SUMMARY
Surveillance activities for ovine scrapie have expanded in the 21st century, following concerns about the potential for a hidden epidemic of bovine spongiform encephalopathy in European sheep populations. Large-scale surveys have been used to estimate the prevalence of scrapie infection. In this study we analyse data from the surveys in Great Britain between 2002 and 2004. When we estimate genotype-specific prevalences for each of the two screening tests used a difference is observed. One test underestimates the number of positive cases in genotypes classically considered to be at a low relative risk of developing clinical disease (ARR- and AHQ-containing genotypes). By comparison, the other test underestimates the number of positive cases in genotypes classically considered to be at an increased relative risk of developing clinical disease (VRQ-containing genotypes). These findings have implications for surveillance, disease control, and diagnostic test evaluation.
INTRODUCTION
Historically the surveillance of scrapie has relied on confirmation of suspected clinical disease, otherwise known as ‘passive’ surveillance. There are several problems associated with such a system, not the least of which is the requirement for suspected cases to be reported in the first place. Hence national estimates derived from this surveillance stream are highly likely to be underestimates of the actual prevalence [1, 2]. In Great Britain an alternative approach was to investigate healthy slaughtered animals using a relatively small-scale abattoir survey [3]. More recently the European Commission (EC) required member states (MS) to implement routine surveillance of both healthy slaughtered sheep and ‘high-risk’ fallen stock [4]. The logistics of such large-scale surveillance programmes require the use of a rapid, sensitive screening test. Five tests were approved for use initially, although none were specifically designed or evaluated for use in pre-clinical ovine prion disease. Meanwhile, an evaluation of the new generation of diagnostic tests specifically for use in small ruminants was instigated. The results of this evaluation have recently been published [5]. Although the prion protein (PrP) genotype is known to be associated with the odds of developing clinical scrapie [6, 7], little is known about the odds, or relative risks, of infection.
The first statutory surveillance for sheep scrapie in Great Britain, to fulfil both national and EC requirements, was conducted from January 2002 until 31 March 2003. This surveillance exceeded the basic EC requirements in two aspects. First, all sheep sampled, both as fallen stock and healthy slaughtered animals, were genotyped. Second, the survey of healthy slaughtered sheep was subdivided into two populations. In one, only brainstem samples were taken and examined by one screening test, the Bio-Rad Platelia ELISA (Bio-Rad Laboratories, Hercules, CA, USA). Positive samples were subject to examination by immunohistochemistry (IHC). In the other population, whole brains were taken, as well as samples of lymphoreticular tissue, to enable an evaluation of sampling and testing protocols. The brain samples in this second population were screened using the Prionics® Check Western (CW) blot test (Prionics AG, Zurich, Switzerland). Samples both positive and negative with the Prionics CW were subject to examination using IHC. The details of this surveillance and basic results have been described previously [8–10]. This surveillance also revealed a number of ovine samples that were positive with the Bio-Rad Platelia ELISA test, but which were not confirmed by the standard immunohistochemical protocol using the anti-PrP rat monoclonal antibody R145 (VLA). There were 28 such samples in the 28911 tested. More importantly, the genotype distribution of these samples was skewed towards the more ‘resistant’ genotypes. Notably samples from sheep with the ARR/ARR haplotype, one that has not been observed in clinical scrapie cases in Great Britain, were found. Subsequent studies using modified R145 IHC protocols or the 2G11 antibody have revealed localized staining of PrP in the trigeminal nucleus (M. Simmons et al., unpublished observations) and forms of PrPSc that are relatively protease-sensitive [11]. These are now designated as ‘atypical’ scrapie [12].
These novel findings stimulated this additional study to investigate the outcome of the two screening tests used, with respect to the PrP genotype. The accumulated results of the surveillance for sheep scrapie in Great Britain from its inception, in January 2002, until 31 December 2004 have been used [9, 13]. Genotype-specific prevalences for each screening test by surveillance stream are estimated using data from the initial surveys, in which the PrP genotype frequencies of the denominator populations were determined. These parameters are then used to predict the number of positive test results stratified by PrP genotype that would have been expected in the total time period, if one or other screening test had been used throughout. The implication of the results for scrapie surveillance and control measures are highlighted in the Discussion.
METHODS
Data
Data was used from the seven ovine scrapie ‘active’ surveillance activities that were completed in Great Britain in the period January 2002 to December 2004 inclusive. They consist of all suitable samples for which both scrapie status and PrP genotype were determined, and are summarized in Table 1. For this reason, numbers may vary from those reported in other publications.
Table 1.
Summary of the seven ovine scrapie surveillance activities in Great Britain between January 2002 and December 2004 (inclusive) from which data was used in these analyses
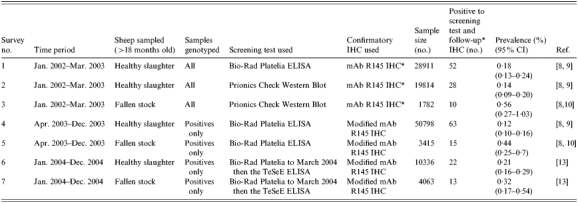
Monoclonal antibody (mAb) R145 immunohistochemistry (IHC) was later followed-up with modified mAb R145 and 2G11 IHC in any initially ‘unclassified’ samples.
Case definition
For the purpose of these analyses, a sample must have had a PrP genotype determined. A ‘positive’ is then a sample that first gave a positive result to any one of the screening tests used: the Bio-Rad Platelia ELISA, later known as the Bio-Rad TeSeE ELISA, and the Prionics CW. Second, the sample must have also given a positive result to IHC follow-up.
Data analysis
Data was manipulated in Microsoft Excel and the statistical software program stata 8 (StataCorp., College Station, TX, USA) was used for the statistical analysis.
The known frequency distributions were examined. The following assumptions were made in order to predict the unknown distributions as stated.
First, it was deemed unlikely that a significant shift in PrP genotype frequency would have occurred in the surveyed populations during the total time period studied. This assumption was based on the fact that the healthy-slaughter population tested for transmissible spongiform encephalopathies (TSE) in Great Britain is predominantly a cull ewe population [9, 13], and that the fallen-stock population will also consist of adult sheep. The National Scrapie Plan for Great Britain was launched in late 2001 and early 2002. Female offspring resulting from the use of rams and ram lambs that had their PrP genotypes determined in late 2001 and early 2002 would have been born in the 2002/2003 lambing season. Thus they would not be aged >18 months (the minimum age for inclusion in the surveys) until mid-2004. The earliest that they could enter the national breeding flock is the 2003/2004 mating season, where they could be expected to remain for at least 3–4 seasons. It is thus improbable that sufficient numbers to affect the PrP genotype frequency distribution would be submitted via either of the two surveillance streams until 2007/2008 at the earliest. It was, therefore, considered appropriate to assume that the sheep in the surveys up to the end of December 2004, subsequent to those conducted between January 2002 and March 2003, had a similar genotype distribution to those observed in surveys 1–3. The genotype frequency distributions from surveys 1 and 2 were combined to provide a standard ‘healthy-slaughter’ population stratified by genotype; i.e. the target population-specific genotype frequency for the period January 2002–March 2003 (inclusive) (Gi,s), where i is one of the 15 PrP genotypes, s is the target survey population (either healthy slaughter=1 or fallen stock=2) and t is the number of TSE-tested samples in time period m (January 2002–March 2003 inclusive=1; April 2003–December 2003 inclusive=2; January 2004–December 2004 inclusive=3) with screening test r (either Bio-Rad ELISA=1 or Prionics CW=2), is calculated thus:
| (1) |
The target population-specific genotype frequencies (Gi,s) were then used to predict the likely numerical genotype frequency distributions (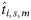 ) for each genotype i in each of surveys 4–7; i.e. the total number of TSE-tested samples with a PrP genotype determined (T) in survey population s during time period m was multiplied by Gi,s:
) for each genotype i in each of surveys 4–7; i.e. the total number of TSE-tested samples with a PrP genotype determined (T) in survey population s during time period m was multiplied by Gi,s:
| (2) |
Second, it was assumed that the test-specific prevalence of positives, stratified by genotype (Vi,s,r) could be estimated for each target population. For the healthy-slaughter population this was estimated directly from surveys 1 and 2, where pi,s,m,r is the number of positives of genotype i in the survey population s, time period m using screen test r
| (3) |
In the fallen-stock population direct estimation could be used for the Prionics CW in survey 3 [see equation (3) above] but for the Bio-Rad Platelia ELISA indirect estimation from surveys 5 and 7 was required:
| (4) |
Although the Bio-Rad TeSeE ELISA was used in later surveys, it was effectively a name change and not a different test. Hence it was assumed that the two versions of the Bio-Rad ELISA would give the same result; they are hereafter referred to as ‘Bio-Rad ELISAs’.
The third and final assumption was that by combining the relevant predicted genotype distributions with the estimated test-specific prevalence, stratified by genotype, for each target population, the number of positive results that would have been expected (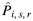 ) in each survey could be predicted for the screening test that was not used:
) in each survey could be predicted for the screening test that was not used:
| (5) |
Consequently, the number of positive cases, stratified by genotype could be predicted for the total time period (January 2002–December 2004 inclusive) for both screening tests. Using these assumptions and the known distributions, the PrP genotype distribution, the Bio-Rad ELISAs positive distribution by genotype, and the Prionics CW positive distribution by genotype were calculated for each of the populations in the EC proscribed surveillance streams. The fallen-stock population was comparatively small and liable to the most error; hence this prediction of the overall number of positive results was only attempted with the healthy-slaughter population.
Pearson's χ2 test with appropriate degrees of freedom was used to compare frequency distributions and overall prevalence estimates.
RESULTS
The genotype frequency distribution of all suitable samples in the two surveillance activities (surveys 1 and 2) between January 2002 and March 2003 did not appear to be biased with respect to the survey population sampled (Table 2). One would not expect there to be a difference as both surveys are derived from the same population – healthy slaughter. There was, however, some evidence (Pearson's χ2P<0·01, 14 d.f.) for a statistically significant difference. Differences in the observed proportions occurred in genotypes present at very low frequencies: namely ARH-containing genotypes (excluding ARH/ARH) and AHQ/AHQ. It was considered that this was due to small numbers, sampling variation and the difference that a single sample can make in such a situation, and that in larger samples this effect would not be observed. Thus, it was deemed valid to combine the genotype-specific frequencies to provide a standard ‘healthy-slaughter’ population stratified by genotype with which to predict the expected genotype frequency distributions in surveys 4 and 6.
Table 2.
Frequency distribution (number and %) by PrP genotype of all suitable samples in the British ovine scrapie healthy-slaughter and fallen-stock surveillance activities from January 2002 to March 2003 tested with either the Bio-Rad Platelia ELISA (Bio-Rad PE) or Prionics Check Western blot (Prionics CW) rapid screening tests
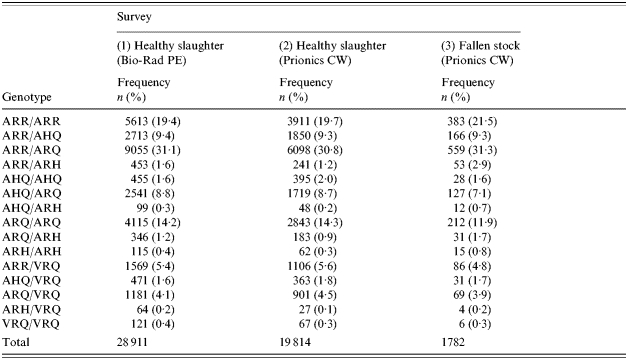
The frequency distribution of genotypes in all suitable samples in the third surveillance activity in this period (survey 3) is taken from a different population – fallen stock. There was statistical evidence (Pearson's χ2P<0·01, with 28 and 14 d.f. respectively) for a difference when the distribution in survey 3 was compared with those of surveys 1 and 2, and the combined standard ‘healthy-slaughter’ population. Differences occurred with several genotypes. As it is probable that ‘fallen stock’ does represent a different population, the resultant frequency distribution was not combined with the healthy-slaughter population. The fallen-stock sample of survey 3, stratified by genotype, was used as the standard in order to predict the expected genotype frequency distributions in surveys 5 and 7.
Within each target population, there was no evidence for a statistically significant difference in the observed prevalence of positives in the different surveys. Thus, within each target population there was no statistically significant difference in the overall prevalence estimates between surveys that used different screening tests. There were, however, notable differences in the genotype-specific distribution of the positives. The test-specific prevalence of positives, stratified by genotype was estimated for each target population (Table 3). Bio-Rad ELISA positives occurred in a range of genotypes, including those traditionally considered to be at low risk of developing clinical disease (namely ARR/ARR ARR/AHQ, ARR/ARQ, AHQ/AHQ and AHQ/ARQ), whilst the Prionics CW positives were restricted to the VRQ-containing genotypes (ARR/VRQ, ARQ/VRQ, ARH/VRQ and VRQ/VRQ) plus ARQ/ARQ. In the VRQ-containing genotypes the Prionics CW genotype-specific prevalence estimates were greater than those of the Bio-Rad ELISAs.
Table 3.
Test-specific prevalence estimates and 95% confidence intervals (95% CI) for each British ovine scrapie surveillance target population stratified by PrP genotype, estimated from the survey data of January 2002 to March 2003 (inclusive) except for the column in italics where prediction of the genotype frequency distribution from the standard fallen-stock population was required in order to estimate the denominator, and positive test results from the fallen-stock surveys between April 2003 and December 2004 were used as the numerator
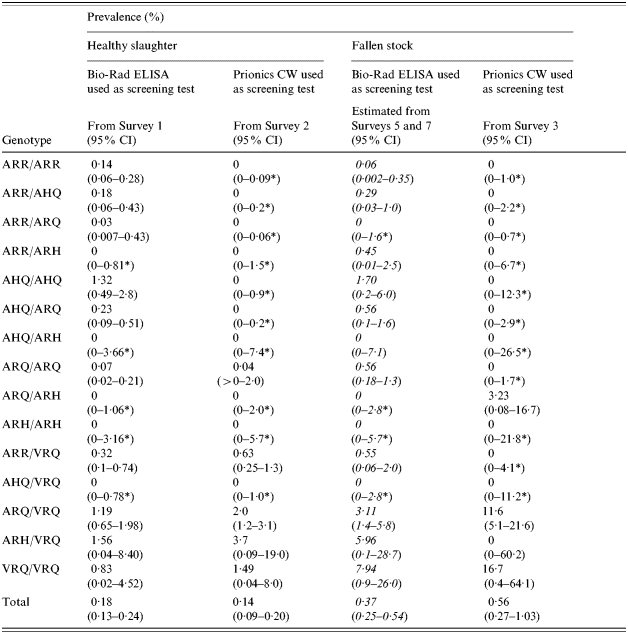
One-sided 97·5% CI.
Within the target population of healthy slaughter, the estimated number of expected positive test results for each screening test, across the four surveys (1, 2, 4 and 6) in the total time period, stratified by genotype were calculated (Table 4). The difference in the genotype distribution of the expected positive test results is apparent. Once again, the overall test-specific prevalence estimates, mask this genotype effect [0·14% (95% CI 0·12–0·17) and 0·18% (95% CI 0·16–0·21)] for the Prionics CW and the Bio-Rad ELISAs respectively [Pearson's χ2 (1 d.f.) P=0·022].
Table 4.
The predicted genotype and test-positive frequency distributions (number) for the complete British ovine healthy-slaughter population surveyed for scrapie during the period 2002–2004 including overall predicted prevalence estimates and 95% confidence intervals (95% CI), by screening test [Bio-Rad ELISA and Prionics Check Western blot (Prionics CW)]. Estimated odds ratios by PrP genotype for clinical (classical) scrapie from a published reference is indicated, as are the genotypes in which atypical cases have been found in Great Britain
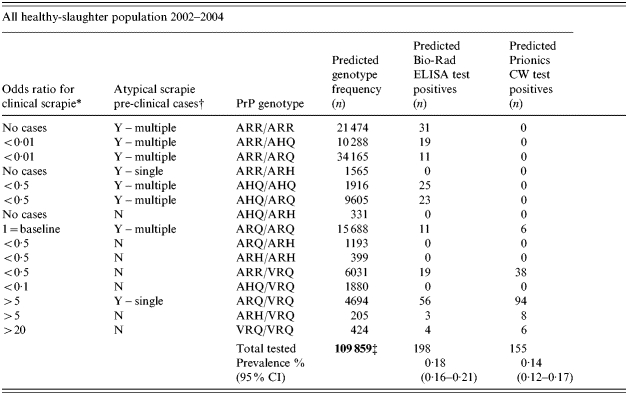
Y, yes; N, no.
Adapted from ref. [7].
Pre-clinical atypical scrapie cases were detected in these genotypes in the ovine healthy-slaughter and fallen-stock scrapie surveillance populations in Great Britain during the period 2002–2004.
Actual number.
The overall prevalence estimate remains similar, whichever screening test is used: the disparities between the two tests are only seen when genotype-specific distributions are examined. The sole use of the Bio-Rad ELISAs as a screening test would apparently have resulted in an underestimate of the prevalence (and therefore numbers) of scrapie-infected sheep in the traditional VRQ-containing genotypes when compared to the Prionics CW. If the Prionics CW had been used as the sole screening test it apparently would not have detected the affected animals in the more traditionally ‘resistant’ or at ‘low risk of clinical disease’ genotypes.
DISCUSSION
Surveillance of incompletely understood diseases that occur at low prevalence and have long incubation periods will always present challenges.
The introduction of wide-scale surveillance inevitably results in improved estimates of prevalence and can possibly identify the full spectrum of infection. This appears to be the case with the enhanced scrapie surveillance. The results from scrapie surveillance in Great Britain between 2002 and 2004 indicate that if either the Bio-Rad ELISAs or the Prionics CW screening tests were used on their own there would have been an underestimate of the prevalence of PrPSc-infected sheep. However, this only becomes apparent when the surveillance results are examined in the context of PrP genotype. The early Bio-Rad ELISA screening tests underestimated the prevalence of VRQ scrapie, but detected PrPSc infection in the, previously designated, ‘resistant’ or ‘low-risk’ genotypes based on studies of clinical scrapie [6, 7].
A possible explanation for this finding is that the Bio-Rad ELISA misclassifies infected sheep of these VRQ-bearing genotypes due to incorrect sampling sites, sampling error and limited PrPSc distribution in early pre-clinical sheep of relevant genotypes. In ‘classical’ scrapie, early PrPSc deposits can be limited to the margins of the dorsal nucleus of the vagus (DNV), which is situated close to the midline. A para-sagittal division of the obex or brainstem (as used in the first survey Bio-Rad ELISA survey – survey 1) may result in a specimen for rapid testing that omits the relevant area, leading to a negative test result. The DNV also does not always extend cranially and caudally sufficiently for detection to occur, particularly when limited PrPSc distribution is combined with a very rostral or caudal sample. In recognition of this potential problem, the sampling protocol for later surveys was changed. Genotype distributions of positive samples and prevalence estimates in these later surveys are, however, similar to those in the early surveys. This fact alone would tend to negate the sampling argument as a reason for the substantial numbers of VRQ scrapie infections apparently undetected by the Bio-Rad ELISAs. In addition, the protocol for surveys that used the Prionics CW also involved hemi-sectioning of the caudal medulla. Any omissions of the target site due to sampling error should therefore have been relatively consistent in the initial surveys and not associated with the screening test used. In order to resolve this matter, an investigation into the rates of sampling error and of occurrence of pre-clinical cases with such limited PrPSc distribution has been carried out (data not shown). The results indicate that the observed genotype-specific differences in numbers of positives detected by the two screening tests cannot be accounted for by these factors alone. Whatever the reason, the Prionics CW is more efficient at detecting VRQ scrapie, but does not detect some of the cases in the low-risk genotypes; those which are currently termed ‘atypical scrapie’.
These genotype-specific differences between the two most commonly used screening tests for scrapie surveillance in this time period, have implications both for surveillance and diagnostic test evaluation. As a result of mandatory scrapie surveillance in EU Member States (MS), several MS have recently identified these ‘atypical’ scrapie cases. Such cases have only been identified in MS using the Bio-Rad ELISA screening tests. These MS include Portugal, France, Germany, Belgium, Ireland and Great Britain [14–17]. Given the results presented here (i.e. the numbers of expected test-positives in VRQ genotypes with the Prionics CW as the rapid screening test are double those of expected test-positives in VRQ genotypes with the Bio-Rad ELISA screening test), these countries could have seriously underestimated the extent of infection within VRQ genotypes and hence the ‘classical’ scrapie population, within their respective surveys.
It could be argued that estimates of the prevalence of scrapie will, for many reasons, always be underestimates. However, the aim should be to obtain the best estimates possible in the circumstances, given limiting factors and with a testing regime that best suits the intended purpose. Whilst the enhanced surveillance was originally introduced to provide estimates of prevalence, the results are often used for case finding. A positive result initiates tracing procedures and control action is implemented in identified affected flocks [4]. With the use of the early Bio-Rad ELISAs as the screening test, not only were authorities presented with the challenge of what control action to take in flocks identified as the source of origin of an ‘atypical’ case, but affected ‘classical’ scrapie-affected flocks will have gone undetected. It is often stated that a screening test should have a good diagnostic sensitivity and be ‘fit for purpose’ [5]. From the observations reported in this study not only does the question arise of the diagnostic sensitivity of these two screening tests at an individual animal level dependent on PrP genotype, but also (and perhaps more importantly) that of: ‘What is the current purpose of the TSE monitoring or surveillance programme?’
Is it to provide a prevalence estimate for ovine scrapie, or one for ovine prion abnormalities? Is it to detect individual cases of either ‘classical’ scrapie, or ‘atypical’ prion disease, or both? And will that case detection then be used for the instigation of control measures? If the latter is the situation then consideration will also need to be given to other epidemiological issues, such as herd-level sensitivity.
The comparison of prevalence estimates between surveys using different tests whether it is within country or between countries will also be compromised. Amongst the myriad of other factors, the genotype distribution of the denominator population and the screening test used will need to be considered. Due to cost, resource and logistical implications, the genotype distribution data for the denominator population is often not determined: without it the disparities observed in this study would not have been detected. This study also highlights the need to monitor surveillance protocols and sampling methodologies carefully. Even though the sample error and pre-clinical limited distribution cases do not occur at sufficiently high rates to account for such discrepancies, without such relevant information it would be difficult to assess potential causes of emerging challenges.
For the genotypes in which both the Bio-Rad ELISAs and the Prionics CW detect positive cases, we do not yet know the extent, if any, of the overlap of detection by the two tests. Whether there is a biochemical, molecular or strain basis for this genotype-specific difference between the two tests also remains be elucidated. Whilst the ‘atypical’ cases have been intensively studied, revealing the relative protease sensitivity [11, 18], the association with codon 141 polymorphisms [19, 20] the immunohistochemical and biochemical differences [14, 17] and the experimental transmissibility to transgenic mice [18], little attention has been paid to the test variation observed in the VRQ-containing genotypes and its potential causes.
Meanwhile, the conclusion from these analyses indicates that there are at least two, possibly overlapping, populations of detectable PrP infection in sheep that are dependent on genotype. These may represent different forms of PrP infection, such as scrapie ‘strains’ or some other molecular variation. In vitro conversion of PrP has been shown to vary with PrP genotype [21]. Just as important is the conclusion that any evaluation and validation of available screening tests must take account of PrP genotype and be conducted in the appropriate target population. At the time of these surveys, the most precise estimate of the prevalence of PrPSc infection in sheep would have been best obtained from the use of both the Prionics CW and the Bio-Rad ELISAs as screening tests. Even then, the resultant estimate would still have been an underestimate. Future diagnostic tests, if evaluated with respect to genotype, should go some way to addressing some of the anomalies identified in this study. The results and recommendations of the evaluation exercise for the current generation of rapid tests, specifically for use in small ruminants, have recently been published by EFSA [5]. In order to monitor their performance in ‘the field’ adequate data on relevant variables, such as PrP genotype distribution in the denominator population, should continue to be collected. Much has been made of the apparent differences in sensitivity between the two screening tests used in early scrapie surveillance programmes [14, 22]. Often this is only determined with respect to test positive samples; without some examination of negative samples the sensitivity (number of positive animals identified as such) cannot be determined.
ACKNOWLEDGEMENTS
The Department for the Environment, Food and Rural Affairs funds ovine TSE surveillance in Great Britain and the work of the authors. The contributions of all those involved in ovine TSE surveillance in Great Britain is gratefully acknowledged as are those of Dr Danny Matthews, for constructive comments on the initial manuscript; Dr Marion Simmons for discussions and data on the influence of the pathological aspects and sampling error and Dr Colin Birch for the mathematical formulae.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Hoinville LJ et al. Descriptive epidemiology of scrapie in Great Britain: results of a postal survey. Veterinary Record. 2000;146:455–461. doi: 10.1136/vr.146.16.455. [DOI] [PubMed] [Google Scholar]
- 2.Sivam SK et al. Descriptive analysis of the results of an anonymous postal survey of the occurrence of scrapie in Great Britain in 2002. Veterinary Record. 2006;158:501–506. doi: 10.1136/vr.158.15.501. [DOI] [PubMed] [Google Scholar]
- 3.Simmons MM et al. Scrapie surveillance in Great Britain: results of an abattoir survey, 1997/98. Veterinary Record. 2000;146:391–395. doi: 10.1136/vr.146.14.391. [DOI] [PubMed] [Google Scholar]
- 4.EC. 2001. pp. 1–40. ). Regulation No. 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain Transmissible Spongiform Encephalopathies. OJ L147 of 31.05.2001, pp. and subsequent amendments.
- 5.EFSA 2005. . EFSA Scientific Report ( ), 31 1-17 on the evaluation of rapid post mortem TSE tests intended for small ruminants.
- 6.Baylis M et al. Risk of scrapie in British sheep of different prion protein genotype. Journal of General Virology. 2004;85:2735–2740. doi: 10.1099/vir.0.79876-0. [DOI] [PubMed] [Google Scholar]
- 7.Tongue SC et al. Estimation of the relative risk for developing clinical scrapie: the role of prion protein (PrP) genotype and selection bias. Veterinary Record. 2006;158:43–50. doi: 10.1136/vr.158.2.43. [DOI] [PubMed] [Google Scholar]
- 8.Wilesmith JW, Matthews D, Ryan J 2003. http://www.defra.gov.uk/animalh/bse/publications/reports/SheepSurveyrpt.pdf. http://www.defra.gov.uk/animalh/bse/publications/reports/SheepSurveyrpt.pdf . Summary of the results of scrapie surveillance in sheep in Great Britain, January 2002–March 2003. ). Accessed November 2004.
- 9.Elliott H et al. Prevalence of scrapie in sheep in Great Britain estimated from abattoir surveys during 2002 and 2003. Veterinary Record. 2005;157:418–419. doi: 10.1136/vr.157.14.418. [DOI] [PubMed] [Google Scholar]
- 10.Del Rio Vilas V et al. Prevalence of scrapie in sheep: results from fallen stock surveys in Great Britain in 2002 and 2003. Veterinary Record. 2005;157:744–745. doi: 10.1136/vr.157.23.744. [DOI] [PubMed] [Google Scholar]
- 11.Everest SJ et al. Atypical prion protein in sheep brain collected during the British scrapie surveillance programme. Journal of General Virology. 2006;87:471–477. doi: 10.1099/vir.0.81539-0. [DOI] [PubMed] [Google Scholar]
- 12.Anon. Opinion of the Scientific Panel on Biological Hazards on the request from the European Commission on classification of atypical Transmissible Encephalopathy (TSE) cases in Small Ruminants. EFSA Journal. 2005;276:1–30. [Google Scholar]
- 13.Wilesmith JW http://www.defra.gov.uk/animalh/bse/publications/reports/SheepSurvey2.pdf. http://www.defra.gov.uk/animalh/bse/publications/reports/SheepSurvey2.pdf Summary of the results of scrapie surveillance in sheep in Great Britain, 2004 ( ). Accessed April 2005.
- 14.Buschmann A et al. Atypical scrapie cases in Germany and France are identified by discrepant reaction patterns in BSE rapid tests. Journal of Virological Methods. 2004;117:27–36. doi: 10.1016/j.jviromet.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Orge L et al. Identification of putative atypical scrapie in sheep in Portugal. Journal of General Virology. 2004;85:3487–3491. doi: 10.1099/vir.0.80246-0. [DOI] [PubMed] [Google Scholar]
- 16.Onnasch H et al. Two Irish cases of scrapie resembling Nor98. (2004) Veterinary Record. 2004;155:636–637. doi: 10.1136/vr.155.20.636. [DOI] [PubMed] [Google Scholar]
- 17.De Bosschere Het al. TSE detected in a Belgian ARR-homozygous sheep via active surveillance. Veterinary Journal2007173449–451. [DOI] [PubMed]
- 18.Le Dur A et al. A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proceedings of the National Academy of Sciences USA. 2005;102:16031–16036. doi: 10.1073/pnas.0502296102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moum T et al. Polymorphisms at codons 141 and 154 in the ovine prion protein gene are associated with scrapie Nor 98 cases. Journal of General Virology. 2005;86:231–235. doi: 10.1099/vir.0.80437-0. [DOI] [PubMed] [Google Scholar]
- 20.Saunders GC et al. PrP genotype of atypical scrapie cases in Great Britain. Journal of General Virology. 2006;87:3141–3149. doi: 10.1099/vir.0.81779-0. [DOI] [PubMed] [Google Scholar]
- 21.Bossers A, de Vries R, Smits MA. Susceptibility of sheep for scrapie as assessed by in vitro conversion of nine naturally occurring variants of PrP. Journal of Virology. 2000;74:1407–1414. doi: 10.1128/jvi.74.3.1407-1414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morignat E et al. Estimates of the prevalence of transmissible spongiform encephalopathies in sheep and goats in France in 2002. Veterinary Record. 2006;158:683–687. doi: 10.1136/vr.158.20.683. [DOI] [PubMed] [Google Scholar]


