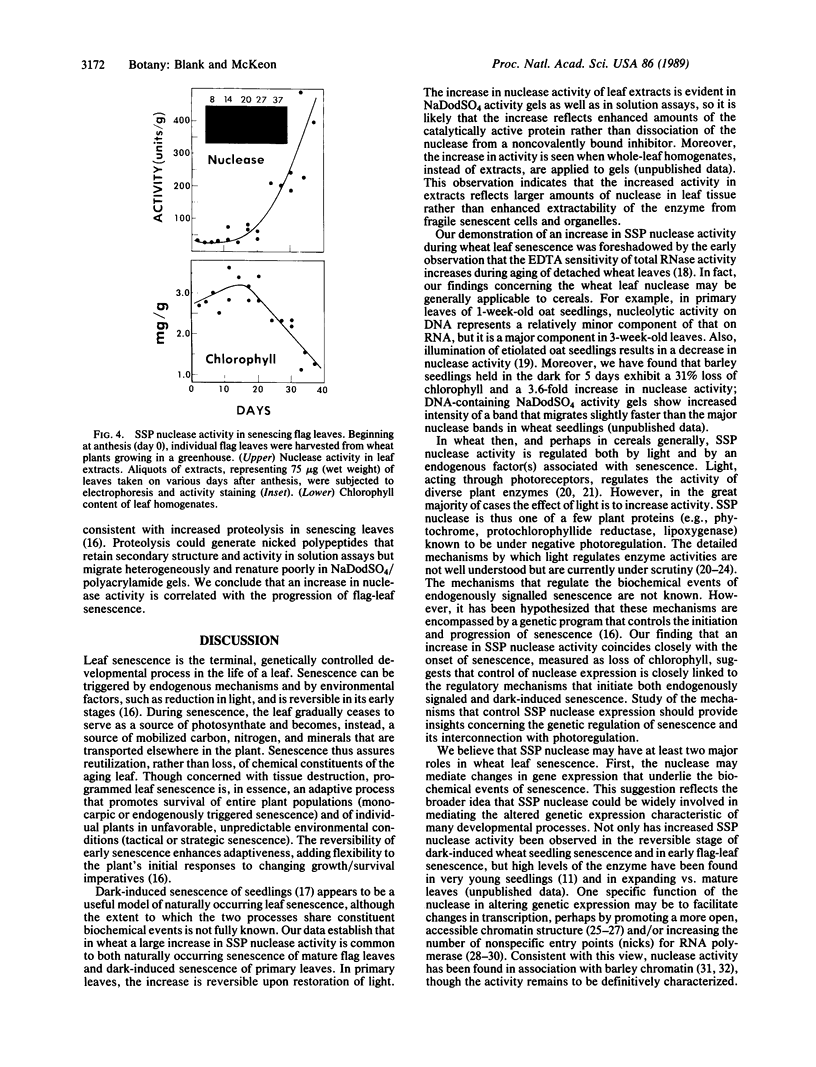
Abstract
Single-strand-preferring nucleases (EC 3.1.30.1) selectively cleave internucleotide bonds in single-stranded regions of predominantly duplex DNA and DNA·RNA hybrids and extensively degrade denatured DNA and RNA. The functions of single-strand-preferring nuclease in plants are unknown. We have monitored this nuclease activity in flag leaves of wheat (Triticum aestivum L. cv. Chinese Spring) undergoing natural senescence and in primary leaves of wheat seedlings undergoing dark-induced senescence. In falg leaves, nuclease activity remained at basal levels during the first 2 weeks after anthesis, while chlorophyll content increased to a maximum. Nuclease activity then rose in concert with a decline in chlorophyll, reaching a 16-fold elevation at 5 weeks post-anthesis, when 53% of the chlorophyll had been lost. When 8-day-old wheat seedlings were induced to senesce by placing them in darkness, nuclease activity rose without apparent lag, reaching a 13-fold elevation in 7 days, when 61% of the chlorophyll had been lost. The increase in nuclease activity was reversible upon reexposure of seedlings to light, a decline beginning without apparent lag. Reversibility was complete for plants that had been held in darkness for 5 days, with activity returning to the control level in 2 days. These senescence-related changes in nuclease activity, measured in conventional assays, were consistent with concomitant analysis by activity staining of sodium dodecyl sulfate/polyacrylamide gels. We conclude that an increase in single-strand-preferring nuclease activity is closely associated with wheat leaf senescence and that nuclease activity is subject to negative photoregulation.
Keywords: Triticum aestivum, photocontrol, activity gels, sodium dodecyl sulfate, polyacrylamide gel electrophoresis, cereals
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayusawa D., Shimizu K., Koyama H., Takeishi K., Seno T. Accumulation of DNA strand breaks during thymineless death in thymidylate synthase-negative mutants of mouse FM3A cells. J Biol Chem. 1983 Oct 25;258(20):12448–12454. [PubMed] [Google Scholar]
- Blank A., Dekker C. A. Ribonucleases of human serum, urine, cerebrospinal fluid, and leukocytes. Activity staining following electrophoresis in sodium dodecyl sulfate-polyacrylamide gels. Biochemistry. 1981 Apr 14;20(8):2261–2267. doi: 10.1021/bi00511a030. [DOI] [PubMed] [Google Scholar]
- Blank A., Silber J. R., Thelen M. P., Dekker C. A. Detection of enzymatic activities in sodium dodecyl sulfate-polyacrylamide gels: DNA polymerases as model enzymes. Anal Biochem. 1983 Dec;135(2):423–430. doi: 10.1016/0003-2697(83)90705-4. [DOI] [PubMed] [Google Scholar]
- Blank A., Sugiyama R. H., Dekker C. A. Activity staining of nucleolytic enzymes after sodium dodecyl sulfate-polyacrylamide gel electrophoresis: use of aqueous isopropanol to remove detergent from gels. Anal Biochem. 1982 Mar 1;120(2):267–275. doi: 10.1016/0003-2697(82)90347-5. [DOI] [PubMed] [Google Scholar]
- Bonven B., Westergaard O. DNase I hypersensitive regions correlate with a site-specific endogenous nuclease activity on the r-chromatin of Tetrahymena. Nucleic Acids Res. 1982 Dec 11;10(23):7593–7608. doi: 10.1093/nar/10.23.7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. H., Ho T. H. Barley aleurone layers secrete a nuclease in response to gibberellic Acid : purification and partial characterization of the associated ribonuclease, deoxyribonuclease, and 3'-nucleotidase activities. Plant Physiol. 1986 Nov;82(3):801–806. doi: 10.1104/pp.82.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright I. L., Abmayr S. M., Fleischmann G., Lowenhaupt K., Elgin S. C., Keene M. A., Howard G. C. Chromatin structure and gene activity: the role of nonhistone chromosomal proteins. CRC Crit Rev Biochem. 1982;13(1):1–86. doi: 10.3109/10409238209108709. [DOI] [PubMed] [Google Scholar]
- Chandler D. W., Gralla J. Specific binding and protection of form II SV40 deoxyribonucleic acid by ribonucleic acid polymerase II from wheat germ. Biochemistry. 1980 Apr 15;19(8):1604–1612. doi: 10.1021/bi00549a012. [DOI] [PubMed] [Google Scholar]
- Chen S. G., Srivastava B. I. Fragmentation of DNA and chromatin during senescence in barley leaves. Mech Ageing Dev. 1986 Mar;34(1):57–61. doi: 10.1016/0047-6374(86)90104-1. [DOI] [PubMed] [Google Scholar]
- Dawson B. A., Lough J. Immunocytochemical localization of transient DNA strand breaks in differentiating myotubes using in situ nick-translation. Dev Biol. 1988 Jun;127(2):362–367. doi: 10.1016/0012-1606(88)90322-3. [DOI] [PubMed] [Google Scholar]
- Eissenberg J. C., Cartwright I. L., Thomas G. H., Elgin S. C. Selected topics in chromatin structure. Annu Rev Genet. 1985;19:485–536. doi: 10.1146/annurev.ge.19.120185.002413. [DOI] [PubMed] [Google Scholar]
- Endo Y., Huber P. W., Wool I. G. The ribonuclease activity of the cytotoxin alpha-sarcin. The characteristics of the enzymatic activity of alpha-sarcin with ribosomes and ribonucleic acids as substrates. J Biol Chem. 1983 Feb 25;258(4):2662–2667. [PubMed] [Google Scholar]
- Farzaneh F., Shall S., Johnstone A. P. The dynamic nature of DNA-strand breaks present in differentiating muscle cells and quiescent lymphocytes. FEBS Lett. 1985 Sep 9;189(1):62–66. doi: 10.1016/0014-5793(85)80842-5. [DOI] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Hanson D. M., Fairley J. L. Enzymes of nucleic acid metabolism from wheat seedlings. I. Purification and general properties of associated deoxyribonuclease, ribonuclease, and 3'-nucleotidase activities. J Biol Chem. 1969 May 10;244(9):2440–2449. [PubMed] [Google Scholar]
- Johnstone A. P. Rejoining of DNA strand breaks is an early nuclear event during the stimulation of quiescent lymphocytes. Eur J Biochem. 1984 Apr 16;140(2):401–406. doi: 10.1111/j.1432-1033.1984.tb08116.x. [DOI] [PubMed] [Google Scholar]
- Kalinski A., Chandra G. R., Muthukrishnan S. Study of barley endonucleases and alpha-amylase genes. J Biol Chem. 1986 Aug 25;261(24):11393–11397. [PubMed] [Google Scholar]
- Lewis M. K., Burgess R. R. Transcription of simian virus 40 DNA by wheat germ RNA polymerase II. Priming of RNA synthesis by the 3'-hydroxyl of DNA at single strand nicks. J Biol Chem. 1980 May 25;255(10):4928–4936. [PubMed] [Google Scholar]
- Nagy F., Kay S. A., Chua N. H. Gene regulation by phytochrome. Trends Genet. 1988 Feb;4(2):37–42. doi: 10.1016/0168-9525(88)90064-9. [DOI] [PubMed] [Google Scholar]
- Reeves R. Transcriptionally active chromatin. Biochim Biophys Acta. 1984 Sep 10;782(4):343–393. doi: 10.1016/0167-4781(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Sierakowska H., Shugar D. Mammalian nucleolytic enzymes. Prog Nucleic Acid Res Mol Biol. 1977;20:59–130. doi: 10.1016/s0079-6603(08)60470-5. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I. Increase in chromatin associated nuclease ctivity of excised barley leaves during senescence and its suppression by kinetin. Biochem Biophys Res Commun. 1968 Aug 13;32(3):533–538. doi: 10.1016/0006-291x(68)90695-5. [DOI] [PubMed] [Google Scholar]
- Touchette N. A., Anton E., Cole R. D. A higher order chromatin structure that is lost during differentiation of mouse neuroblastoma cells. J Biol Chem. 1986 Feb 15;261(5):2185–2188. [PubMed] [Google Scholar]
- Wittenbach V. A. Induced senescence of intact wheat seedlings and its reversibility. Plant Physiol. 1977 Jun;59(6):1039–1042. doi: 10.1104/pp.59.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreschner D. H., James T. C., Silverman R. H., Kerr I. M. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2'p)nA) in interferon-treated cells. Nucleic Acids Res. 1981 Apr 10;9(7):1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyen N. V., Erdei S., Farkas G. L. Isolation from Avena leaf tissues of a nuclease with the same type of specificity towards RNA and DNA. Accumulation of the enzyme during leaf senescence. Biochim Biophys Acta. 1971 Mar 25;232(3):472–483. doi: 10.1016/0005-2787(71)90601-0. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Bear D. G., Morgan W. D., McSwiggen J. A. Protein-nucleic acid interactions in transcription: a molecular analysis. Annu Rev Biochem. 1984;53:389–446. doi: 10.1146/annurev.bi.53.070184.002133. [DOI] [PubMed] [Google Scholar]






