Taxonomic studies on some atypical strains of Brucella suis. British Veterinary Journal. 1984;140:34–43. doi: 10.1016/0007-1935(84)90055-1. [DOI] [PubMed] [Google Scholar]
- 27.García Carrillo C, Turovetzky A, Lucero N. Brucella species and biotypes isolated from humans in Argentina: confirmation of human infection by B. abortus biotype 4 [in Spanish] Medicina (Buenos Aires) 1985;45:20–21. [PubMed] [Google Scholar]
- 28.Lucero NE et al. A new variant of Brucella melitensis. Clinical Microbiology and Infectious Diseases. 2006;12:593–596. doi: 10.1111/j.1469-0691.2006.01386.x. [DOI] [PubMed] [Google Scholar]
- 29.Baumgarten D. Brucellosis: a short review of the disease situation in Paraguay. Veterinary Microbiology. 2002;90:63–69. doi: 10.1016/s0378-1135(02)00246-8. [DOI] [PubMed] [Google Scholar]
- 30.Luna-Martinez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Veterinary Microbiology. 2002;90:19–30. doi: 10.1016/s0378-1135(02)00241-9. [DOI] [PubMed] [Google Scholar]
- 31.Poester PF, Gonçalvez PVS, Lage PA. Brucellosis in Brazil. Veterinary Microbiology. 2002;90:55–62. doi: 10.1016/s0378-1135(02)00245-6. [DOI] [PubMed] [Google Scholar]
- 32.Rivera SA, Ramirez MC, Lopetegui PI. Eradication of bovine brucellosis in the 10th Region de Los Lagos, Chile. Veterinary Microbiology. 2002;90:45–53. doi: 10.1016/s0378-1135(02)00244-4. [DOI] [PubMed] [Google Scholar]
- 33.Vargas OFJ. Brucellosis in Venezuela. Veterinary Microbiology. 2002;90:39–44. doi: 10.1016/s0378-1135(02)00243-2. [DOI] [PubMed] [Google Scholar]
- 34.Servicio Nacional de Sanidad y Calidad Agroalimentaria http://www.senasa.gov.ar. http://www.senasa.gov.ar . Sanidad Animal. Autorízase la importación, producción y el uso de la vacuna Rev. 1, en las condiciones que determinen la Dirección Nacional de Sanidad Animal y la Dirección de Laboratorios y Control Técnico. Resolución 216/2006. ( ). Accessed 26 April 2007.
- 35.Lucero NE, Greco G, Carrete P. Brucella melitensis biovar 3 en Argentina. Revista Argentina de Microbiología. 1999;31:58–59. (Suppl. 1); [PubMed] [Google Scholar]
- 36.Lucero NE IX Congreso Argentino de Microbiología. Buenos Aires: 2001. Brucelosis asociada a la ingestión, en el extranjero, de lácteos sin pasteurizar. . Poster 220. [Google Scholar]
- 37.Cabral M, Maldonado F, Di Lorenzo C. Comportamiento epidemiológico de la brucelosis canina en un criadero. Revista del Colegio de Veterinarios de la provincia de Buenos Aires. 2006;35:47–48. [Google Scholar]
- 38.Lucero NE et al. Diagnosis of human brucellosis caused by Brucella canis. Journal of Medical Microbiology. 2005;54:457–461. doi: 10.1099/jmm.0.45927-0. [DOI] [PubMed] [Google Scholar]


