SUMMARY
Lyssaviruses cause acute, progressive encephalitis in mammals. Current rabies vaccines offer protection against the lyssaviruses, with the notable exceptions of Mokola virus (MOKV), Lagos bat virus (LBV) and West Caucasian bat virus (WCBV). Here we describe the cross-protective and cross-reactive immune responses induced by experimental recombinant vaccinia viruses encoding the glycoprotein genes of rabies virus (RABV), MOKV and WCBV, either singly or in dual combinations. Constructs expressing a single glycoprotein gene protected mice against lethal intracranial challenge with homologous virus. Similarly, recombinants expressing glycoprotein genes from two different lyssaviruses offered mice protection against both homologous viruses. VNAb induced by vaccines that included a MOKV glycoprotein gene cross-neutralized LBV, but not WCBV. We concluded that a single recombinant poxvirus-vectored vaccine including MOKV and RABV glycoprotein genes, should be a major addition to available rabies biologics and should offer broad protection against all of the lyssaviruses, except WCBV.
INTRODUCTION
The Lyssaviruses belong to the family Rhabdoviridae within the order Mononegavirales (i.e. mono- single; nega- negative genome) and are the aetiological agents of rabies encephalitis in supposedly all warm-blooded animals and humans [1, 2]. These viruses are classified into seven genotypes (species) based on antigenic characteristics and molecular sequence analysis of nucleo-, phospho- and glyco- (G) protein genes of the virus [1, 2]. These are: genotype (gt) 1, Rabies virus (RABV); gt 2, Lagos bat virus (LBV); gt 3, Mokola virus (MOKV); gt 4, Duvenhage virus (DUVV); gt 5, European bat lyssavirus-1 (EBLV-1); gt 6, European bat lyssavirus-2 (EBLV-2) and gt 7, Australian bat lyssavirus (ABLV). In addition, four novel lyssaviruses that were isolated from different bat species from central Asia and Russia have been described since 2003 [3–6]. It has been suggested that these four viruses, namely Aravan (ARAV), Khujand (KHUV), Irkut (IRKV) and West Caucasian bat virus (WCBV) could be considered as separate genotypes based on the current criteria for lyssavirus taxonomy [1, 2]. Furthermore, considering phylogeny (comparison of glycoprotein sequences), immunogenicity and virulence of isolates representing the range of lyssaviruses, members of the genus were proposed to be separable into two distinct phylogroups [7]. This division into phylogroups generally correlates with the pattern of vaccine cross-protection observed for lyssaviruses described prior to 2003 [7–9]. The first phylogroup is represented by isolates from genotypes 1, 4, 5, 6 and 7, and also include ARAV, KHUV and IRKV [5, 6, 8]. Commercial vaccines and biologicals, administered according to the WHO prescribed regimens for pre- and post-exposure, are considered to be effective against infections of viruses from this group (reviewed in [9]). However, it should be noted that equivalent data are not available for all the phylogroup 1 viruses, and for example in the case of ARAV, KHUV and IRKV, assumptions can only being made based on the characteristics of single isolates [8]. In addition, vaccine studies with DUVV have been very limited indeed (reviewed in [9]), and further validation of vaccine cross-protection against these viruses is becoming increasingly pressing, especially considering the recent DUVV-induced human fatality from South Africa in 2006 [10]. It is generally accepted that commercial vaccines and biologics for rabies do not offer full protection against infection with the viruses outside of the proposed lyssavirus phylogroup 1, i.e. the African non-rabies lyssaviruses of genotypes 2 and 3 [7–9]. In addition, WCBV is recognized as the most phylogenetically divergent lyssavirus compared to classic rabies virus (and other phylogroup 1 viruses), but also exhibits limited relatedness to genotypes 2 and 3 viruses. Laboratory evidence indicated little or no cross-neutralization of anti-RABV sera with this isolate [4, 8].
The objective of this study was to construct recombinant vaccinia viruses expressing single and dual copies of the glycoprotein genes of RABV, MOKV and WCBV and to investigate the protective and cross-protective value of these candidate vaccines in an attempt to demonstrate broader protection against a range of lyssaviruses discovered to date. Although these are experimental vaccines, they would represent the first cross-protective lyssavirus vaccines to fall within an already approved vaccine class.
MATERIAL AND METHODS
Viruses and cells
Parental Vaccinia Copenhagen (Vacc Cop) and recombinant vaccinia viruses were passaged on Vero cell culture (CCL-81). All cell cultures used in this study were grown in Minimal Essential Medium (MEM) supplemented with 4 mm glutamine and 2× MEM vitamin solution (all from Gibco, Invitrogen, Carlsbad, CA, USA). The medium was supplemented with 1× antibiotics (100 μg/ml penicillin, 100 μg/ml streptomycin and 250 μg/ml amphotericin) (Gibco) and 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA). Cultures were kept at 37°C and at an atmosphere of 0·5% or 5% CO2. RABV (Cynictis penicillata isolate ARC-OVI M710/90), MOKV (ARC-OVI RA361) and WCBV were amplified in suckling mice and titred in 3- to 4-week-old ICR mice according to previously described methods [11].
Animals used in the study
Outbred ICR mice (H2d-restricted, female, different ages) were obtained from Harlan Sprague–Dawley (Indianapolis, IN, USA). Animals were housed and handled according to protocols approved by the Institutional Animal Care and Use Committee of the CDC.
Molecular cloning of full-length lyssavirus glycoprotein genes
Molecular cloning of a RABV and MOKV glycoprotein genes
The RABV glycoprotein gene was obtained from previous clones and inserted into the BamHI site of the vaccinia virus transfer vector pGVWR-gptNew to generate pGVWR-RG [12]. The MOKV glycoprotein gene was digested from pBUDCE4-MokG [11] with PstI (New England Biolabs, Beverly, MA, USA) and inserted into the PstI site of pGVWR-gptNew to generate pGVWR-MG. The molecules were ligated with T4 DNA ligase and rapid ligation buffer and transformed in competent JM109 cells, following a heat-shock protocol (Promega, Madison, WI, USA). Clones were screened using a rapid nucleic acid isolation method [13] and possible recombinants were screened and the direction of gene insertions verified by plasmid isolation using the QIAprep Spin miniprep kit (Qiagen, Hilden, Germany) and restriction enzyme digestion.
Molecular cloning of the WCBV glycoprotein gene
Total RNA was extracted from an infected mouse brain, using TRIzol™ reagent (Invitrogen) according to the manufacturer's recommendations. cDNA was generated by reverse transcription with a sense primer (ACTCAACAATCTGAAGAAGATG), for 90 min at 42°C with dNTPs (2·5 mm each) and 0·5 units of AMV reverse transcriptase (Roche, Mannheim, Germany). The PCR primers, WCBV G forward (CTCATCTCAGAGAAATGGC) and WCBV G reverse (CCCTTGAAGAATTCAATACC) were designed and used for the PCR amplification of the full-length WCBV G protein gene. The PCR product was cloned into pGEM®-TEasy vector (Promega) as before. WCBV glycoprotein gene of recombinant clones was sequenced using M13 universal primers (Promega). The sequencing reactions were prepared with the BigDye® terminator kit version 1.0 as prescribed by the manufacturer and sequences were resolved on an ABI Prism 377 gel automatic sequencer (Applied Biosystems, Foster City, CA, USA). Sequences were examined for PCR-incorporated errors and blasted against sequences on the NCBI Genbank. An intact WCBV glycoprotein gene was digested from a recombinant pGEM clone and inserted into the EcoRI site of the mammalian expression vector, pCINeo (Promega) for purposes not relevant to this study. The gene was retrieved by digestion of the pCINeo clone with XhoI and SalI (New England Biolabs) and inserted into the SalI site of pGVWR-gptNew to generate pGVWR-WG. The direction of the insertion was determined by digestion with EcoRV restriction enzyme (New England Biolabs).
Generation and isolation of recombinant vaccinia viruses expressing lyssavirus glycoprotein genes
The methods for the generation, isolation and purification of recombinant vaccinia viruses were adapted from previously described protocols [14, 15]. The activated-dendrimer transfection reagent, Superfect® (Qiagen) was used to prepare transfection reactions, according to the manufacturer's suggestions. To generate recombinant vaccinia viruses expressing a single copy of the RABV, MOKV or WCBV glycoprotein gene, Vacc Cop-infected cell culture was transfected with the recombinant transfer vectors. Similarly, the recombinants expressing dual copies of a RABV glycoprotein gene, or a RABV and MOKV or a RABV and WCBV glycoprotein gene were generated after transfection of cell culture infected with another experimental vaccinia recombinant with a RABV gene inserted into the haemagglutinin site of the Vacc Cop genome (construct available from Rabies Unit, CDC, Atlanta, GA, USA). All the genes are expressed under regulation of the p7·5 vaccinia virus promoter. Recombinant viruses were plaque purified in the presence of cell culture medium containing 25 μg/ml mycophenolic acid, 250 μg/ml xanthine and 15 μg/ml hypoxanthine (all from Sigma Aldrich, Munich, Germany). The homogeneity of the recombinant isolates was analysed with PCR [16] targeting the thymidine kinase region of the Vacc Cop genome. Genomic DNA was extracted from virus isolates from subsequent passages as described elsewhere [17]. Expression of the different glycoprotein genes from the homogenous recombinants was confirmed with an indirect immunofluoresence assay (IFA). Mouse anti-WCBV hyperimmune serum or RABV or MOKV specific monoclonal antibodies was used to test for the expression of protein. Recombinant vaccinia viruses expressing MOKV and WCBV glycoproteins were identified and designated VV-MG and VV-WG. Recombinant vaccinia viruses expressing two G protein genes, two RABV, RABV and MOKV or RABV and WCBV were identified and named VV-RGRG; VV-RGMG and VV-RGWG. These viruses, together with parental strains were propagated, semi-purified through ultracentrifugation at 19 000 g through a 36% sucrose cushion, resuspended in MEM with 1× antibiotics and stored in aliquots at −80°C.
Immunization and challenge study
Six-week-old ICR mice received 107 plaque-forming units (p.f.u.) of the vaccine viruses or their parent viruses in 50 μl MEM with 1× antibiotic. The administrations were given intramuscularly in the right quadriceps muscle with a 0·5 cc tuberculin syringe and an 8 mm 31-gauge needle. All animals received a booster of 105 p.f.u. of the same vaccine virus 14 days after the primary immunization. Mock controls were included by immunizing mice with only MEM or Vacc Cop preparations. Blood was collected via the retro-orbital route on days 0, 7 and 21, using heparinized microhaematocrit capillary tubes (Becton Dickinson and Company, Franklin Lakes, NJ, USA) or heparinized Natalson blood collecting capillaries (Fisher Scientific, Houston, TX, USA). Sera were separated in Microtainer® serum separation tubes with SST™ (Becton Dickinson and Company) as suggested by the manufacturer, and stored at −20°C until analysis. On day 28 each animal received 100 MICLD50 of RABV, MOKV or WCBV intracranially. The inoculations were in volumes of 30 μl each, and performed with a 0·5 cc tuberculin syringe (8 mm, 31-gauge needle) or 1 cc syringe (27-gauge needle) (Becton Dickinson and Company). The animals were monitored for up to 30 days post-challenge and post-mortem diagnosis of rabies virus infection was confirmed using the direct fluorescent antibody test with FITC anti-rabies monoclonal globulin (Fujirebio Diagnostics, Malvern, PA, USA) [18].
Analysis of humoral responses: rapid fluorescent focus inhibition test (RFFIT)
Preparation and interpretation of the RFFIT was carried out as described previously [19]. In modification of this protocol, challenge virus included not only the challenge virus standard (CVS-11), but tests were also prepared with MOKV, LBV and WCBV as challenge viruses. Cell-culture-adapted isolates of these viruses were diluted to 50 fluorescent foci doses per 100 μl before use.
Statistical analysis of data
Survival data was analysed with conservative confidence intervals with 90% confidence to compare the different groups pair-wise. VNAb titre data was analysed by the Kruskal–Wallis non-parametric method at α=0·05. The statistical treatments were prepared by Statomet (University of Pretoria, South Africa).
RESULTS
Molecular cloning of full-length lyssavirus glycoprotein genes and generation of recombinant vaccinia viruses
The integrity of all constructs and cloned genes described was confirmed by comprehensive sequencing analysis. Recombinant vaccinia viruses that carry MOKV (VV-MG) or WCBV G (VV-WG) protein gene were generated using Vacc Cop as the parent virus. The homogenous recombinants were subsequently isolated after six rounds under MPA resistance selection. The purity of these recombinants was monitored by PCR of the TK region of the parent genome (results not shown). In addition, recombinant viruses that carried two RABV (VV-RGRG) or a RABV and MOKV (VV-RGMG) or a RABV and WCBV (VV-RGWG) glycoprotein genes were also constructed. An experimental recombinant vaccinia virus encoding a rabies virus glycoprotein gene (VV-RG) was used as a parent virus for the construction of these double antigen-encoding recombinants and the recombinants were generated upon transfection of cell culture infected with VV-RG. The recombinants were also isolated similarly to the single gene recombinants. Expression of the different glycoprotein genes was confirmed for all the vaccine constructs, by probing infected cells with antibodies that bind specifically to the RABV, MOKV or WCBV glycoproteins in IFA assays (results not shown).
Survival and VNAb responses in mice
The single antigen expressing recombinant vaccines (i.e. VV-RG, VV-MG and VV-WG) significantly protected mice against lethal intracerebral challenge with homologous virus, compared to control groups that succumbed to the challenge (Tables 1–3). These constructs elicited measurable virus-neutralizing responses by day 7 after primary immunization (Tables 1–3). In addition, up to a fivefold increase in VNAb titre was measured on day 21 (7 days after a booster immunization), for animals immunized with VV-RG and VV-WG. Up to a sixfold increase in titre was measured for the animals that received the VV-MG vaccine. Similarly, the double antigen expressing vaccines (i.e. VV-RGRG, VV-RGMG, VV-RGWG) also protected mice against lethal intracerebral challenge with homologous viruses (Tables 1–3). The RABV VNAb responses increased three-, five- and sevenfold, respectively, after the second dose of VV-RGRG, VV-RGWG and VV-RGMG was administered (Tables 1–3). The average levels of RABV VNAb elicited after vaccination with any of the vaccines expressing RABV G alone or in combination with another G, did not differ significantly (α=0·05). The same was true for MOKV VNAb titres after vaccination with VV-MG and VV-RGMG, and WCBV VNAb titres in mice that received VV-WG or VV-RGWG (α=0·05).
Table 1.
Pre-challenge RABV VNAb titres and survival of mice vaccinated with recombinant vaccinia viruses and challenged with street RABV
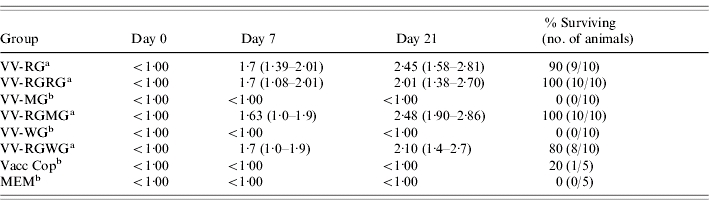
The VNAb titres of groups indicated with (a) or (b) differ statistically at α=0·05. The titres are noted for day 0 (naive sera), day 7 (7 days after primary vaccination) and day 21 (7 days after booster vaccinations) and are expressed as log base 10 of the geometric mean titres (with the range of the values provided in parentheses). The number of surviving animals per group is also indicated in parentheses.
Table 3.
Pre-challenge WCBV VNAb titres and survival of mice vaccinated with recombinant vaccinia viruses and challenged with WCBV
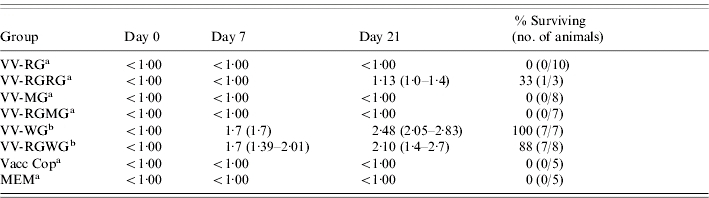
The VNAb titres (day 21 titres used for calculations) of groups indicated with (a) or (b) differ statistically at α=0·05.
In vitro cross-reactivity and in vivo cross-protection studies
Animals that were immunized with VV-RG were not protected against challenge with MOKV or WCBV (Tables 2 and 3). These results were mirrored in the lack of neutralization of MOKV or WCBV with sera collected from these animals. Similar observations were made for mice that received VV-MG. This vaccine did not elicit protection against RABV or WCBV challenge and the results were corroborated by the lack of anti-RABV or WCBV VNAb in the respective sera (Tables 1 and 3).
Table 2.
Pre-challenge MOKV VNAb titres and survival of mice vaccinated with recombinant vaccinia viruses and challenged with MOKV
The VNAb titres (day 21 titres used for calculations) of groups indicated with (a) or (b) differ statistically at α=0·05.
One animal vaccinated with VV-RGRG survived challenge with WCBV, but this result was not found to be statistically significant compared with the other groups (α=0·05). Although this single survival was not statistically significant, it does hold clinical significance. Although the sera of the surviving animal collected on day 7 after primary vaccination did not cross-neutralize WCBV, the sera collected on day 21, did. Indeed, a low level of WCBV neutralizing antibodies was demonstrated (Table 3) and the sera of the surviving animal (collected on day 21) were shown to cross-neutralize WCBV in vitro. The same VV-RGRG vector did not induce any measurable VNAb responses against MOKV and all the animals that received this vaccine and were challenged with MOKV, succumbed. In addition, the VV-RGMG vaccine did not afford any protective responses in mice challenged with WCBV.
Unexpectedly, two of the seven animals that received the VV-WG vaccine survived challenge with MOKV. Once again statistical treatment of these results failed to confirm its significance, but both surviving animals had low levels of MOKV VNAb on day 21, providing supporting evidence for the clinical importance of this finding (Table 3).
To establish cross-reactivity with the final member of the lyssavirus phylogroup 2, sera were also tested against LBV for cross-reactivity (Table 4). The sera collected from mice vaccinated with VV-MG and VV-RGMG indeed significantly cross-neutralized LBV in the RFFIT (α=0·05). Although the geometric mean titres elicited by VV-RGMG were lower than those elicited by VV-MG on both days 7 and 21, these differences were not statistically significant (α=0·05).
Table 4.
LBV VNAb titres of mice vaccinated with recombinant vaccinia viruses
The VNAb titres (day 21 titres used for calculations) of groups indicated with (a) or (b) differ statistically at α=0·05.
DISCUSSION
The veterinary and public health threat of the non-rabies lyssaviruses may appear to be of inconsequential status compared to RABV, and this is reflected in the lack of surveillance of, in particular, the African non-rabies lyssaviruses. Generally, the lack of epidemiological data on these viruses is attributable to poor or complete lack of surveillance in countries where these viruses have been isolated before [4, 20]. As such, LBV has not yet been isolated from humans and MOKV from humans is considered extremely rare [20–26]. Even so, several pressures continue to lead to the emergence of lyssaviruses (and other RNA viruses) and therefore the burden of these viruses may become more significant in time (reviewed in [27]). In addition, considering the distribution of the postulated reservoirs of these viruses, the epizootiology of these viruses may well be of future importance for animal and human health [5, 6, 9]. The isolation of MOKV from a diversity of hosts, including shrews (Crocidura spp.), a dog, domestic cats and a rodent (Lophuromys sikapusi) demonstrates this virus's ability to cross the species barrier and its potential for establishing in a new host range, similar to the spillover that probably established the global distribution of terrestrial RABV [21, 28]. The same argument applies to LBV, another lyssavirus that is continually encountered in bats in southern Africa and have now also been isolated from a mongoose – representing the first ever such recognized case in terrestrial wildlife [20, 29]. In 2006 a second human fatality of DUVV, another southern African lyssavirus, was reported – presumably from an insectivorous bat [10]. This particular event marks the first reported case of DUVV since 1982. From these perspectives, investigation into the development of animal and human RABV vaccines and other biologicals that offer protection against the broad spectrum of lyssaviruses is a worthwhile objective. In this study different recombinant vaccinia-based lyssavirus vaccines were generated and the cross-protective capacity of these vaccines evaluated. The vaccinia virus system was chosen for this proof-of-concept study since it is particularly well studied for the expression of RABV proteins and has been applied to and evaluated in a large variety of animal models. Moreover, recombinant vaccinia viruses are effective carriers of RABV glycoproteins – and one such recombinant vector, V-RG, occupies a significant niche in oral vaccination of wildlife [30, 31].
Previous studies investigating cross-protective lyssavirus vaccines explored the utility of recombinant subunit vaccines expressing MOKV (previously recognized as the most divergent lyssavirus) glycoprotein [11, 32]. Such vaccines offered protection against lethal MOKV challenge, but not against RABV challenge. The construction of chimeric lyssavirus glycoproteins consisting of half of a RABV and half of a MOKV glycoprotein gene provided protection against challenge with both viruses [33, 34]. Sera collected from animals immunized with these chimeric constructs were tested for cross-reactivity against other lyssaviruses. Sera significantly cross-neutralized LBV and EBLV-2, but only slightly cross-neutralized DUVV and EBLV-1 [34]. The role of the lyssavirus nucleoprotein in enhancing cross-protective immunity has also been explored. The lyssavirus nucleoprotein gene is highly conserved, even amongst the different genotypes and carries various epitopes, including T helper cell epitopes [35, 36]. The conclusion from a number of studies employing nucleoprotein recombinant subunit vaccines [11, 37–39], is that the nucleoprotein may play some role in protecting against peripheral virus challenge, but there is little evidence for an enhancement of responses, particularly of a cross-protective nature. The lyssavirus glycoprotein remains the single most important component of any lyssavirus vaccine. Presence of the glycoprotein (subunit vaccine) or the glycoprotein gene (genetic or live vector-based vaccine) affords a fully protective immune response against homologous challenge in various different models evaluated (reviewed in [9]).
Our recombinant vaccinia viruses expressing RABV, MOKV or WCBV glycoproteins were efficacious in mice via the intramuscular route. Not only were specific VNAb measured only a week after primary immunization, but all these experimental vaccines elicited strong anamnestic responses after booster administrations. As expected, these vaccines protected mice against lethal intracranial challenge with homologous lyssavirus. However, no protection against heterologous challenge was observed for any of these single lyssavirus glycoprotein-encoding recombinant vaccines. The presence or lack of VNAb responses, recognized as the single most important immune response to confer immunological protection [40, 41], and the corresponding survival data confirms the consistency of the immune responses examined in this set of experiments. The finding that our RABV G protein vaccine failed to elicit cross-reactive serological responses and failed to cross-protect against MOKV or against WCBV in vivo, substantiate the findings of related studies which utilized somewhat different experimental approaches [8, 11]. By the same token, sera from animals that received the RABV glycoprotein vaccine did not cross-react with LBV in RFFITs, again in support of related experimental findings on the failure of experimental or commercial rabies vaccines to protect against this member of the phylogroup 2 lyssaviruses. The failure of the MOKV vaccine to protect against RABV, and vice versa, was expected and in agreement with previous findings [11, 32]. Sera from animals that received the MOKV vaccine significantly neutralized LBV in RFFITs, and underline the relationship between these two African viruses. However, the MOKV vaccine did not provide any protection or exhibit any cross-neutralizing activities against WCBV challenge. This finding underscores the divergence of WCBV from not only classical RABV but also the viruses belonging to the putative phylogroup 2.
The vaccines expressing two different heterologous lyssavirus glycoprotein genes, i.e. MOKV plus RABV and WCBV plus RABV G protein genes, elicited specific VNAb responses that were comparable to those elicited by the single antigen expressing vaccines and each of the vaccines offered dual protection against challenges with either viruses. Previously, a recombinant RABV which express the RABV glycoprotein gene in duplicate, were found to concomitantly produce more glycoprotein and consequentially elevated neutralizing responses in a back-titration study, as opposed to a RABV expressing only one RABV glycoprotein gene [42]. This observation was supported by survival studies in a mouse model. Where conventional rabies vaccines yielded very high total VNAb titres, some neutralization of MOKV, LBV, and particularly WCBV has been reported [8]. By inference, a double RABV G protein expressing vaccinia virus may have been expected to induce not only more neutralizing responses, but possibly confer enhanced neutralization of non-rabies lyssaviruses. However, our double expression vaccinia recombinant elicited titres of RABV neutralizing responses that were comparable with the single glycoprotein-expressing vaccine and did not enhance neutralization of MOKV, LBV or WCBV at the dosage of vaccine tested.
Apart from not observing elevated VNAb levels, enhanced cross-reactivity of sera induced by the double RABV glycoprotein vaccine with MOKV, LBV and WCBV was not observed. Only a single animal that received the double RABV glycoprotein vaccine, survived a heterologous challenge, namely with WCBV and this animal indeed presented a low anti-WCBV antibody titre 21 days after vaccination. While the taxonomic position of WCBV is under discussion, our data provide evidence in support of the idea that WCBV does not belong to either phylogroups 1 or 2, based on the generally accepted criteria for such a grouping [7]. Indeed, apart from phylogenetic and serological distance, we found WCBV to be separable from MOKV and LBV (the sole members of phylogroup 2) based on the responses to the new set of vaccines studied here. Specifically, sera from animals vaccinated with a MOKV-specific vaccine strongly and consistently cross-neutralized a LBV isolate. These same sera completely failed to cross-neutralize WCBV, effectively distancing WCBV from the other two viruses. Unexpectedly, two of the seven animals that received the VV-WG vaccine survived challenge with MOKV. Once again statistical treatment of these results failed to confirm its significance, but both surviving animals had low levels of MOKV VNAb on day 21, providing supporting evidence for the clinical importance of this finding (Table 3). Due to this divergent nature of WCBV, the dual WCBV plus RABV vaccine did not offer the protection desired for a broader spectrum of lyssaviruses in Africa.
Instead, the dual MOKV plus RABV, or a hypothetical LBV plus RABV should offer more complete protection against all the known lyssaviruses, with the exception of WCBV. Such a vaccine would, based on our findings, be as effective against RABV, the major agent for which these vaccines will be intended, as those vaccines that are directed at RABV only. Studies to determine the immunogically important epitopes on the WCBV glycoprotein, or common epitopes on all the lyssaviruses could contribute to the development of poly-epitope vaccines [33, 34]. The move towards newer vaccines for lyssaviruses may well be precipitated in our modern era through the increased detection of the activities of non-rabies lyssaviruses in animal reservoirs/targets and through non-rabies human fatalities, as reported in recent times. In addition to an alternative approach to vaccine development, future research may also focus more on the development of antivirals that could be used post-exposure and should be active against the spectrum of lyssaviruses.
ACKNOWLEDGEMENTS
The authors thank the following people for contributions to the study: Dr A. I. Wandeler (Centre of Expertise for Rabies, Canadian Food Inspection Agency, Canada) for providing anti-rabies virus monoclonals for expression studies and Dr C. T. Sabeta (Agricultural Research Council, Onderstepoort Veterinary Institute) for providing rabies virus isolate used in the study. The authors sincerely thank members of the Rabies Unit, CDC, in particular Mr J. S. Self and Mr M. Niezgoda. J. W. was supported by a Regular Fellowship (Level 42) at the CDC. This work was supported in part by a grant from the US National Vaccine Program Office. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agency.
REFERENCES
- 1.Tordo N, Fauquet CM Virus taxonomy: the classification and nomenclature of viruses. The 8th Report of the International Committee on Taxonomy of Viruses. San Diego: Oxford Academic; 2006. Lyssaviruses; pp. 623–629. , pp. [Google Scholar]
- 2.WHO Geneva: 2005. pp. 15–19. . World Health Organization Expert Consultation on Rabies, 5–8 October 2004, First report. World Health Organization Technical report series 931. : World Health Organization, , pp. . 105. [Google Scholar]
- 3.Arai YY et al. New lyssavirus genotype from lesser mouse-eared bats (Myotis blythi), Kyrghyzstan. Emerging Infectious Disease. 2003;9:333–337. doi: 10.3201/eid0903.020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botvinkin AD et al. Novel lyssaviruses isolated from bats in Russia. Emerging Infectious Disease. 2003;9:1623–1625. doi: 10.3201/eid0912.030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuzmin IV et al. Bat lyssaviruses (Aravan and Khujand) from Central Asia: phylogenetic relationships according to N, P and G gene sequences. Virus Research. 2003;97:65–79. doi: 10.1016/s0168-1702(03)00217-x. [DOI] [PubMed] [Google Scholar]
- 6.Kuzmin IV et al. Phylogenetic relationships of Irkut and West Caucasian bat viruses within the Lyssavirus genus and suggested quantitative criteria based on the N gene sequence for lyssavirus genotype definition. Virus Research. 2005;111:28–43. doi: 10.1016/j.virusres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Badrane H et al. Evidence for two lyssavirus phylogroups with distinct pathogenicity and immunogenicity. Journal of Virology. 2001;75:3268–3276. doi: 10.1128/JVI.75.7.3268-3276.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanlon CA et al. Efficacy of rabies biologics against new lyssaviruses from Eurasia. Virus Research. 2005;111:44–54. doi: 10.1016/j.virusres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Nel LH. Vaccines for lyssaviruses other than rabies. Expert Reviews of Vaccines. 2005;4:553. doi: 10.1586/14760584.4.4.533. [DOI] [PubMed] [Google Scholar]
- 10.Paweska JT et al. Fatal human infection with rabies-related Duvenhage virus, South Africa. Emerging Infectious Disease. 2006;12:1965–1967. doi: 10.3201/eid1212.060764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nel LH et al. A comparison of DNA vaccines for the rabies-related virus, Mokola. Vaccine. 2003;21:2598–2606. doi: 10.1016/s0264-410x(03)00036-7. [DOI] [PubMed] [Google Scholar]
- 12.Weyer J Pretoria, South Africa: University of Pretoria; 2006. pp. 127–156. . Immune responses to recombinant vaccinia viruses expressing full-length lyssavirus glycoprotein genes [Thesis]. , pp. [Google Scholar]
- 13.Beuken E, Vink C, Bruggeman CA. One step procedure for screening recombinant plasmids by size. BioTechniques. 1998;24:748–750. doi: 10.2144/98245bm10. [DOI] [PubMed] [Google Scholar]
- 14.Earl PL Current Protocols in Molecular Biology. Indianapolis, IN: John Wiley and Sons; 1998. Preparation of cell cultures and vaccinia virus stocks; pp. 16.16.1–16.20.2. , pp. [DOI] [PubMed] [Google Scholar]
- 15.Byrd CM, Hruby DE, Isaacs SN. Vaccinia virus and poxvirology. Methods and Protocols. Totowa, NJ: Humana Press; 2005. Construction of recombinant vaccinia virus. Cloning into the thymidine kinase locus; pp. 31–39. , pp. [DOI] [PubMed] [Google Scholar]
- 16.Pasamontes L et al. Direct identification of recombinant virus plaques by PCR. Journal of Virological Methods. 1991;35:137–141. doi: 10.1016/0166-0934(91)90129-n. [DOI] [PubMed] [Google Scholar]
- 17.Esposito J, Condit R, Objeski J. The preparation of orthopoxvirus DNA. Journal of Virological Methods. 1981;2:175–179. doi: 10.1016/0166-0934(81)90036-7. [DOI] [PubMed] [Google Scholar]
- 18.Dean DJ, Abelseth MK, Atanasiu P, Meslin F-X, Kaplan MM, Koprowski H. Laboratory Techniques in Rabies. 4th edn. Geneva: World Health Organization; 1996. The fluorescent antibody test; pp. 88–95. , pp. [Google Scholar]
- 19.Smith JS, Yager PA, Baer GM, Meslin F-X, Kaplan MM, Koprowski H. Laboratory Techniques in Rabies. 4th edn. Geneva: World Health Organization; 1996. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus–neutralizing antibody; pp. 181–192. , pp. [Google Scholar]
- 20.Markotter W et al. Lagos Bat virus, South Africa. Emerging Infectious Diseases. 2006;12:504–506. doi: 10.3201/eid1203.051306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nel L et al. New cases of Mokola virus infection in South Africa: a genotypic comparison of Southern African virus isolates. Virus Genes. 2000;20:103–106. doi: 10.1023/a:1008120511752. [DOI] [PubMed] [Google Scholar]
- 22.Familusi JB, Moore DL. Isolation of a rabies-related virus from the cerebrospinal fluid of a child with aseptic meningitis. African Journal of Medical Science. 1972;3:93–96. [PubMed] [Google Scholar]
- 23.Familusi JB et al. A fatal human infection with Mokola virus. American Journal of Tropical Medicine and Hygiene. 1972;21:959–963. doi: 10.4269/ajtmh.1972.21.959. [DOI] [PubMed] [Google Scholar]
- 24.Boulger IR, Porterfield JS. Isolation of a virus from Nigerian fruit bats. Transcripts of the Royal Society of Tropical Medicine and Hygiene. 1958;52:421–424. doi: 10.1016/0035-9203(58)90127-5. [DOI] [PubMed] [Google Scholar]
- 25.Sureau P, Tignor GH, Smith AL. Antigenic characterization of the Bangui strain (ANCB-672d) of Lagos bat. Annals of Virology. 1980;131:25–32. [Google Scholar]
- 26.Mebatsion T, Cox JH, Frost JW. Isolation and characterization of 115 street rabies virus isolates from Ethiopia by using monoclonal antibodies: identification of 2 isolates as Mokola and Lagos bat viruses. Journal of Infectious Diseases. 1992;166:972–977. doi: 10.1093/infdis/166.5.972. [DOI] [PubMed] [Google Scholar]
- 27.Fooks AR. The challenge of emerging lyssaviruses. Expert Review of Vaccines. 2004;3:89–92. doi: 10.1586/14760584.3.4.333. [DOI] [PubMed] [Google Scholar]
- 28.Badrane H, Tordo N. Host switching in Lyssavirus history from the Chiroptera to the Carnivora orders. Journal of Virology. 2001;75:8096–8104. doi: 10.1128/JVI.75.17.8096-8104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markotter W et al. Isolation of Lagos bat virus from water mongoose. Emerging Infectious Diseases. 2006;12:1913–1918. doi: 10.3201/eid1212.060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieny M-P et al. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984;312:163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- 31.Rupprecht CE, Kieny M-P, Campbell JB, Charlton KM. Rabies. Boston, USA: Kluwer Academic Publishers; 1988. Development of a vaccinia-rabies glycoprotein recombinant virus vaccine; pp. 335–364. , pp. [Google Scholar]
- 32.Tordo N et al. Structure and expression in baculovirus of the mokola virus glycoprotein: an efficient recombinant vaccine. Virology. 1993;194:59–69. doi: 10.1006/viro.1993.1235. [DOI] [PubMed] [Google Scholar]
- 33.Bahloul C et al. DNA-based immunization for exploring the enlargement of immunological cross-reactivity against the lyssaviruses. Vaccine. 1998;16:417–425. doi: 10.1016/s0264-410x(97)00204-1. [DOI] [PubMed] [Google Scholar]
- 34.Jallet C et al. Chimeric lyssavirus glycoprotein genes with increased immunological potential. Journal of Virology. 1999;73:225–233. doi: 10.1128/jvi.73.1.225-233.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourhy H, Kiss B, Tordo N. Molecular diversity of the lyssavirus genus. Virology. 1993;194:70–81. doi: 10.1006/viro.1993.1236. [DOI] [PubMed] [Google Scholar]
- 36.Goto H et al. Expression of the nucleoprotein of rabies virus in Escherichia coli and mapping of antigenic sites. Archives of Virology. 1995;140:1061–1074. doi: 10.1007/BF01315415. [DOI] [PubMed] [Google Scholar]
- 37.Dietzschold B et al. Induction of protective immunity against rabies by immunization with rabies virus ribonucleoprotein. Proceedings of the National Academy of Sciences USA. 1987;84:9165–9169. doi: 10.1073/pnas.84.24.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lodmell DL, Smith JS, Esposito JJ. Cross-protection of mice against a global spectrum of rabies virus variants. Journal of Virology. 1995;69:4957–5962. doi: 10.1128/jvi.69.8.4957-4962.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drings A et al. Is there and advantage to including the nucleoprotein in a rabies glycoprotein subunit vaccine? Vaccine. 1999;17:1549–1557. doi: 10.1016/s0264-410x(98)00357-0. [DOI] [PubMed] [Google Scholar]
- 40.Perry LL, Lodmell DL. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. Journal of Virology. 1991;65:3429–3434. doi: 10.1128/jvi.65.7.3429-3434.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hooper DC et al. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. Journal of Virology. 1998;72:3711–3719. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faber M et al. Over-expression of the rabies virus glycoprotein results in enhancement of apoptosis and antiviral immune responses. Journal of Virology. 2002;76:3374–3381. doi: 10.1128/JVI.76.7.3374-3381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


