SUMMARY
Skin infections are highly prevalent in many Australian Aboriginal communities. This study aimed to determine the prevalence of group A streptococcus (GAS) and Staphylococcus aureus in skin sores of Indigenous people living in an urban setting. We undertook a cross-sectional study of 173 children and youths attending the Wuchopperen Clinic (Cairns) for treatment of skin infections. Participants were interviewed using a structured questionnaire, and a skin lesion swab obtained. The median age was 5·3 years, with 42% identifying themselves as Torres Strait Islanders and 34% as Aboriginal. Impetigo (65%) was the most frequent diagnosis reported followed by scabies (19%); 79% of the lesions had erythema and 70% had exudate. Of 118 lesions, 114 were positive for pathogenic bacteria, with GAS isolated in 84 cases and S. aureus in 92; both these species were recovered from 63 lesions. Significant diversity of emm-types of GAS was associated with skin lesions in Indigenous patients (22 emm-types identified). Fifteen of the 92 S. aureus isolates were suggestive of being community-acquired on the basis of antimicrobial susceptibility profile and nine of these strains were co-cultured from nine lesions. These results have implications for future changes of antibiotic policies for the treatment of skin infections in this population.
Streptococcus pyogenes (group A streptococcus; GAS) and Staphylococcus aureus are the most important organisms causing skin infections. In Australia, rates of skin infection are much higher in many remote Aboriginal communities [1] than in the Caucasian population and some epidemiological studies have reported the prevalence of pyoderma (impetigo or skin sores) to be as high as 70% [1–3]. These studies have also revealed GAS-associated pyoderma to be the most common skin infection in the Aboriginal and Torres Strait Islander (hereafter referred to as Indigenous) people of Central and Northern Australia [1, 3]. Nevertheless, there is some evidence that S. aureus, as the primary pathogen, may be increasing in more affluent urban settings with community-acquired methicillin-resistant S. aureus (CA-MRSA) rising in incidence [4].
In 2001, 73% of the Australian Indigenous population lived in major cities or regional areas [5]. Although there have been numerous studies examining skin infections in remote Aboriginal communities [1, 3], the epidemiology of skin infections among Indigenous people living in urban settings has been less well studied [6]. To describe the pattern of skin infections affecting Indigenous children and youths living in an urban setting, we conducted a study at the Wuchopperen Health Service in Cairns (Far North Queensland). The primary aim was to determine the prevalence of GAS and CA-MRSA among patients with skin sores, and to describe the genetic diversity of GAS isolates obtained from these.
The Wuchopperen Clinic (Wuchopperen Health Services) is an Indigenous Health Service providing care to about 20 000 Indigenous people in Far North Queensland. The primary service is based in Cairns with a smaller clinic in Atherton on the Tablelands, west of Cairns. We conducted a cross-sectional study of children and youths attending the Wuchopperen Clinic in Cairns for treatment of skin infections. Data collection spanned an 8-month period from November 2005 to June 2006. We aimed to recruit all patients aged <20 years attending the clinic with a skin infection. Doctors had the help of an Indigenous research assistant to collect study data. Ethical approval was gained from the Queensland Institute of Medical Research Human Ethics Committee and the Cairns Base Hospital Ethics Committee. In addition, we sought approval from the Wuchopperen Health Service Board to conduct the study. Completed signed consent from participants (parents/carers for those aged <18 years) was required prior to participation in the study. Doctors or the study's research assistant were responsible for explaining the study, gaining informed consent and collecting the data.
There was no strict protocol for diagnosis or definition of skin infections. Doctors working at the Wuchopperen Clinic assessed patients clinically and made diagnoses based on their usual clinical practice. Study participants were interviewed using a structured questionnaire. Questions addressed basic demographic characteristics, clinical history of skin lesion, clinical examination, assessment and treatment. In addition, one skin lesion swab was obtained from each patient. Patients who did not have a skin infection with an exudate (i.e. scabies, ringworm, or simple cellulitis) or had an injury (i.e. animal bite) were not swabbed (17 cases); 38 cases were not swabbed due to an oversight by the doctor. Swab collection and processing was as used for clinical routine sample collection [7], and swabs were transported to the local QML Pathology Laboratory in Cairns for culture and antimicrobial susceptibility testing. Bacterial isolates were identified and antimicrobial susceptibility determined using standard laboratory methods. S. aureus isolates were classified as possible CA-MRSA using current clinical diagnostic criteria based on the antimicrobial susceptibility testing, i.e. isolates resistant to ⩽2 classes of non β-lactam antibiotics (macrolides, quinolones aminoglycosides, etc.). Pathology reports were sent to both the Wuchopperen Clinic for insertion in the patient's medical records and to the Queensland Institute of Medical Research for data entry.
GAS isolates were sent to the Queensland Institute of Medical Research's Bacterial Pathogenesis Laboratory for emm-typing. Briefly, chromosomal DNA was extracted from overnight cultures using the DNAeasy tissue kit (Qiagen, Valencia, CA, USA). The emm-gene was PCR amplified and nucleotide sequence determined. The emm-type of each isolate was subsequently determined by comparison to the emm-type database at the Centers for Disease Control (http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm).
Data were entered into a Microsoft® Access database and all calculations performed using the SPSS statistical software package, version 13 (SPSS Inc., Chicago, IL, USA). Mean and standard deviation were calculated for normally distributed data (e.g. household size, duration of symptoms), median and range for data not normally distributed (e.g. age), and odds ratios (OR) and 95% confidence intervals (CI) to describe associations. Point prevalences of GAS and CA-MSRA were calculated by dividing the number of positive cultures by the total number of swabs. The number of people and bedrooms in the household (as reported by subjects) was used as a measure of household crowding and patients sharing the same address were grouped together. Skin lesions diagnosed within a month of each other were counted as within the same time period.
A total of 184 eligible patients attended the Wuchopperen Clinic with skin infections between November 2005 and May 2006, 173 of whom were included in the study; 11 refused to take part and two were deemed ineligible as the attendances were follow-up visits, giving a response rate of 94%. The majority of patients were aged 0–9 years (80%); ages ranged from 1 month to 20 years (median 5·3 years); 48% were males. All patients were Indigenous; 42% identified themselves as Torres Strait Islanders, 34% as Aboriginal and the remainder Aboriginal and Torres Strait Islanders. The average household size was 6·6 people (s.d.=2·8, range 2–20) and houses had on average 2·9 bedrooms.
The average duration of symptoms was 8·6 days (95% CI 7·0–10·1), ranging from 1 to 60 days; 24 (19%) patients reported fever and 93 (69%) had observed pus at the site of the lesion; 34 (29%) patients had been prescribed previous treatments. Impetigo (65%) was the most frequent diagnosis reported followed by scabies (19%). Of 111 patients with impetigo, 73 had swabs taken: 71 (71%) had exudate and 29 (29%) did not have exudate (information was missing for 11 patients). Twenty-five patients had both scabies and impetigo. At clinical assessment, 79% of the lesions had erythema, 70% had exudate, 64% swelling, 60% tenderness to palpation, and 23% had enlarged lymph nodes. Most skin sores were on the limbs (36%), followed by feet (11%) and hands (9%); 25% were on multiple sites of the body.
Of the swabs collected from 118 lesions, 114 (97%) were positive for pathogenic bacteria. GAS was recovered in 84 cases (71%) and S. aureus in 92 (78%). Antimicrobial susceptibility testing revealed that 15 of the 92 (16%) S. aureus isolates were methicillin-resistant (MRSA). Thirteen of these 15 (87%) MRSA isolates were resistant to only β-lactam antibiotics, while the remaining two isolates were resistant to only one other antimicrobial. While this resistance pattern is typical of circulating Australian CA-MRSA isolates [4, 7], confirmation by multilocus sequence typing (MLST) and staphylococcal chromosomal cassette mec (SCCmec) typing was not performed. Both GAS and S. aureus were isolated from 63 swabs and of the 25 patients who had both impetigo and scabies, GAS was recovered in 82%, and S. aureus in 77% of cases. Of the 15 putative CA-MRSA positive lesions, nine also had GAS.
GAS was recovered in 82% of cases with impetigo compared to 47% of cases with other diagnoses (OR 5·2, 95% CI 2·2–12·2, P<0·001); GAS and S. aureus together were recovered in 60% of cases with impetigo compared to 40% of others (OR 2·5, 95% CI 1·0–5·0, P=0·042). For S. aureus alone and MRSA the associations were negative and statistically not significant (P=0·246 and P=0·783, respectively). Patients with impetigo with exudate were 3·5 times more likely (95% CI 0·94–12·3, P=0·051) to have GAS recovered from their lesions and 1·9 times more likely (95% CI 0·56–6·28) to have both GAS and S. aureus than impetigo patients without exudate. However, chance could not be ruled out. For S. aureus alone and MRSA alone, the associations were negative and statistically not significant (P=0·392 and P=0·712, respectively).
emm-typing of the GAS isolates revealed 22 different emm-types with emm54.0 being the most prevalent (six isolates) followed by emm41.2 and emm42.0 (four isolates each). The presence or absence of scabies, erythema, odour, or swelling did not appear to be associated with any specific emm-type. The only exception was a group of five patients diagnosed with both abscess and boil or furuncle; emm42.0 was identified in two, emm41.2 in two and emm100.0 in one.
Ninety-five of the 173 patients came from different households and 78 shared a household with one or more patients; there were 28 shared households with 2–5 patients each. The Table gives the results for patients who shared a household with another patient in the study, and had microbiologically confirmed skin infections diagnosed within a month of each other. Five households had two patients with the same emm-type of GAS, and one household had two patients with putative CA-MRSA isolated from skin lesions. The average number of people living in the house was similar for those with infected lesions compared with those with negative culture (P=0·591). Likewise, average household sizes for those with lesions infected with GAS, S. aureus and putative CA-MRSA were not statistically different.
Table.
Group A streptococcal emm-types, S. aureus and putative community-acquired methicillin-resistant S. aureus (CA-MRSA): results of patients who shared households at the time of presentation to the Wuchopperen Clinic
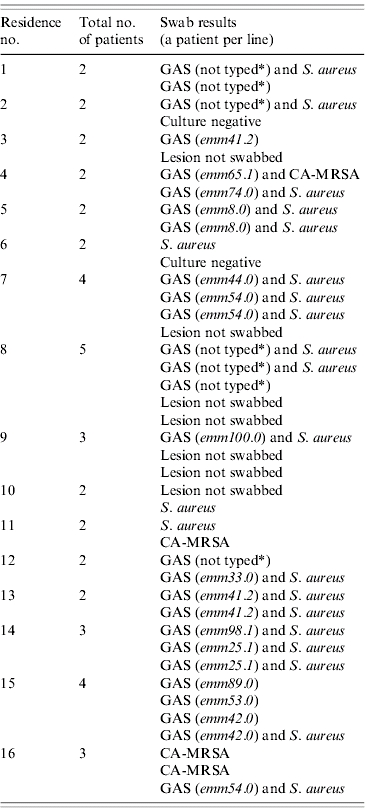
GAS, Goup A streptococcus; CA-MRSA, community-acquired methicillin-resistant S. aureus.
15% of the GAS strains could not be typed.
In 2004, Cairns had a resident population of about 125 000 people, with 7·6% representing the Indigenous population (http://www.cairns.qld.gov.au/community/profiles). The Wuchopperen Clinic was the only Indigenous controlled health service in the region at the time the study was conducted. This study is one of the few to describe skin infections affecting Indigenous children and youths in an urban setting. In this setting Indigenous households tend to be larger than non-Indigenous households, with an average household size of 3·5 people compared with 2·6 for all Australian households [5] and this is reflected here. Nevertheless, we found no association between household size and positive culture for either GAS or S. aureus from skin lesions.
GAS have been further separated on the basis of differences in the surface expressed M protein [8] and these have been elucidated through nucleotide sequencing of the emm-genes which correlate well with M protein serotypes [9]. Certain M-types are commonly, but not exclusively, associated with specific clinical presentations (i.e. pyoderma but not pharyngitis) [10]. There was significant diversity of emm-types of GAS associated with skin lesions in this study (Table). However, patients with skin lesions from the same household did not necessarily share strains of the same emm-types, which suggests that acquisition of strains was mostly through community interaction, rather than spread within the household. The diversity of emm-types reported here is a feature of GAS epidemiology in the Indigenous communities in the North of Australia, and has also been reported in such groups in other geographical regions [10, 11]. Comparing our results to a large study conducted in the Northern Territory [10] where 350 GAS isolates were emm-typed, only 10 of 22 emm-types identified in our sample were also identified in the remote communities and several emm-types were associated with both throat and skin infections in Aboriginal people. Based on studies conducted in other populations, about eight M protein serotypes have been associated with rheumatic heart disease [12]; none was identified in this study sample. However, it is worth pointing out that in remote Aboriginal communities in Australia where rates of acute rheumatic fever and rheumatic heart disease are high, pharyngitis caused by GAS is rare. One hypothesis is that recurrent skin infections may immunize individuals against throat infections. McDonald et al. [13] suggest that in this population, group C and G β-haemolytic streptococci could have a pathogenic role in acute rheumatic fever.
Our data demonstrating the presence of multiple emm-types of GAS in skin sores highlights the dangers of transposing epidemiological features to new populations that may have different social, socioeconomic conditions. Other studies undertaken in the North of Australia [14] have also documented genetic differences between Australian ‘emm-types’ and other emm-types. This further highlights the need for more extensive population studies in affected communities and geographic regions. The high prevalence of multiple emm-types also emphasizes the need for a conserved GAS vaccine, rather than M-type specific, that targets multiple serotypes in this population [15]. In fact none of the emm-types reported in this study are represented in the 26-valent vaccine developed by Hu et al. [16].
The role of putative CA-MRSA as a skin pathogen in the study is also intriguing. Although CA-MRSA has emerged globally as an important pathogen and some forecast epidemics in many areas of the world [4], there have been few studies of its prevalence in our Indigenous population. Vlack et al. [7] found a high prevalence of CA-MRSA carriage in schoolchildren from a Queensland Indigenous community while McDonald et al. [17], investigating the epidemiology of CA-MRSA in remote Aboriginal communities of Northern Australia, reported that 59% of children with skin sores had S. aureus identified and 29% had both S. aureus and GAS; they also found 23% of S. aureus isolates were methicillin resistant. Here GAS and S. aureus were recovered in 60% of cases with impetigo and 14% of S. aureus were putative CA-MRSA.
This was not a large study. The goal was to include all patients attending the Wuchopperen Clinic and 94% of those invited took part. Even though there is a potential for selection bias it is unlikely that the types of lesion, causative bacterial species or genetic subtype were subject to bias. It is not known whether the results of the study can be extrapolated to other urban settings but they do suggest a significant diversity of emm-types of GAS associated with skin lesions in Indigenous patients living in an urban setting, as is the case for remote Aboriginal communities in the Northern Territory [18]. Our findings have implications for future changes of antibiotic policies for the treatment of skin infections in this population. Given that the majority of the Indigenous population of Queensland live in cities, further investigation of the prevalence and antibiotic susceptibility patterns of S. aureus isolates to guide selection of antibiotic therapy is warranted. Pyoderma has historically been treated with penicillin G, and remains the drug of choice for GAS infection. However, when additional coverage against S. aureus is required, the choice for empirical therapy is less clear. Indeed, the recent study in Northern Australia that documented high rates of macrolide resistance suggests that clindamycin may not be the ideal empirical agent [17]. In contrast, cotrimoxazole would be expected to show good cover against putative CA-MRSA, but its activity against GAS is less certain.
ACKNOWLEDGEMENTS
The authors thank Ms Diane Rudkin for data collection, Mrs Valerie Logan for technical support and Ms Leona Daley from QML laboratory in Cairns for processing the swabs. This work has been produced as part of the activities of the Co-operative Research Centre for Aboriginal Health, a collaborative partnership partly funded by the CRC Programme of the Commonwealth Department of Education, Science and Technology. Michael Batzloff is supported by a Postdoctoral Fellowship from the National Heart Foundation of Australia. Patricia Valery was supported by a National Health and Medical Research Council Public Health (Australia) Training Fellowship (NHMRC ID 339461). We are particularly grateful to the children and their families who participated in the study, without whom this work would not have been possible.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Currie BJ, Carapetis JR. Skin infections and infestations in Aboriginal communities in northern Australia. Australasian Journal of Dermatology. 2000;41:139–143. doi: 10.1046/j.1440-0960.2000.00417.x. [DOI] [PubMed] [Google Scholar]
- 2.Gardiner DL, Sriprakash KS. Molecular epidemiology of impetiginous group A streptococcal infections in aboriginal communities of northern Australia. Journal of Clinical Microbiology. 1996;34:1448–1452. doi: 10.1128/jcm.34.6.1448-1452.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nimmo GR et al. Group A streptococcal infection in an aboriginal community. Medical Journal of Australia. 1992;157:521–522. doi: 10.5694/j.1326-5377.1992.tb137346.x. [DOI] [PubMed] [Google Scholar]
- 4.Gosbell IB. Epidemiology, clinical features and management of infections due to community methicillin-resistant Staphylococcus aureus (cMRSA) Internal Medical Journal. 2005;35:S120–135. doi: 10.1111/j.1444-0903.2005.00985.x. (Suppl. 2): [DOI] [PubMed] [Google Scholar]
- 5.ABS Canberra: Australia Bureau of Statistics; 2005. . The health and welfare of Aboriginal and Torres Strait Islander peoples. . Report No.: 47040.0. [Google Scholar]
- 6.Heath DL, Panaretto KS. Nutrition status of primary school children in Townsville. Australian Journal of Rural Health. 2005;13:282–289. doi: 10.1111/j.1440-1584.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 7.Vlack S et al. Carriage of methicillin-resistant Staphylococcus aureus in a Queensland Indigenous community. Medical Journal of Australia. 2006;184:556–559. doi: 10.5694/j.1326-5377.2006.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham MW. Pathogenesis of group A streptococcal infections. Clinical Microbiology Reviews. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Facklam R et al. emm typing and validation of provisional M types for group A streptococci. Emerging Infectious Diseases. 1999;5:247–253. doi: 10.3201/eid0502.990209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDonald MI et al. Molecular typing of Streptococcus pyogenes from remote Aboriginal communities where rheumatic fever is common and pyoderma is the predominant streptococcal infection. Epidemiology and Infection 2007 doi: 10.1017/S0950268807008023. . Published online: 19 February . . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tewodros W, Kronvall G. M protein gene (emm type) analysis of group A beta-hemolytic streptococci from Ethiopia reveals unique patterns. Journal of Clinical Microbiology. 2005;43:4369–4376. doi: 10.1128/JCM.43.9.4369-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisno AL., Mandell GL. Principles and Practice of Infectious Diseases. New York: Churchill Livingstone; 1995. Non-suppurative poststreptococcal sequelae: rheumatic fever and glomerulonephitis; pp. 1799–1810. , pp. [Google Scholar]
- 13.McDonald MI et al. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian aboriginal communities where acute rheumatic fever is hyperendemic. Clinical Infectious Diseases. 2006;43:683–689. doi: 10.1086/506938. [DOI] [PubMed] [Google Scholar]
- 14.McGregor KF et al. Group A streptococci from a remote community have novel multilocus genotypes but share emm types and housekeeping alleles with isolates from worldwide sources. Journal of Infectious Diseases. 2004;189:717–723. doi: 10.1086/381452. [DOI] [PubMed] [Google Scholar]
- 15.McMillan DJ, Chhatwal GS. Prospects for a group A streptococcal vaccine. Current Opinion in Molecular Therapeutics. 2005;7:11–16. [PubMed] [Google Scholar]
- 16.Hu MC et al. Immunogenicity of a 26-valent group A streptococcal vaccine. Infection and Immunity. 2002;70:2171–2177. doi: 10.1128/IAI.70.4.2171-2177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald M et al. Use of a single-nucleotide polymorphism genotyping system to demonstrate the unique epidemiology of methicillin-resistant Staphylococcus aureus in remote aboriginal communities. Journal of Clinical Microbiology. 2006;44:3720–3727. doi: 10.1128/JCM.00836-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald MI et al. The dynamic nature of group A streptococcal epidemiology in tropical communities with high rates of rheumatic heart disease. Epidemiology and Infection 2007 doi: 10.1017/S0950268807008655. . Published online: 31 May . . doi: [DOI] [PMC free article] [PubMed] [Google Scholar]


