SUMMARY
This study aimed to: (1) investigate whether non-ruminant wildlife interfacing with dairy sheep and goats of four Greek flocks endemically infected with Mycobacterium avium subspecies paratuberculosis (MAP) harboured MAP and (2) genetically compare the strains isolated from the wildlife to those isolated from the small ruminants of these flocks. We cultured and screened, by polymerase chain reaction (PCR), pooled-tissue samples from 327 wild animals of 11 species for the MAP-specific IS900 insertion sequence. We also cultured faecal samples from 100 sheep or goats from each of the four flocks. MAP was detected in samples from 11 sheep, 12 goats, two mice, two rats, a hare and a fox. Only one rat had histopathological findings. Genetic typing categorized 21 isolates as cattle-type strains and two, from a house mouse and a goat respectively, as sheep-type strains; this is the first report of a rodent harbouring a sheep-type strain. The MAP types that were most frequently isolated amongst the sheep and goats of each flock were also the ones isolated from sympatric rodents; those isolated from the fox and hare also belonged to the predominant ruminant strains.
INTRODUCTION
Spillover of infectious organisms from domestic herds to wildlife is of concern to wildlife managers and livestock keepers, both because it affects wildlife health and because wild animals may serve as potential reservoirs or disseminators of infection to livestock. Wildlife may become exposed to Mycobacterium avium subsp. paratuberculosis (MAP) via feeding on contaminated grain, forage in pastures, faeces, or on infected prey. Potential routes of transmission of MAP from wildlife back to livestock include faecal contamination of feed and forage, both in farm buildings and on pastures [1].
Paratuberculosis has long been considered a disease of ruminants only. Apart from domestic ruminants, the disease is also well documented in several wild ruminants [2–6]. It is only recently that isolations of MAP from non-ruminant wildlife species, with or without typical pathology suggesting natural paratuberculosis, are increasingly being reported. MAP has been isolated from lagomorphs [7–10], rodents, badgers, racoons, nine-banded armadillos, opossums, northern short-tailed shrew, striped skunks [10–12], wild boars [13, 14], a rhinoceros [15] and several bird species [10–12]. The typical pathology of paratuberculosis has been noted in wild rabbits, wood mice, foxes, stoats, weasels and crows [8, 11, 16–19].
DNA-based subtyping techniques, such as IS900 restriction fragment length polymorphism (IS900 RFLP), IS1311 polymerase chain reaction–restriction endonuclease analysis (IS1311 PCR–REA) and pulsed-field gel electrophoresis (PFGE) have been applied to reveal the genetic variation of MAP isolates and differentiate among strains infecting different populations [20–23]. Based on these and the cultural characteristics, two major groups of MAP strains have been identified, the sheep or Type-I strains, including slow-growing strains that appear to have a strong host preference for sheep, and the cattle or Type-II strains, including faster growing strains commonly isolated from cattle and a broad host range including humans.
Most Greek dairy sheep and goat flocks are kept under semi-intensive management systems. Typically, animals graze on pastures throughout most of the year and are offered a concentrate supplement. They spent most of the day outside and are moved into their sheds during the night. Most of these flocks are endemically infected with paratuberculosis [24]. Their management provides several opportunities for inter-species transmission of MAP between sheep, goats and wildlife which cohabit the same pastures or sheds. Before this study commenced, Greig et al. [17, 18] showed that wild rabbits living on the grazing grounds of beef cattle in Scotland harboured and excreted MAP, and, therefore, potentially acted as a maintenance reservoir for the infection in these farms. A similar scenario is possible in Greek dairy sheep and goat flocks, where other non-ruminant wildlife, such as rodents, hares and foxes, that frequently cohabit the sheds (rodents) and the grazing grounds (rodents, hares, foxes), may be involved. However, this possibility had never been investigated before. In this study we screened for MAP non-ruminant wildlife that lived in the sheds and on the grazing grounds of endemically infected dairy sheep and goat flocks. At the same time, we screened for MAP samples collected from the sheep and goats of these flocks. The strains isolated from the small ruminants and the wildlife were genetically characterized by IS1311 PCR–REA.
METHODS
Study site and collection of samples
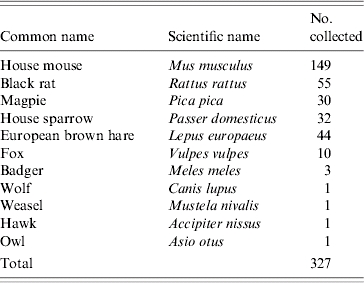
Four dairy sheep and goat flocks, each with 250–350 animals, located in Central Greece (longitude: +39° 22′, latitude: +22° 00′), situated at a distance of >10 km away from each other, were selected as study sites. One flock consisted entirely of sheep while another consisted entirely of goats with the remaining two flocks mixed, one with 60:40 and the other with 40:60 sheep:goat ratios. All flocks had a history of clinical paratuberculosis, but vaccination against MAP had never been applied. The sheep and goats were kept under semi-intensive management, for the purposes of milk production. The farmers selected breeding replacements amongst the daughters of the high-yielding ewes and goats. The males bought into the flocks originated from high-yielding animals from other flocks. The annual replacement rate in these flocks was around 20%, which was similar to the annual culling rate; this was because farmers received subsidies on the basis of flock size. The flocks were intensively monitored for 2 years. Prior to launching the study, the flock owners were informed about the study's tasks and accepted that two of the authors (P.K and M.F.) would visit their flock sheds to trap wildlife every second day from September 2001 to June 2003. The monitoring did not interfere with routine management or affect management decisions in any way. Prior to sampling wildlife, the endemic level of infection in the flocks was confirmed both serologically, on sera obtained from 100 animals per flock randomly selected among animals aged >1 year (IDEXX ELISA test; IDEXX Laboratories, Portland, ME, USA), and bacteriologically, on gut samples collected at slaughter from animals aged >1 year with macroscopic lesions suggestive of paratuberculosis. During the study period, a total of 327 wild animals, from 11 species (Table 1), either were captured and euthanized, by using authorized, species-appropriate methods (live-captured traps and shooting), or were found dead in the sheds (mice, rats, house sparrows) or on the grazing grounds of the flocks (badgers, foxes, hares, magpies, a wolf, a weasel, a hawk and an owl). All animals were subjected to full necropsy, during which all gross lesions of the intestine and other tissues were noted. The last parts of the duodenum and the ileum, the first parts of the caecum and the colon, the ileocaecal and the colic lymph nodes and the liver were collected, and pooled for each animal, put into sterile bags and preserved at −20°C for culture and direct IS900 PCR. Parts of all samples, except those originating from animals found dead in advanced autolysis, were fixed in 10% neutral buffered formalin for histopathology. In addition to wildlife sampling, from September 2002 to February 2003, 100 clinically healthy animals, aged >1 year from each flock, were faecally sampled. Faecal samples of ∼10 g were collected from the rectum of each animal. Samples were separated and put into sterile bags and preserved at −20°C for culture.
Table 1.
Wildlife trapped (rodents), shot (hares) or found dead in the sheds and on the grazing grounds of four endemically infected with MAP Greek sheep and goat flocks, from September 2001 to June 2003
Culture
Faecal and pooled tissue samples were cultured on Lowenstein–Jensen (LJ) medium, using a slight modification of the procedure described by Whitlock & Rosenberger [25], and on Herrold's Egg-Yolk medium (HEYM), using the procedure described by Greig et al. [17]. More specifically, for culture on LJ medium, 2–5 g faecal samples from sheep or goats were placed in a tube with 15 ml sterile normal saline, mixed and allowed to stand for 30 min. From wildlife, 0·5 cm3 of finely chopped tissues were homogenized for 30 s in 10 ml sterile distilled water with a Colworth Stomacher 80 (Seward Medical, London, UK). Subsequently, 5 ml of the faecal supernatants and the tissue homogenates were transferred to a fresh tube containing 25 ml of 0·9% hexadecylpyridinium chloride (Sigma-Aldrich, Athens, Greece) in half-strength brain heart infusion (BHI) broth (Oxoid, Basingstoke, UK) and allowed to stand at 37°C for 24 h. Subsequently, the supernatants were centrifuged at 1000 g for 30 min and the formed pellet was collected and re-suspended in 1 ml half-strength BHI broth, containing vancomycin (100 mg/ml), nalidixic acid (100 mg/ml) and amphotericin B (50 mg/ml) (all Sigma antibiotics). The tube containing the pellet with the mixture of antibiotics was incubated for 48 h at 37°C. The prepared sediment was divided in three equal portions and ∼300 μl (six drops) were inoculated onto each of three slopes of LJ medium (two with mycobactin and pyruvate, and one plain).
For culture on HEYM, faecal samples (1–2 g) or 0·5 cm3 of finely chopped tissues were homogenized for 30 s in 10 ml sterile distilled water with a Colworth Stomacher 80 (Seward Medical). The homogenates were decontaminated by adding 10 ml of 1·5% hexadecylpyridinium chloride (Sigma-Aldrich) and left to stand overnight at room temperature. The resulting supernatants were centrifuged at 704 g for 20 min at 4°C, and each pellet was re-suspended in 10 ml sterile distilled water. The centrifugation step was repeated, and each formed pellet was re-suspended in 1 ml sterile distilled water. Suspensions were then transferred to a microfuge tube and centrifuged at 13 226 g for 5 min at room temperature. The pellets were re-suspended in 0·5 ml sterile distilled water. Following decontamination procedures, two slants of HEYM supplemented with mycobactin J and antibiotics (Becton Dickinson product code: 222233), were inoculated with 0·1 ml of the prepared suspension.
The inoculated slants were incubated for at least 7 months at 37°C. Growth was determined, both visually and microscopically, at weeks 1, 2, 4, 6, 8, 10, 12, 16, 20, 24 and 30. Smears were prepared, using sterile procedures, from the inoculation surface of the medium and stained by the Ziehl–Nielsen (ZN) method.
DNA extraction
MAP DNA extraction procedures were identical for IS900, IS1311 and IS1245 PCR assays. DNA extraction from pooled-tissue samples and from colonies grown on LJ medium was performed using the Genomic DNA Purification Kit (Puregene, Minneapolis, MN, USA), according to the manufacturer's instructions. To prepare DNA for analysis from HEYM cultures, one or two colonies were lifted off the media, taking care not to remove any media, added to 100 μl sterile water in a sterile 1·5 ml tube. The contents of the tube were mixed vigorously for 1 min on a vortex mixer (IKA-Works Inc. Wilmington, NC, USA), heated at 100°C for 30 min to lyse the bacteria and centrifuged at 15 339 g for 10 min. The supernatant was transferred to another sterile 1·5 ml tube and the pellet was discarded. DNA samples were placed at 4°C, when analysed immediately, or were stored at −20°C.
PCR and REA reactions
All culture isolates and pooled-tissue samples were subjected to PCR for the MAP-specific IS900 insertion sequence. Subsequently, IS1311 PCR followed by REA with HinfI and IS1245 PCR were performed on all IS900-positive isolates. The IS1311 PCR–REA was performed to discriminate cattle- and sheep-type strains. The IS1245 PCR was used to identify Mycobacterium avium subsp. avium (M. avium). The sequences of the primers used for each PCR are given in Table 2. All PCR reactions were performed as previously described [26, 27], using a slightly modified protocol. Each PCR reaction mixture contained 10 μl genomic DNA, 10 μl 10× PCR buffer (Invitrogen, Paisley, UK), 2 mm MgCl2 (Invitrogen), 200 μm of a dNTP mix (Invitrogen), 7·5 U (for IS900 PCR) or 11·25 U (for IS1311 and IS1245 PCR) of Taq polymerase (Invitrogen), and 30 pmol of the primers. The reaction volume was adjusted to 100 μl with DEPC-treated water. The thermal cycler parameters included an initial denaturation step at 94°C for 3 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 62°C for 30 s (for IS900 PCR) or 15 s (for IS1311 and IS1245 PCR), extension at 72°C for 2·5 min (for IS900 PCR) or 1 min (for IS1311 and IS1245 PCR) and a final extension of 5 min at 72°C. A total of 10 μl of each PCR product were analysed by electrophoresis on a 2% agarose gel and stained with ethidium bromide (0·5 μg/ml). A 100 bp DNA ladder (Invitrogen) was analysed on the same gel to serve as a size marker. The expected sizes of the products were 414 bp for IS900 PCR, 268 bp for IS1311 PCR and 198 bp for IS1245 PCR.
Table 2.
Summary of the target, the specificity and the sequences of the primer pairs, for IS900-, IS1311- and IS1245-PCR; the relevant references are included in square brackets
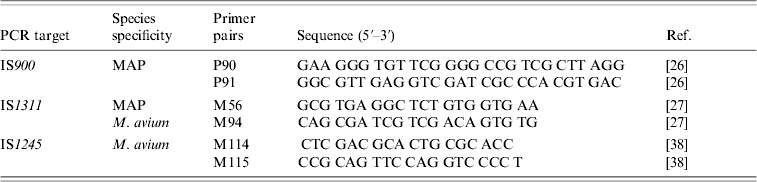
The IS900 PCR was used to identify the insertion sequence which is specific to MAP, the IS1311 PCR, followed by REA, was used to discriminate cattle from sheep-type strains, while the IS1245 PCR was used to identify the insertion sequence which is found only in M. avium.
All IS900 PCR products were gel-purified (Qiaquick Gel Extraction kit; Qiagen, West Sussex, UK) and sequenced in both directions by MWG Biotech (Ebersberg, Germany), using the forward and reverse PCR primers.
REA reactions were prepared, as described by Marsh et al. [27], by adding 10 μl IS1311 PCR product, 2 U HinfI, 1·6 μl of buffer (supplied with restriction endonuclease) and made up to 16 μl with sterile purified water. Restriction digests were incubated at 22°C for 2 h and were assessed by electrophoresis in 4% agarose gel prestained with ethidium bromide (0·5 μg/ml). The expected sizes of the REA products were 268, 218 and 50 bp for the cattle strain digestion pattern and 268 bp for the sheep strain digestion pattern.
Histopathology
The formalin-fixed samples were dehydrated through graded alcohols and embedded in paraffin wax. Then, sections were cut and stained with haematoxylin and eosin (HE), for routine histopathological examination and by the ZN method for detection of acid-fast bacteria (AFB).
RESULTS
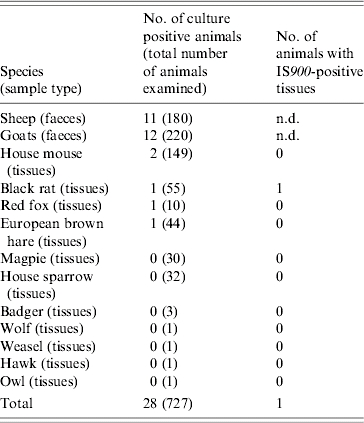
MAP was isolated from the samples of five wild animals and 23 small ruminants (Table 3). The isolates from three sheep, three goats and two house mice grew only on LJ medium and the remaining isolates only on HEYM. One faecal sample from a goat was positive on both culture media. All isolates were re-cultured on HEYM slants, but eventually only those initially grown on HEYM grew again.
Table 3.
Summary of results from the culture of faecal samples from clinically healthy sheep and goats of four flocks and of pooled tissue samples from wildlife
n.d., Not done.
Each pooled-tissue sample consisted of the last part of the duodenum and ileum, the first part of the caecum and colon, the ileocaecal and colic lymph nodes and the liver of each animal. The pooled-tissue samples were also screened by PCR for the MAP-specific IS900 insertion sequence. The isolates grew either on Lowenstein–Jensen medium or on Herrold's Egg-Yolk medium and were confirmed as MAP by IS900 PCR. The isolate from a goat faecal sample grew on both media. A pooled-tissue sample from a culture-negative black rat was positive to IS900 PCR.
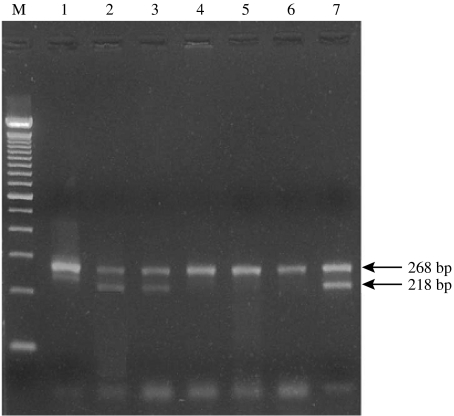
All isolates were positive for IS900. The IS900 insertion sequence was also detected in the pooled-tissue sample from one culture-negative black rat (Table 3). Sequence analysis of the IS900 insertion sequence confirmed that the isolates were indeed MAP isolates. IS1311 PCR–REA categorized 21 isolates into cattle-type and two into sheep-type strains (Table 4). The latter originated from a goat and a house mouse (Fig.). Seven isolates were not typed, possibly due to inadequate quantity of DNA. The positive samples from one of the mixed flocks originated from two sheep, a goat, a black rat and a house mouse, and were all classified as cattle-type strains. Those from the other mixed flock came from four goats, a sheep and a house mouse. The latter was classified as sheep-type and the former as cattle-type strains. In the goat-only flock, three goats were infected with cattle-type strains and one, which was positive in both culture media, by both strain types. The isolates from the sheep-only flock, were from four sheep and a black rat and belonged to the cattle-type strains (Table 4). The isolates from the fox and the hare, found dead and shot, respectively, on the grazing grounds of the flocks, were typed as cattle strains (Fig.). None of the isolated strains had the M. avium specific IS1245 insertion sequence.
Table 4.
Results of genetic characterisation with IS1311 PCR and REA with HinfI, of 23 of the isolates arranged by sampling location and origin of the isolate. The goat sample (from flock 2), which was positive on both culture media harboured both MAP types
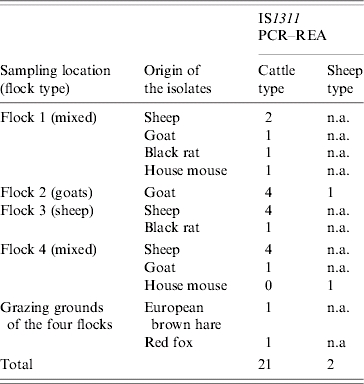
n.a., Non-applicable.
Fig.
Typical IS1311 PCR-REA patterns with HinfI-digested amplicons in adjacent lanes. MAP cattle-type strains (lanes 2, 3) recovered from a fox and a hare respectively and MAP sheep-type strains (lanes 4 and 5) recovered from a house mouse and a goat are shown. Lanes 1 and 6 represent positive controls for sheep-type strains (ileum from sheep previously positive by IS900 PCR and IS1311 PCR–REA), and lane 7 a positive control for cattle-type strains (MAP strain ATCC19698). Lane M, 100 bp ladder.
No gross lesions of paratuberculosis were noted at necropsy in any of the wildlife animals examined. The formalin-fixed samples from the IS900-positive wild animals were examined histologically. No AFB or granulomatous infiltrates consistent with MAP infection were seen in tissue, with the exception of one animal, a black rat. In this rat intestine there were small numbers of AFB-positive macrophages scattered throughout the intestinal lamina propria. Only rarely were >5 AFB seen in the cytoplasm of these cells.
DISCUSSION
We detected or isolated MAP from four different wildlife species cohabiting the sheds and the grazing grounds of paratuberculosis-affected flocks. Specifically, MAP was recovered from two black rats and two house mice trapped in the sheds and from a fox and a hare found dead or shot on the grazing grounds of the flocks. We failed to recover MAP from magpies, badgers, a wolf, a weasel, a hawk and an owl but the available number of animals from these species was very small (Table 1). We found no gross lesions of paratuberculosis in any of the wild animals examined. However, there were AFB in the intestinal macrophages of one culture-negative black rat, which was found to be IS900-positive by direct tissue PCR. This finding indicates a probable infection, not a ‘passing-through’ of recently ingested contaminated material. Beard et al. [11] also found numerous AFB-containing macrophages in a culture-positive wood mouse. Other authors have also noted the disagreement between histological and culture results [10, 11]. This has also been evident in experimentally infected mice and rats, which did not consistently develop clinical disease and histological lesions [28].
Stevenson et al. [23] characterizing a large battery of isolates from non-ruminant wildlife, by IS1311 PCR–REA, classified all of them as cattle or type-II strains. All but one of our isolates were also classified into the cattle group. However, the isolate from a house mouse belonged to the sheep group. To our knowledge this is the first report of a sheep-type strain isolated from non-ruminant wildlife. Therefore, it is likely that non-ruminant wildlife may be susceptible to infection with either type of MAP. The mouse was trapped in the shed of a flock where only cattle-type strains had been isolated from MAP-shedding sheep and goats. This may indicate either that our sampling and isolation protocols failed to detect infection of the flock with sheep-type strains or that the mouse was infected with MAP elsewhere. Evidently, the latter is epidemiologically more important. Whittington et al. [29] reported that the recovery of MAP from environmental samples in sheep and goat farms in New Zealand was very low 5 months after the infected stock were removed, but infected wildlife with longer lifespans, such as mice, rats, hares and foxes, may continue shedding at some level beyond this period of time.
Haydon et al. [30] suggested that genetic characterization of pathogens isolated from different populations provides a powerful tool for identifying key components of probable reservoirs. They proposed that a reservoir should be defined as one or more epidemiologically connected populations or environments in which the pathogen can be permanently maintained and from which infection may be transmitted to the defined target population. The animal populations in a reservoir may include different species which are directly or indirectly connected to each other. In Scotland, wild rabbits were initially suspected as a possible maintenance reservoir for paratuberculosis in beef cattle [31, 32], because molecular genetic typing by RFLP and PFGE could not discriminate between rabbit and cattle isolates from the same or different farms [18]. This was later proved to be the case through genetic characterization. Most of our isolates from the four flocks and the non-ruminant wildlife were classified as cattle-type strains. Interestingly, the majority of the wildlife isolates from each flock belonged to the same MAP type with the sheep and/or goat isolates from the same flock. The isolates from the fox and the hare also belonged to the predominant cattle-type strains among sheep and goats. The rodents probably received MAP infection through scavenging livestock feed on floors contaminated with livestock faeces. The fox probably became infected with MAP when consuming contaminated prey, e.g. rodents and hares. These findings indicate that inter-species transmission may have occurred and that rodents, foxes and hares and their environment may have a potential role in a complex maintenance reservoir for MAP infection of small ruminant populations. Daniels et al. [1, 33] reported that infected wild rabbits in Scotland excreted up to 4 million c.f.u./g faeces, an amount which was sufficient to constitute an infective dose, and that cattle did not avoid grazing on pasture contaminated by rabbit faecal pellets. Clearly, since the grazing behaviour of sheep and goats is different from cattle, further studies considering this and the pathogenesis of MAP in non-ruminant wildlife are needed to determine whether non-ruminant wildlife species represent true threats for infection of small ruminants. It is suggested that infected wildlife may have the greatest effect on the epidemiology of MAP infection on farms that have eliminated all infected livestock from the premises or on MAP-free farms located in the same geographic area as infected farms.
We isolated both MAP types from the faecal samples of a goat. Mixed infections with MAP strains, belonging to different RFLP types, have already been reported in a cow [34]. Moreover, Corn et al. [10] recovered three distinct short sequence repeat genotypes from an armadillo and two from a feral cat. The goat in our study belonged to a flock from which only cattle-type strains were recovered. This goat may have received this strain elsewhere, because the flock was not operating as a completely closed flock, or whilst grazing on communal pastures where different flocks have the potential to interchange different MAP strains. Of course, it is also possible that both MAP types existed in this and the other three study flocks, but we underestimated the prevalence of sheep-type strains because: (1) they are very difficult to culture, as there is still disagreement on the most appropriate culture media for MAP [35, 36] and (2) routine decontamination protocols decreased the number of sheep-type strains isolated [37].
In conclusion, we examined a wide range of wildlife species co-habiting four infected flocks of sheep and goats for the presence of MAP. We found that wildlife belonging to four different species (mice, rats, foxes and hares) and the domestic livestock exposed to this wildlife shed and/or harboured the organism. The mice harboured both MAP types, whereas, only cattle-type strains were detected in the other wildlife species. This is the first report of a sheep-type strain being isolated from non-ruminant wildlife. The MAP types most frequently isolated from the sheep or goats of each flock were also the ones isolated from sympatric rodents. These facts strongly suggest that inter-species transmission of MAP may have occurred and substantiate a potential role of wildlife in the epidemiology of sheep and goat paratuberculosis. Although further studies considering the grazing behaviour of sheep and goats and the pathogenesis of MAP in non-ruminant wildlife are needed to determine whether the non-ruminant wildlife species represent true threats for the infection of small ruminants, our results strongly indicate that the wildlife may have the greatest effect on farms that have eliminated all infected livestock from the premises or on MAP-free farms located in the same geographic area as infected farms.
ACKNOWLEDGEMENTS
This research was part of a project entitled ‘The role of wildlife in the epidemiology of Mycobacterium avium subspecies paratuberculosis in domestic ruminants in Europe’ funded by the European Commission (contract no. QLK2-CT-2001-00879). M.F. acknowledges financial support from the Greek Ministry of Education (Project ‘Herakleitos’).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Daniels MJ et al. Do non-ruminant wildlife pose a risk of paratuberculosis to domestic livestock and vice versa in Scotland? Journal of Wildlife Diseases. 2003;39:10–15. doi: 10.7589/0090-3558-39.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Williams ES, Spraker TR, Schoonveld GG. Paratuberculosis (Johne's disease) in bighorn sheep and a rocky mountain goat in Colorado. Journal of Wildlife Diseases. 1979;15:221–227. doi: 10.7589/0090-3558-15.2.221. [DOI] [PubMed] [Google Scholar]
- 3.Jessup DA et al. Paratuberculosis in tule elk in California. Journal of the American Veterinary Medical Association. 1981;179:1252–1254. [PubMed] [Google Scholar]
- 4.Chiodini RJ, Vankruiningen HJ. Eastern white-tailed deer as a reservoir of ruminant paratuberculosis. Journal of the American Veterinary Medical Association. 1983;182:168–169. [PubMed] [Google Scholar]
- 5.Delisle GW, Yates GR, Collins DM. Paratuberculosis in farmed deer: case reports and DNA characterization of isolates of Mycobacterium paratuberculosis. Journal of Veterinary Diagnostic Investigation. 1993;5:567–571. doi: 10.1177/104063879300500411. [DOI] [PubMed] [Google Scholar]
- 6.Buergelt CD et al. The pathology of spontaneous paratuberculosis in the North American bison (Bison bison) Veterinary Pathology. 2000;37:428–438. doi: 10.1354/vp.37-5-428. [DOI] [PubMed] [Google Scholar]
- 7.Mathews PRJ, Sargent A. The isolation of mycobacteria from the brown hare (Lepus europaeus) British Veterinary Journal. 1977;133:399–404. doi: 10.1016/s0007-1935(17)34041-1. [DOI] [PubMed] [Google Scholar]
- 8.Beard PM et al. Natural paratuberculosis infection in rabbits in Scotland. Journal of Comparative Pathology. 2001;124:290–299. doi: 10.1053/jcpa.2001.0466. [DOI] [PubMed] [Google Scholar]
- 9.Raizman EA et al. Mycobacterium avium subsp. paratuberculosis from free-ranging deer and rabbits surrounding Minnesota dairy herds. Canadian Journal of Veterinary Research. 2005;69:32–38. [PMC free article] [PubMed] [Google Scholar]
- 10.Corn JL et al. Isolation of Mycobacterium avium subspecies paratuberculosis from free-ranging birds and mammals on livestock premises. Applied and Environmental Microbiology. 2005;71:6963–6967. doi: 10.1128/AEM.71.11.6963-6967.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beard PM et al. Paratuberculosis infection of non-ruminant wildlife in Scotland. Journal of Clinical Microbiology. 2001;39:1517–1521. doi: 10.1128/JCM.39.4.1517-1521.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deutz A et al. Mycobacterium avium subsp. paratuberculosis in wild animal species and cattle in Styria/Austria. Berliner und Münchener tierärztliche Wochenschrift. 2005;118:314–320. [PubMed] [Google Scholar]
- 13.Machackova M et al. Wild boar (Sus scrofa) as a possible vector of mycobacterial infections: review of literature and critical analysis of data from Central Europe between 1983 and 2001. Veterinarni Medicina. 2003;48:51–65. [Google Scholar]
- 14.Alvarez J et al. Mycobacterium avium subspecies paratuberculosis in fallow deer and wild boar in Spain. Veterinary Record. 2005;156:212–213. doi: 10.1136/vr.156.7.212. [DOI] [PubMed] [Google Scholar]
- 15.Cousins DV et al. DNA fingerprinting of Australian isolates of Mycobacterium avium subsp. paratuberculosis using IS900 RFLP. Australian Veterinary Journal. 2000;78:184–190. doi: 10.1111/j.1751-0813.2000.tb10590.x. [DOI] [PubMed] [Google Scholar]
- 16.Angus K. Intestinal lesions resembling paratuberculosis in a wild rabbit (Oryctolagus cuniculus) Journal of Comparative Pathology. 1990;103:22–23. doi: 10.1016/s0021-9975(08)80140-5. [DOI] [PubMed] [Google Scholar]
- 17.Greig A et al. Paratuberculosis in wild rabbits (Oryctolagus cuniculus) Veterinary Record. 1997;140:141–143. doi: 10.1136/vr.140.6.141. [DOI] [PubMed] [Google Scholar]
- 18.Greig A et al. Epidemiological study of paratuberculosis in wild rabbits in Scotland. Journal of Clinical Microbiolgy. 1999;37:1746–1751. doi: 10.1128/jcm.37.6.1746-1751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beard PM et al. Evidence of paratuberculosis in fox (Vulpes vulpes) and stoat (Mustela erminea) Veterinary Record. 1999;145:612–613. [Google Scholar]
- 20.Collins DM, Gabric DM, De Lisle GE. Identification of two groups of Mycobacterium paratuberculosis strains by restriction endonuclease analysis and DNA hybridization. Journal of Clinical Microbiology. 1990;28:1591–1596. doi: 10.1128/jcm.28.7.1591-1596.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittington RJ et al. Polymorphisms in IS1311, an insertion sequence common to Mycobacterium avium and Mycobacterium avium subsp. paratuberculosis, can be used to distinguish between and within these species. Molecular and Cellular Probes. 1998;12:349–358. doi: 10.1006/mcpr.1998.0194. [DOI] [PubMed] [Google Scholar]
- 22.Pavlik I et al. Standardisation of restriction fragment length polymorphism analysis for Mycobacterium avium subspecies paratuberculosis. Journal of Microbiological Methods. 1999;38:155–167. doi: 10.1016/s0167-7012(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson K et al. Molecular characterization of pigmented and nonpigmented isolates of Mycobacterium avium subsp. paratuberculosis. Journal of Clinical Microbiology. 2002;40:1798–1804. doi: 10.1128/JCM.40.5.1798-1804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimarelli Z et al. A survey of ovine and caprine paratuberculosis in the Thessaloniki area, Greece. Paratuberculosis Newsletter. 1991;3:8–9. [Google Scholar]
- 25.Whitlock RH, Rosenberger AE. Proceedings of the 94th Annual Meeting of the US Animal Health Association. U.S. Animal Health Association; Denver, Colorado: 1990. Fecal culture protocol for Mycobacterium paratuberculosis a recommended procedure; pp. 280–285. ; pp. [Google Scholar]
- 26.Millar DS et al. Solid-phase hybridization capture of low-abundance target DNA sequences: application to the polymerase chain reaction detection of Mycobacterium paratuberculosis and Mycobacterium avium subsp. silvaticum. Analytical Biochemistry. 1995;226:325–330. doi: 10.1006/abio.1995.1232. [DOI] [PubMed] [Google Scholar]
- 27.Marsh I, Whittington R, Cousins D. PCR-restriction endonuclease analysis for identification and strain typing of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium based on polymorphisms in IS1311. Molecular and Cellular Probes. 1999;13:115–126. doi: 10.1006/mcpr.1999.0227. [DOI] [PubMed] [Google Scholar]
- 28.Harris NB, Barletta RG. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clinical Microbiology Reviews. 2001;14:489–512. doi: 10.1128/CMR.14.3.489-512.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittington RJ et al. Isolation of Mycobacterium avium subsp. paratuberculosis from environmental samples collected from farms before and after destocking sheep with paratuberculosis. Australian Veterinary Journal. 2003;81:559–563. doi: 10.1111/j.1751-0813.2003.tb12887.x. [DOI] [PubMed] [Google Scholar]
- 30.Haydon DT et al. Identifying reservoirs of infection: a conceptual and practical challenge. Emerging Infectious Diseases. 2002;8:1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Judge J et al. Clustering of Mycobacterium avium subsp. paratuberculosis in rabbits and the environment: how hot is a hot spot? Applied and Environmental Microbiology. 2005;71:6033–6038. doi: 10.1128/AEM.71.10.6033-6038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Judge J et al. Routes of intraspecies transmission of Mycobacterium avium subsp. paratuberculosis in rabbits (Oryctolagus cuniculus): a field study. Applied and Environmental Microbiology. 2006;72:398–403. doi: 10.1128/AEM.72.1.398-403.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniels MJ et al. The grazing response of cattle to pasture contaminated with rabbit faeces and the implications for the transmission of paratuberculosis. Veterinary Journal. 2001;161:306–313. doi: 10.1053/tvjl.2000.0550. [DOI] [PubMed] [Google Scholar]
- 34.Pavlik I et al. Characterization by restriction endonuclease analysis and DNA hybridization using IS900 of bovine, ovine, caprine and human dependent strains of Mycobacterium paratuberculosis isolated in various localities. Veterinary Microbiology. 1995;45:311–318. doi: 10.1016/0378-1135(94)00130-o. [DOI] [PubMed] [Google Scholar]
- 35.Whittington RJ et al. Evaluation of modified BACTEC 12B radiometric medium and solid media for culture of Mycobacterium avium subsp. paratuberculosis from sheep. Journal of Clinical Microbiology. 1999;37:1077–1083. doi: 10.1128/jcm.37.4.1077-1083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Juan L et al. Comparison of four different culture media for isolation and growth of type II and type I/III Mycobacterium avium subsp. paratuberculosis strains isolated from cattle and goats. Applied and Environmental Microbiology. 2006;72:5927–5932. doi: 10.1128/AEM.00451-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddacliff LA, Vadali A, Whittington RJ. The effect of decontamination protocols on the numbers of sheep strain Mycobacterium avium subsp. paratuberculosis isolated from tissues and faeces. Veterinary Microbiology. 2003;95:271–282. doi: 10.1016/s0378-1135(03)00181-0. [DOI] [PubMed] [Google Scholar]
- 38.Collins DM, Cavaignac S, De Lisle GW. Use of four DNA insertion sequences to characterize strains of the Mycobacterium avium complex isolated from animals. Molecular and Cellular Probes. 1997;11:373–380. doi: 10.1006/mcpr.1997.0131. [DOI] [PubMed] [Google Scholar]