SUMMARY
We conducted a seroprevalence survey in Belgium, Finland, England & Wales, Italy and Poland on 13 449 serum samples broadly representative in terms of geography and age. Samples were tested for the presence of immunoglobulin G antibody using an enzyme immunoassay. The age-specific risk of infection was estimated using parametric and non-parametric statistical modelling. The age-specific risk in all five countries was highest in children aged 7–9 years and lower in adults. The average proportion of women of child-bearing age susceptible to parvovirus B19 infection and the risk of a pregnant women acquiring B19 infection during pregnancy was estimated to be 26% and 0·61% in Belgium, 38% and 0·69% in England & Wales, 43·5% and 1·24% in Finland, 39·9% and 0·92% in Italy and 36·8% and 1·58% in Poland, respectively. Our study indicates substantial epidemiological differences in Europe regarding parvovirus B19 infection.
INTRODUCTION
Parvovirus B19 is the infectious agent of erythema infectiosum, commonly known as slapped cheek syndrome or fifth disease [1]. The disease in children and teenagers is usually mild, but infection with parvovirus B19 during pregnancy has been associated with miscarriage, intrauterine fetal death, fetal anaemia and non-immune hydrops [2]. Parvovirus B19 infection has also been associated with acute arthropathy in adults [3], with aplastic crisis in sickle-cell disease patients and with chronic anaemia in immunodeficient patients [4]. It is mainly transmitted through the respiratory route, but blood-borne and nosocomial transmission occurrences have been documented [4, 5].
While some basic features of the epidemiology of parvovirus B19 infection in temperate countries are known – infection in childhood is common, infection continues at a lower rate in adulthood, epidemics occur at intervals of a few years [4] – very few studies have actually tried to address and estimate the burden of parvovirus B19 infection for the population as a whole in a more precise and systematic manner. Most studies have predictably focused on risk factors in pregnant women because of the risk to the fetus [6].
Although vaccine development has shown promising initial results [7], there is currently no vaccine available against parvovirus B19. Because immunoglobulin G (IgG) antibodies following infection are thought to persist for a lifetime [4], seroprevalence profiles from large cross-sectional surveys provide estimates of past exposure to parvovirus B19 infection and can be used to derive the age-specific annual risk of acquiring infection – also known as the force of infection (FOI) – which has been found to vary with age for a number of infectious diseases [8].
The principal aim of our study was to determine age-specific B19 seroprevalence profiles in five European countries (Belgium, England & Wales, Finland, Italy, Poland) by testing blood samples from large serum banks for the presence of IgG antibodies against parvovirus B19 using the same assay to avoid possible inter-assay variation. This study allowed us to compare the epidemiology of parvovirus B19 in the five countries by estimating age-specific seroprevalence of parvovirus B19 infection, derive the age-specific FOI and determine the overall risk of women having a parvovirus B19 infection during pregnancy by linking our analysis with publicly available demographic data.
METHODS
Study population
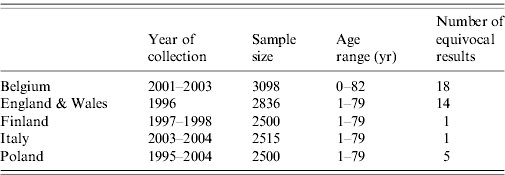
In the five countries testing for parvovirus B19 IgG antibody was performed on large representative national serum banks during 2005–2006. The sera were collected between 1995 and 2004 and were obtained from residual sera collected during routine laboratory testing. Sera covered all age groups, were approximately evenly distributed between males and females and were geographically representative of each country (see Table 1).
Table 1.
Characteristics of serum banks used in our study
Comparison of two commercial parvovirus B19 IgG assays
Prior to testing the large serum banks we compared the diagnostic performance of two commercial parvovirus B19 IgG enzyme immunoassays (EIA) – Mikrogen recomWell (Martinsried, Germany) and Biotrin (Dublin, Ireland) on a subsample of 180 Finnish sera having the same age distribution as the final Finnish data used in this study. Sera were tested according to the manufacturers' instructions and discordant results were retested using the Mikrogen Western blot assay (recomBlot Parvovirus B19 IgG).
Main serum bank testing
The main serum banks were tested using the recomWell Parvovirus B19 IgG assay kits (Mikrogen) from the same batch by five laboratories in each country according to the manufacturer's instructions. Quantitative results were calculated from the optical densities and plate-dependent corrections according to manufacturer's instructions. Samples with antibody activity levels >24 U/ml were considered positive, samples with antibody activity levels <20 U/ml were considered negative and samples in between were considered equivocal.
The cut-off defined by the manufacturer to discriminate negative from positive samples was checked and validated using histograms of the quantitative results on a logarithmic scale.
Modelling seroprevalence and FOI
Seroprevalence was estimated separately for each country, sex and age group. The 95% confidence intervals were calculated based on the exact binomial method.
Parametric and non-parametric curves with confidence bands for the seroprevalence and FOI were estimated using equation (1)
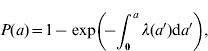 |
(1) |
where P(a) is the prevalence and λ(a) is the FOI at age a. For the parametric estimates of the FOI we initially chose a piecewise constant model with intervals [0·5, 5), [5, 10), [10, 25), [25, 40) and [40+) assuming that maternal antibodies last until age 6 months. The age groups correspond approximately to the 20th, 40th, 60th and 80th percentiles of the age distribution of serum donors.
For the non-parametric estimates of the seroprevalence and FOI, local quadratic polynomials were used with a data-driven automatic procedure to choose the local bandwidth [9]. The 95% confidence intervals were obtained by a bootstrap procedure with 1000 replications.
Estimating number of parvovirus B19 infections in pregnant women
The age-dependent number of infections in pregnancy was estimated using Eurostat data of live births in 1997 (http://epp.eurostat.cec.eu.int), the most recent year with data available for all five countries. The number of pregnant women of age a with B19 infection, I(a), was estimated using equation (2) [10]
| (2) |
where λ(a) is the estimated FOI at age a and 1−P(a) is the estimated proportion of susceptibles of age a according to the local quadratic model, L(a) is the number of live births for mothers of age a, and the scalar 0·75 represents the duration of 9 months of an average pregnancy in yearly units.
RESULTS
Comparison of assays
Qualitative agreement (the proportion of concordant results: 91%) and quantitative correlation (0·87) between the two assays was high. The major reason for discordant results was sera positive by the Biotrin EIA and negative by the Mikrogen EIA. Donors of discordant sera were significantly younger than donors of sera with concordant results. Antibody reactivity in the Biotrin assay on these sera was also significantly lower than for concordant positive results. Retesting with the Mikrogen Western blot assay tended to agree with the Mikrogen EIA assay. Seroprofiles by age of tested sera were similar between the two assays except in the youngest age groups, where the Biotrin assay estimated a slightly higher seroprevalence. Based on these results, the Mikrogen assay was chosen for testing the five main serum banks.
Analysis of the cut-off used for the main serum bank
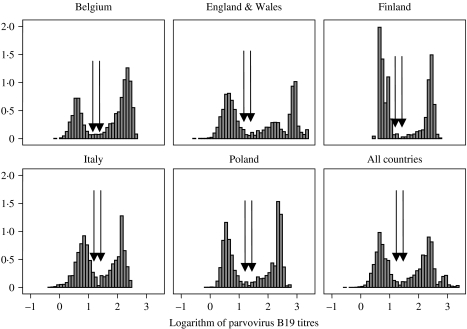
For each country's main serum bank, the Mikrogen assay produced two distinct populations of seropositives and seronegatives (Fig. 1). The equivocal range indicated by the manufacturer was found in the trough between the two populations modes, such that there was no need to apply further modelling techniques as suggested in a previous study based on a different laboratory assay [10]. For further analysis all samples in the equivocal range were excluded (see Table 1). The distributions of quantitative antibody data was very similar between countries, although the data from Finland displayed a smaller variation within each distribution of positives and negatives and the fraction of the data of positives from England & Wales appeared to yield higher titres than found in any of the other countries.
Fig. 1.
Histograms of log parvovirus B19 antibody reaction level distribution of national serum banks in five countries. Left and right arrows indicate negative and positive cut-offs, respectively, as indicated by the manufacturer.
Seroprevalence and the FOI
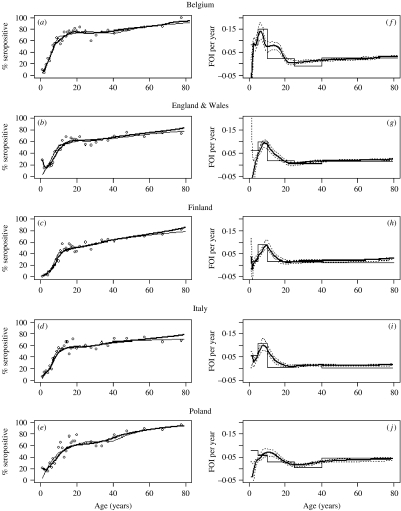
Figure 2(a–e) shows the seroprevalence profiles in the five countries which follow a broadly similar pattern. In all countries we observed that in the first three age groups (i.e. those aged 1–3 years), the seroprevalence data and model estimates appear to be constant or even decrease to some extent resulting in locally negative estimates of the FOI. Again in all five countries, the seroprevalence then increases quite rapidly in older children and teenagers. In older teenagers and young adults the seroprevalences start to level off and reach a plateau for young adults followed by a further increase in age groups about >25–30 years. There is little difference between the seroprevalence estimates of parametric and non-parametric models.
Fig. 2.
Panels (a–e) show different estimates of parvovirus B19 seroprevalence profiles in Belgium, England & Wales, Finland, Italy and Poland, respectively. The open symbols (○) indicate point estimates for each age group, the thick line indicates the local quadratic model and the thin line indicates the piece-wise constant model. Panels (f–j) show the force-of-infection (FOI) estimates corresponding to the seroprevalence profiles in panels (a–e), respectively. The thick line indicates the local quadratic model; the dotted line represents 95% confidence intervals of the local quadratic model, and the thin line the piecewise constant model.
The FOI estimates in Figure 2(f–j) show a similar picture, with a peak occurring in children, followed by a decline in teenagers and a marginal increase again in the late twenties and thirties. Again the parametric and non-parametric models have similar shapes although it is obvious that the non-parametric curves of the local quadratic method allow more smooth and flexible shapes than the parametric piecewise constant method.
When the seroprevalence curves are compared between the countries in more detail, some interesting and unexpected features emerge (Fig. 3a). It is striking that for persons aged between 5 and 45 years, B19 seroprevalence for Finland is always lowest, whereas the prevalence in Belgium is highest and Poland, Italy and England & Wales intermediate. In Finland and Belgium, the difference in seroprevalence estimates in these age groups is statistically significant (data not shown), suggesting that the epidemiology could be different in these two countries. Based on estimates from the local quadratic model, the seroprevalences range from 35% in Finland to 58% in Belgium at age 10 years, from 51% in Finland to 75% in Belgium at age 20 years, and from 57% in Finland to 73% in Belgium at age 30 years, which is approximately the mean age of pregnant women (see Fig. 4). The plateau in the seroprevalence for young adults seems to be more pronounced for Belgium, England & Wales and Italy than for Finland and Poland.
Fig. 3.
(a) Comparison of age-specific parvovirus B19 seroprevalence profiles in Belgium, England & Wales, Finland, Italy and Poland estimated using the local quadratic model. (b) Comparison of age-specific parvovirus B19 force-of-infection (FOI) estimates from the local quadratic model in Belgium, England & Wales, Finland, Italy and Poland.
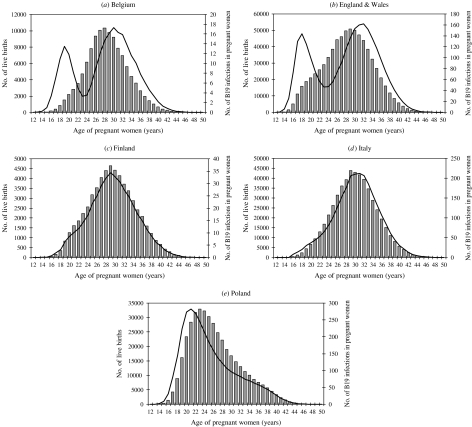
Fig. 4.
Distribution of live births by age of mother in 1997 (shown by histograms, y-axis on the left) according to Eurostat, and distribution by age of annual estimated number of B19 infections occurring during pregnancy based on the local quadratic model of seroprevalence and force of infection (––––, y-axis on the right).
The FOI estimates of the local quadratic model reflect the differences observed in the seroprevalence profiles for children and adolescents. In Belgium there was quite a narrow and sharp peak at 7 years with a maximum value of 0·14. England & Wales and Finland had very similar looking peaks at 9 years with a maximum value of 0·09 per year while for Italy this peak occurs at 8 years with a maximum value of 0·10 per year. The peak in Poland was much wider than in other countries with a FOI estimate of around 0·06 for children aged 8–14 years. Interestingly the Belgian data was unique in showing a shoulder effect in the 12–18 years age group with a FOI estimate of about 0·08. The lowest FOI estimates among adults was found at age 24 years (Belgium, England & Wales, Italy), 22 years (Finland) and 27 years (Poland). Following this decline in early adulthood, the quadratic model suggested another slight increase of the FOI for the 25–40 years age groups. The Polish data was unique in sustaining a much higher FOI (0·03) in persons aged ⩾40 years, compared to the other countries.
Sex-specific differences within countries in seroprevalence and FOI estimates were generally minor and mostly non-significant except in the ⩾40 years age group, where the FOI estimates tended to be slightly higher for women than for men.
Estimating the burden of parvovirus B19 infections in pregnant women
Whereas the age distribution of pregnant women leading to live births in Belgium, Finland and Italy is very symmetric with a mode at 28–30 years, the distribution in England & Wales differs mainly because of a higher proportion of teenage pregnancies and the age distribution of pregnancies in Poland is skewed to the left (Fig. 4). In Finland and Italy, the expected distribution of B19 cases occurring in pregnant mothers follows very closely the actual age distribution of pregnant mothers. In Poland, due to the higher FOI in older teenagers, the peak of expected B19 infections occurs 2 years earlier than the peak of actual pregnancies. Most interestingly in Belgium and England & Wales two distinct peaks appear, the first in teenagers due to a higher FOI and a high rate of teenage pregnancies in the United Kingdom and a second peak which follows more closely the distribution of pregnant women.
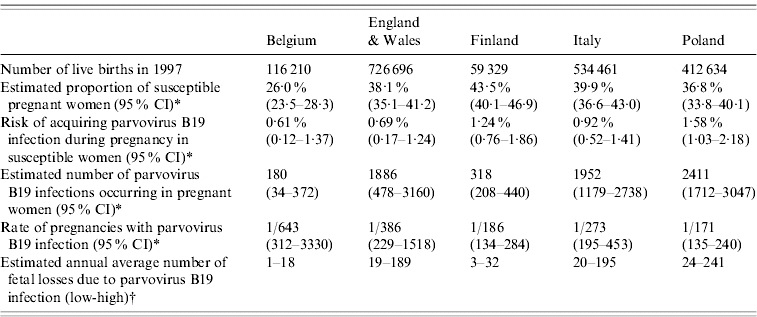
Table 2 shows some summary measures of the total burden of parvovirus B19 among pregnant women in the five countries. Belgium has the lowest estimated proportion of pregnant women susceptible to parvovirus B19 infection and the lowest associated FOI. The risk of acquiring infection during pregnancy is significantly higher in Poland and Finland. This is also reflected in the ratio of maternal infections which varies from 1/643 pregnancies in Belgium to 1/171 pregnancies in Poland. Note that we are only able give a very broad estimate of the total number of fetal deaths due to parvovirus B19 infection during pregnancy because of the wide range of estimates reported in the literature for this parameter [1, 11].
Table 2.
Estimated burden of pregnancy-related parvovirus B19 infection in five European countries
DISCUSSION
To our knowledge this study presents the first comprehensive epidemiological analysis of parvovirus B19 infections from large representative population-wide serum banks using a common laboratory assay in several European countries.
Our seroepidemiological study has revealed several previously unavailable interesting features of parvovirus B19 infection: while the age-specific seroprevalence profiles in the five countries appear to follow broadly similar patterns (i.e. a rapid increase of seroprevalence in childhood, followed by a plateau in young adults and then another increase), we observed notable differences in the FOI profiles in childhood and adolescence, as well as the risk of acquiring infection during pregnancy. Possible epidemiological explanations for the differences could include cultural and behavioural differences that lead to different contact rates in key transmission groups.
In addition to there being real country-specific epidemiological differences, our findings might have been influenced by the serological techniques used to measure parvovirus IgG exposure [12, 13]. In our pilot study, the diagnostic performance of the Mikrogen assay was very similar to the widely used Biotrin assay. Further the fact that antibody reactivity distributions obtained from different laboratories looked very similar suggests that inter-laboratory variation did not play a role in our findings. However, it is interesting to note that in all countries seroprevalence appeared to stay constant or decrease in very young children (aged <4 years), whereas one would expect it to increase following the loss of maternal antibodies in the first year of life. The reasons for this are unclear, but could include a lack of assay specificity for this age group exposed to many other viral agents including possibly the recently discovered human bocavirus belonging to the family Parvoviridae [14].
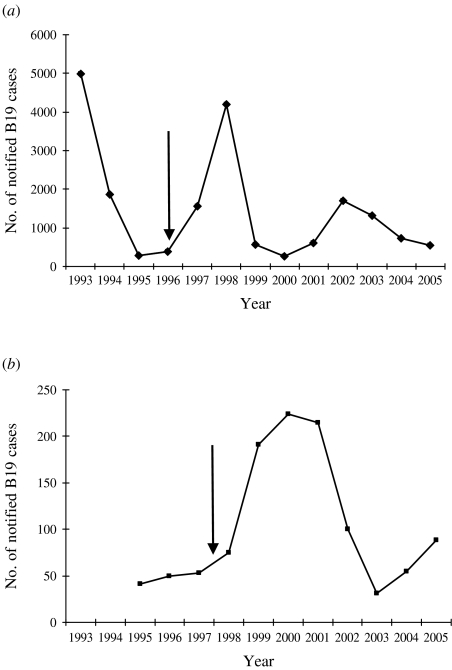
As far as the differing FOI profiles in childhood are concerned, there is a possibility that our estimates could have be influenced by the timing of the sample collection with respect to the epidemic pattern of parvovirus B19 infection, because these derivations assume that the infection is in endemic equilibrium [15]. Figure 5 shows the recent annual number of notified parvovirus B19 infection: for England & Wales the sera were collected 2 years after a very large outbreak in 1993, whereas the collection in Finland occurred just before a major outbreak; unfortunately no similar data are available for Belgium, Italy or Poland. It is possible that such ‘epidemic bias’ could influence our findings such that FOI estimates in Finland might have been larger if the sample collection had been carried out in 2002 after the epidemic. The Polish data are less sensitive to this ‘epidemic cycle’ bias because the serum samples were collected over a 9-year period and therefore the effects of epidemics are likely to have been averaged out. This could explain why the FOI peak in Polish children is much wider than in other countries. Previous modelling studies have claimed that such timing bias has little influence on infectious disease parameters [15], but the authors based their findings on infections with shorter inter-epidemic periods than parvovirus B19. Other authors have also noted the interplay between timing of sample collection and epidemic cycles on seroprevalence [16].
Fig. 5.
Incidence of notified B19 infections in (a) England & Wales, (b) Finland. Arrows indicate timing of sera collection.
While our estimates of seroprevalence in the age groups of childbearing women are broadly similar to those found in other similar studies [10, 13, 17–19], our estimates of risk of maternal infection are generally smaller than those observed in other studies [6, 11, 17]. This could be due to the fact that our cross-sectional estimates are based on an average previous exposure, whereas other study designs could be potentially biased by the epidemic nature of parvovirus B19 transmission.
We have observed some major differences in risk of maternal infection in Europe. This discrepancy can be partly explained by higher rates of infection in children and teenagers (e.g. in Belgium, kindergarten attendance is very high from a young age), and, for Poland at least, a younger average maternal age. Our study shows that potentially greater proportions of maternal infection are expected in countries where teenage pregnancy is more prevalent (where susceptibility is markedly higher than in pregnant women aged ⩾20 years).
Regardless of the differences observed between the countries, our study suggests that parvovirus B19 infection and its effects on maternal outcome are a poorly documented public health issue in Europe. This is principally due to lack of collection of routine epidemiological data as occurs for most vaccine-preventable infections: indeed, parvovirus B19 infection is not a notifiable disease and only limited laboratory confirmation data are collected at national or European level, if at all. With an annual estimate of possibly up to 900 fetal deaths in the five countries studied, this could amount to an annual average of up to several thousand fetal losses when considering Europe as a whole. Exacerbated by the fact that the majority of fetal losses will occur during epidemics every 3–5 years, it is clear that enhanced surveillance of rash fever illness and laboratory notifications of parvovirus B19 infections in women of childbearing age would be necessary to obtain more accurate estimates of the overall burden of parvovirus B19 infection in the population.
This survey is, to our knowledge, the most comprehensive seroprevalence study of parvovirus B19 ever carried out in multiple European countries using a common laboratory methodology. Moreover, in conjunction with direct survey results on social contacts [20, 21], the current study will serve for basic parameterization of dynamic transmission models for close-contact infectious diseases [22, 23]. Results from this study could also serve as an essential specific input in mathematical models evaluating the disease burden of parvovirus B19, as well as the effectiveness and cost-effectiveness of different parvovirus B19 vaccination strategies, in the event of a vaccine becoming available.
ACKNOWLEDGEMENTS
This study was financially supported by grant SP22-CT-2004-502084 of the FP7 programme of the European Commission, DG Research and the Flemish Fund for Scientific Research (FWO grant G.0393.04). The authors are grateful to Veronik Hutse, Robert Vranckx (Scientific Institute of Public Health, Belgium) and Marcello Guido for carrying laboratory testing.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Broliden K, Tolfvenstam T, Norbeck O. Clinical aspects of parvovirus B19 infection. Journal of Internal Medicine. 2006;260:285–304. doi: 10.1111/j.1365-2796.2006.01697.x. [DOI] [PubMed] [Google Scholar]
- 2.Tolfvenstam T et al. Frequency of human parvovirus B19 infection in intrauterine fetal death. Lancet. 2001;357:1494–1497. doi: 10.1016/S0140-6736(00)04647-X. [DOI] [PubMed] [Google Scholar]
- 3.Isa A et al. Prolonged activation of virus-specific CD8+T cells after acute B19 infection. PLoS Medicine. 2005;2:e343. doi: 10.1371/journal.pmed.0020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young NS, Brown KE. Parvovirus B19. New England Journal of Medicine. 2004;350:586–597. doi: 10.1056/NEJMra030840. [DOI] [PubMed] [Google Scholar]
- 5.Zaaijer HL, Koppelman MH, Farrington CP. Parvovirus B19 viraemia in Dutch blood donors. Epidemiology and Infection. 2004;132:1161–1166. doi: 10.1017/s0950268804002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valeur-Jensen AK et al. Risk factors for parvovirus B19 infection in pregnancy. Journal of the American Medical Association. 1999;281:1099–1105. doi: 10.1001/jama.281.12.1099. [DOI] [PubMed] [Google Scholar]
- 7.Ballou WR et al. Safety and immunogenicity of a recombinant parvovirus B19 vaccine formulated with MF59C.1. Journal of Infectious Diseases. 2003;187:675–678. doi: 10.1086/368382. [DOI] [PubMed] [Google Scholar]
- 8.Farrington CP. Modelling forces of infection for measles, mumps and rubella. Statistics in Medicine. 1990;9:953–967. doi: 10.1002/sim.4780090811. [DOI] [PubMed] [Google Scholar]
- 9.Shkedy Z et al. Modeling the force of infection using monotone local polynomials. Journal of the Royal Statistical Society, Series C (Applied Statistics) 2003;52:469–485. [Google Scholar]
- 10.Gay NJ et al. Age specific antibody prevalence to parvovirus B19: how many women are infected in pregnancy? Communicable Disease Report. CDR Review. 1994;4:R104–107. [PubMed] [Google Scholar]
- 11.van Gessel PH et al. Incidence of parvovirus B19 infection among an unselected population of pregnant women in the Netherlands: a prospective study. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2006;128:46–49. doi: 10.1016/j.ejogrb.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson M, Heath A. Report of a collaborative study to calibrate the Second International Standard for parvovirus B19 antibody. Biologicals. 2004;32:207–212. doi: 10.1016/j.biologicals.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Kelly HA et al. The age-specific prevalence of human parvovirus immunity in Victoria, Australia compared with other parts of the world. Epidemiology and Infection. 2000;124:449–457. doi: 10.1017/s0950268899003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning A et al. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. Journal of Infectious Diseases. 2006;194:1283–1290. doi: 10.1086/508219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitaker HJ, Farrington CP. Estimation of infectious disease parameters from serological survey data: the impact of regular epidemics. Statistics in Medicine. 2004;23:2429–2443. doi: 10.1002/sim.1819. [DOI] [PubMed] [Google Scholar]
- 16.Nascimento JP et al. The prevalence of antibody to human parvovirus B19 in Rio de Janeiro, Brazil. Revista do Instituto de Medicina Tropical de São Paulo. 1990;32:41–45. doi: 10.1590/s0036-46651990000100007. [DOI] [PubMed] [Google Scholar]
- 17.Koch WC, Adler SP. Human parvovirus B19 infections in women of childbearing age and within families. Pediatric Infectious Diseases Journal. 1989;8:83–87. [PubMed] [Google Scholar]
- 18.Alanen A et al. Seroprevalence, incidence of prenatal infections and reliability of maternal history of varicella zoster virus, cytomegalovirus, herpes simplex virus and parvovirus B19 infection in South-Western Finland. BJOG: An International Journal of Obstetrics and Gynaecology. 2005;112:50–56. doi: 10.1111/j.1471-0528.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- 19.Enders M, Weidner A, Enders G. Current epidemiological aspects of human parvovirus B19 infection during pregnancy and childhood in the western part of Germany. Epidemiology and Infection. 2007;135:563–569. doi: 10.1017/S095026880600731X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edmunds WJ, O'Callaghan CJ, Nokes DJ. Who mixes with whom? A method to determine the contact patterns of adults that may lead to the spread of airborne infections. Proceedings of the Royal Society of London, Series B: Biological Sciences. 1997;264:949–957. doi: 10.1098/rspb.1997.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beutels P et al. Social mixing patterns for transmission models of close contact infections: exploring self-evaluation and diary-based data collection through a web-based interface. Epidemiology and Infection. 2006;134:1158–1166. doi: 10.1017/S0950268806006418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson NM et al. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallinga J, Teunis P, Kretzschmar M. Using data on social contacts to estimate age-specific transmission parameters for respiratory-spread infectious agents. American Journal of Epidemiology. 2006;164:936–944. doi: 10.1093/aje/kwj317. [DOI] [PubMed] [Google Scholar]
- 24.Miller E et al. Immediate and long term outcome of human parvovirus B19 infection in pregnancy. British Journal of Obstetrics and Gynaecology. 1998;105:174–178. doi: 10.1111/j.1471-0528.1998.tb10048.x. [DOI] [PubMed] [Google Scholar]