SUMMARY
Epidemiological studies have demonstrated high hospitalization rates attributable to influenza and RSV in children aged <24 months. We prospectively recruited 977 children, aged <6 years, presenting with influenza-like illnesses at a hospital during two winter seasons between 2002 and 2004. Nasopharyngeal aspirates were performed and sent for viral immunofluorescence and PCR. Influenza and respiratory syncytial virus (RSV) rates were stratified by age, in-patient/outpatient status and clinical diagnosis. The influenza A and RSV hospitalization incidence rates were 4 and 11/10 000 person-months respectively, whereas the accident and emergency (A&E) outpatient rates were both 32/10 000 person-months in those aged <6 years. The influenza A and RSV hospitalization rates were highest in children aged <24 months and <12 months (9·1 and 35·7/10 000 person-months) respectively. Infection rates were particularly high in those aged <6 months for both viruses. The age-specific influenza A and RSV A&E admission rates were highest in those aged >6 months and those aged <12 months, respectively (43 and 92·5/10 000 person-months, respectively). In conclusion, these high paediatric RSV and influenza incidence rates can be used to inform UK policy on childhood influenza immunization and subsequent RSV immunization in the future.
INTRODUCTION
Documentation of the incidence and morbidity of paediatric influenza and respiratory syncytial virus (RSV) infections informs immunization policy. In the United States, such epidemiological data have been used to great effect in bringing about recommendations for universal paediatric influenza immunization and will be used to create RSV immunization recommendations when an ideal vaccine becomes available [1–5]. In continental Europe there are limited paediatric influenza data available for Germany, [6] Spain [7] and France [8] but there is no consensus on universal immunization. In the United Kingdom, a recent study involving children <72 months of age in a large hospital in Leicestershire [9] revealed that 5·4% of children presenting to the hospital with acute respiratory illness had a diagnosis of influenza infection.
RSV remains the commonest cause of acute lower respiratory tract infections in infants in the industrial world. It is responsible for hospitalization rates of about 30–40/1000 infants per year in Europe and North America, and 59/1000 infants per year in Japan in those with no risk factors [10–14]. Few population-based RSV epidemiological studies exist in Europe and the impact has largely been demonstrated in the context of severe disease and hospitalization rates, particularly with regards to high-risk groups [15, 16].
Furthermore, the interpretation of influenza and RSV incidence rates and morbidity data is often constrained by the knowledge that there may have been a degree of under-reporting. In order to obtain outpatient visit and hospitalization rates attributable to paediatric influenza, differences from the baseline rates in a Massachusetts health maintenance organization were calculated over five seasonal study periods in a retrospective study [4]. The rates of outpatient visits attributable to influenza and RSV among infants and children aged 6–23 months were 1·8 and 4·6/100 person-months, respectively, while for hospitalization this excess rate was 3·9 and 8/10 000 person-months, respectively.
There still remain differences in opinion within European countries about universal influenza vaccination for children [17]. Paediatric influenza and RSV incidence and morbidity data gathered at the local or regional level may help to inform vaccination policy not only at those levels but also at the national level provided it is considered to be representative. We gathered hospital data prospectively during two seasons 2002–2003 and 2003–2004 from a large paediatric population in the catchment area of the Royal London Hospital (RLH), Tower Hamlets, East London in the United Kingdom, a country where universal paediatric influenza immunization is not yet recommended.
METHODOLOGY
Study design
This study was conducted at the RLH in East London. It was a prospective, descriptive study of infants and young children aged <6 years presenting with symptoms suggesting influenza. The study was performed over two winter seasons from 14 October 2002 for 31 weeks and from 17 November 2003 for 24 weeks. The local research ethics committee of the East London and City Health Authority gave ethical approval.
Study population
The main catchment area of the RLH is the London borough of Tower Hamlets, which accounts for about 17 889 resident infants and children aged <6 years [18]. Of those routinely seen at RLH Accident and Emergency Department (A&E), about 80% reside in Tower Hamlets. By contrast, a small proportion of infants and children (1·3%) that were resident in Tower Hamlets were treated routinely at the A&E of the Homerton and Newham Hospitals during the study periods. These are general hospitals on the fringes of the borough of Tower Hamlets. All the children were recruited from six paediatric hospital wards, the high dependency unit or the paediatric A&E of the RLH.
Inclusion and exclusion criteria
Any infant or child aged <6 years was eligible for recruitment if presenting to the hospital with a history of one or more of the following conditions [which collectively define ‘influenza-like illnesses’ (ILI) in children for the purpose of this study] for <7 days (168 hours).
Inclusion criteria were:
acute upper respiratory tract infections including colds, sinusitis, pharyngitis (red sore throat), tonsillitis (inflamed tonsils) and acute otitis media;
acute lower respiratory tract illnesses including croup, tracheitis, bronchiolitis (wheeze age <12 months), virally induced wheeze, asthma and pneumonia (asthma was defined as a child aged >2 years with a history of asthma or newly diagnosed during the study);
any seizure;
any acute febrile gastroenteritis without rebound tenderness or guarding;
apnoea or any life-threatening event in infants aged <12 months;
any acute febrile illness (temperature ⩾38·0°C) but excluding any condition with an acute rash or known non-respiratory viral illness.
Exclusion criteria included any contraindication for nasopharyngeal aspiration (NPA), i.e. recent nasopharyngeal surgery or acute epiglottitis. Those presenting to A&E outside study recruiting times (08:00–18:00 hours) were not recruited overnight but if admitted to hospital, they were eligible for recruitment the next day. Those with difficult social problems such as child protection issues were not approached. In order to obtain written informed consent parents or guardians had to be fluent and literate in English or Bengali (Bengali interpreters were used). Patients were also excluded if consent was refused.
Study team
One clinician and two research nurses were employed during the two seasons. The hospital-based recruitment took place between Monday and Friday in both seasons and also on Saturday in the first season.
Patient enrolment and evaluation
Medical staff in the A&E and the paediatric wards notified the research team about eligible participants. These staff undertook diagnosis and management, according to RLH standard clinical protocols. Once informed consent was obtained enrolment required:
Completion of an electronic standardized questionnaire recording patient demography, past medical history, clinical findings, investigations and diagnosis details. Details of pre-hospital and hospital management were also collected.
A NPA was performed within 24 h according to standard procedures and samples were sent for viral immunofluorescence (IF) and polymerase chain reaction (PCR). The latter was performed twice on every sample; molecular testing of samples was performed using the assays described for influenza by Poddar et al. [19], for RSV by Osiowy [20], for enterovirus by Shen et al. [21] and for adenovirus by Echavarria et al. [22]
-
Telephone follow-up with parents took place at 7 (±2) days, 14 (±2) days and 21 (±2) days following discharge home and weekly until there was objective evidence of resolution of the clinical syndrome. A structured questionnaire was administered during these follow-ups. During telephone contacts, test results were relayed and information was collected on:
the child's clinical state;
whether or not they required re-attendance at a health-care facility;
whether or not additional medications had been procured;
school or day-care absenteeism;
parental time taken off work;
other direct societal costs of treatment.
An anonymized database was set up for all eligible children that were not recruited so that baseline comparisons and extrapolations could be made between the recruited and the unrecruited. This database recorded the date the child was seen, the age, sex, diagnoses and whether admitted to hospital.
Virological studies
Indirect IF methods were used to detect RSV, influenza viruses A & B, parainfluenza virus and adenovirus on fresh NPA aspirates. Separate samples were collected in buffer-containing Eppendorf bottles and stored. A multiplex PCR technique was performed on stored samples to detect RSV, influenza virus A & B, parainfluenza 1, 2 and 3 viruses and enterovirus RNA. PCR analysis was performed twice on each NPA sample in the two seasons.
Statistical analysis
All statistical tests were carried out in stata 8.0 [23]. Incidence rates were calculated for the population of Tower Hamlets as the denominator population was reasonably accurately described. As virology was not available for non-recruited cases, their virology infection rates were estimated from knowledge of the in-patient/outpatient status, clinical diagnosis, and age, using corresponding data of virology status in those recruited. Numerators were corrected to reflect the proportions of eligible cases presenting to A&E and residing in Tower Hamlets (i.e. 80% of all attendees resided in the borough of Tower Hamlets). Numerators were also corrected to reflect the fact that 86·5% and 81·5% of children with influenza and RSV-positive virology were from the borough, respectively. Further extrapolations were made as some children had more than one diagnosis and this was done by adjusting the estimated number of cases based on the actual number of observed cases in each age stratum. Age-specific incidence rates were calculated as cases/10 000 person-months and 1000 person-years, and specific infection rates per total number of ILI recruited were further calculated as both percentages and cases/100 person-years.
Influenza and RSV incidence rates were also calculated for children with asthma using the same methods as above. The numerator was calculated using recruited children presenting with a new diagnosis of asthma or known to have asthma from the history. The age-band populations were based on an asthma prevalence of 9% [24]. The influenza transmission period spanned 13 and 14 weeks in the respective seasons and the RSV season spanned 24 and 27 weeks in the respective seasons. This accounted for a person-time of 6·23 and 11·77 person-months for influenza and RSV, respectively. Pearson's and Fisher's exact χ2 tests were used to compare differences in the Asian, White and Black ethnic groups. Confidence intervals (CI) were computed assuming the Poisson distribution for incidences data.
RESULTS
Patient characteristics
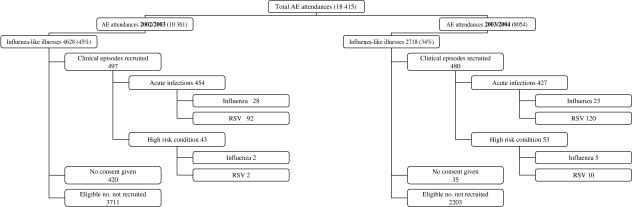
Over the two seasons there were 7346 clinical episodes that presented with ILI out of a total of 18 415 (40%) paediatric A&E episodes, which were aged <6 years. Complete age and diagnostic data were available for 4593 out of 4628 ILI presenting in the first season (over 31 weeks) and 497 clinical episodes were screened; in 443 recruited children (10%). In the second season complete data were available for 2705 out of 2718 ILI (cases over 24 weeks) and 480 clinical episodes were screened: in 438 recruited children (16%) (some children had multiple attendances). Figure 1 shows a flowchart of the recruitment process.
Fig. 1.
Flowchart for the recruitment of children into the study.
Table 1 shows baseline comparisons between recruited and non-recruited clinical episodes. Children with recruited episodes were on average 6 months younger (P<0·001) and were 17 times more likely to be admitted than those with non-recruited episodes (P<0·001). There was an overall decrease in the proportion of those with eligible clinical episodes seen in A&E between seasons: 4628 out of 10 361 (45%) during the first season and 2718 out of 8054 (34%) in the second. This decline was mostly due to the large number of the clinical episodes presenting as gastroenteritis during the first season. When gastroenteritis cases were excluded eligible episodes accounted for 35% and 32% in the first and second seasons, respectively. In the study, 86·5% and 81·5% of respective influenza-A- and RSV-infected children resided in Tower Hamlets. As previously stated, 80% of all clinical episodes presenting to the A&E during the study period were aged <72 months and resided in the Tower Hamlets catchment area. There were 455 eligible clinical episodes that were not recruited due to refusal of carer to give consent, non-fluency in English or Bengali of the carer, and social problems. The mean age of this group was 20·4 months, the male:female ratio was 1·65:1 and the in-patient:outpatient ratio was 1:1·2. These clinical episodes were included in the extrapolation exercise as were other non-recruited episodes. There were 56 children aged <1 year and born prematurely. Of these, 20 had RSV and two had influenza.
Table 1.
Baseline comparisons between recruited and unrecruited eligible clinical episodes added <72 months seen in hospital A&E departments
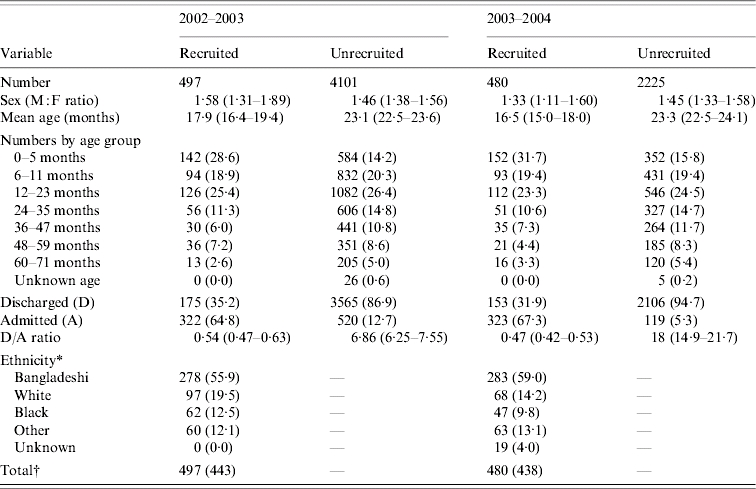
Values within parentheses are percentages or 95% confidence intervals.
Only known for recruited cases.
Total: No. of clinical episodes, () no. of patients recruited.
Children with high-risk medical conditions for influenza
Underlying high-risk factors were identified in 96 (9·8%) of all children recruited of which seven (7·3%) had influenza, and 12 (12·5%) had RSV identified in their NPA. Of the high-risk cases, 40 children had asthma, 26 congenital heart disease, 21 bronchopulmonary dysplasia, six cystic fibrosis, six sickle cell disease, six were on steroid therapy, five had chronic renal disease, three had thalassaemia, and two suffered from immunodeficiencies. Fourteen children had more than one high-risk condition, (asthma accounted for 42% of all high-risk conditions recruited into the study, i.e. 4·1% of all episodes recruited). Of the children known to have asthma, one had influenza and two had RSV identified in their NPA, although all three did not present with asthma exacerbations.
Virology
Over the two seasons, a total of 315 (33%) viruses were identified in 965 samples taken from children aged <72 months. The viral yield was greater in 2003–2004 than in 2002–2003 (P<0·001, Fisher's exact test): 28% of the samples were positive in 2002–2003 compared with 38% in 2003–2004. The virus-specific rates/100 ILI recruited into the study are shown in Tables 2 a and 2 b.
Table 2(a).
Samples (%) detected positive for virus in 2002–2003 (first season): 491 samples tested in children aged <6 years
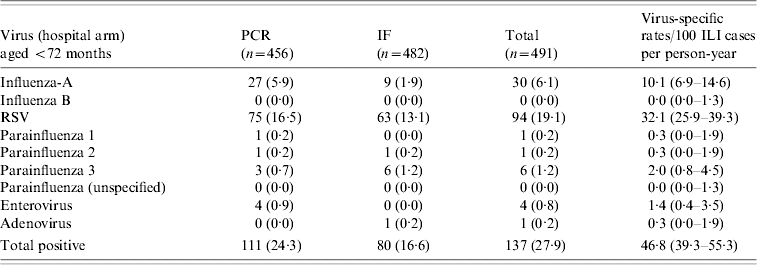
PCR, Polymerase chain reaction; IF, immunofluorescence; ILI. Influenza-like illness; RSV, respiratory syncytial virus.
Values within parentheses are percentages or 95% confidence intervals.
Table 2(b).
Samples (%) detected positive for virus in 2003–2004 (second season): 474 samples tested in children aged <6 years

PCR, Polymerase chain reaction; IF, immunofluorescence; ILI. Influenza-like illness; RSV, respiratory syncytial virus.
Values within parentheses are observed numbers, percentages or 95% confidence intervals.
The overall virus-specific rate/100 ILI for influenza A infections (aggregated 2002–2003 and 2003–2004) (using recruited patients as the denominator) was 11·3/100 person-years (95% CI 8·6–14·6). There was no difference in the virus-specific infection rates/100 ILI cases between the two seasons (P=0·4). RSV infection rates were significantly higher than influenza A infection rates in both seasons. RSV infection rates were higher in 2003–2004 when compared with the 2002–2003 season.
Laboratory-confirmed influenza admissions were seen in Asians (5·5%), Whites (0·9%), Blacks (8·0%) and other groups (4·3%) (χ2=5·6, P=0·03), whereas the A&E outpatient cases were seen in Asians (9·7%), Whites (7·3%), Blacks (7·8%) and other groups (2·8%) (χ2=0·42, P=0·87). Laboratory-confirmed RSV admissions were seen in Asians (23·1%), Whites (29·7%), Blacks (22·7%) and other groups (19·6%) (χ2=2·22, P=0·35), whereas the RSV A&E outpatient cases were seen in Asians (18·9%), Whites (27·3%), Blacks (23·5%) and other groups (16·5%) (χ2=1·95, P=0·37).
Influenza and RSV incidence rates
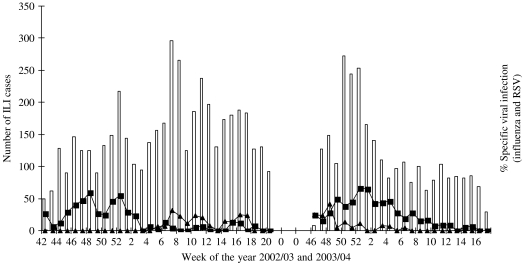
Extrapolated influenza A and RSV hospital incidence rates in children aged <72 months residing in Tower Hamlets (based on the Tower Hamlets population size) and those seen in A&E during the study period are shown in Tables 3 and 4. Tables 5 and 6 show the influenza A and RSV incidence rates for children aged between 2 and 5 years who were known to have asthma. Table 7 shows the age-specific band influenza and RSV rates/1000 per year for the borough population and hospital population. Figure 2 shows the periods of influenza and RSV circulation by week.
Table 3.
Age-specificinfluenza incidence rates
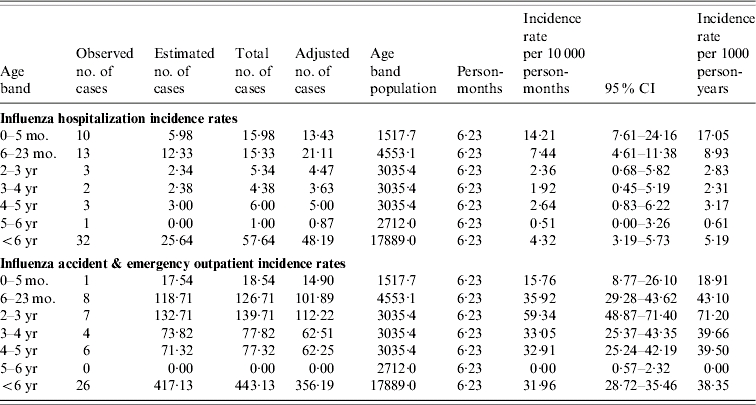
CI, Confidence interval.
The influenza adjusted no. of cases: observed and estimated number of cases were multiplied by a factor of 0·865 and 0·80 respectively to reflect the number of cases residing in Tower Hamlets borough.
Table 4.
Age-specific respiratory syncytial virus (RSV) incidence rates

CI, Confidence interval.
The RSV adjusted no. of cases: observed and estimated number of cases were multiplied by a factor of 0·815 and 0·80 respectively to reflect the number of cases residing in Tower Hamlets borough.
Table 5.
Age-specific influenza incidence rates in children aged 2–5 years with asthma
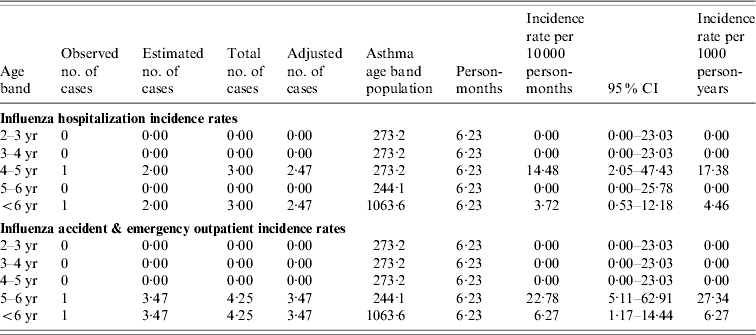
CI, Confidence interval.
The influenza adjusted no. of cases: observed and estimated number of cases were multiplied by a factor of 0·865 and 0·80 respectively to reflect the number of cases residing in Tower Hamlets borough.
The asthma age-band population was based on a prevalence of asthma of 9% in children aged 2–5 years in the United Kingdom.
The definition of a child with asthma for the purpose of the study was a child diagnosed with asthma during the study or a child known to have asthma.
Table 6.
Age-specific respiratory syncytial virus (RSV) incidence rates in children aged 2–5 years with asthma
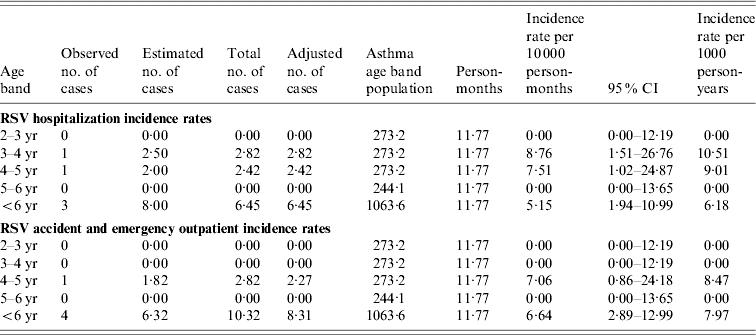
CI, Confidence interval.
The RSV adjusted no. of cases: observed and estimated number of cases were multiplied by a factor of 0·815 and 0·80 respectively to reflect the number of cases residing in Tower Hamlets borough.
The asthma age-band population was based on a prevalence of asthma of 9% in children aged 2–5 years in the United Kingdom.
The definition of a child with asthma for the purpose of the study was a child diagnosed with asthma during the study or a child known to have asthma.
Table 7.
Burden of influenza A and respiratory syncytial virus (RSV) childhood infections
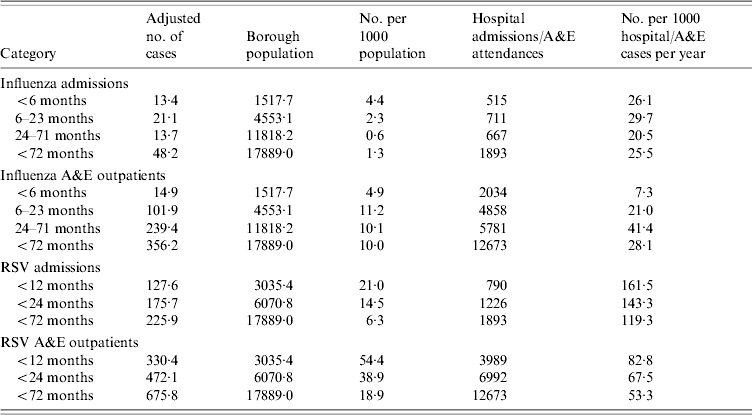
A&E, Accident and Emergency department.
The no. of influenza and RSV cases per 1000 population is per year (i.e. is divided by a factor of 2).
The <2-year-old influenza hospitalization rate per 1000 is 2·8/1000.
The <2-year-old influenza A&E outpatient rate per 1000 is 1·45/1000.
The number of hospital admissions and A&E attendances are over the winter season (i.e. the study periods).
Fig. 2.
Weekly influenza-like illnesses (ILI) and specific viruses in 2002–2003 and 2003–2004 winter seasons. □, Weekly number of influenza-like cases; –■–, weekly (%) of respiratory syncytial virus cases; –▲–, weekly (%) of influenza cases recruited.
DISCUSSION
RSV and influenza infections are the two most common viral causes of ILI in childhood in the industrial world but their exact contributions are unknown [25]. We prospectively studied infants and young children presenting with respiratory illnesses and other influenza-related presentations to the Royal London Hospital paediatric A&E over two winter seasons.
The percentage of laboratory-confirmed influenza in children enrolled into our study was 6% which is similar to the 5·4% estimated for Leicestershire in the 2001–2002 season [9], suggesting low influenza transmission over three consecutive seasons in the United Kingdom. These findings are further supported by Health Protection Agency influenza surveillance statistics [26, 27].
Our influenza hospitalization rates, and laboratory-confirmed influenza percentages were comparable to those documented in the United States [1, 3, 28, 29], Germany [30], France [8, 31], Italy [32] and South Korea [33]. Noticeably, these were lower than those documented in a number of other studies carried out in Spain [7], the United States [34, 35], Japan [36] and Hong Kong [37]. In contrast, our rates were higher than some studies performed in the United States, Finland and a study performed in the United Kingdom in the late 1970s [2, 4, 38, 39]. Some of these studies may have been carried out during different intensities of influenza transmission when compared to our study. Part of the discrepancy in rates may also be explained by the different methodologies used in some of these studies, as most of the large studies were retrospective and/or ecological studies.
RSV hospitalization rates in infants were comparable to previous studies; 21/1000 infants per year vs. 30–40/1000 infants per year in most studies in Europe and the United States. But lower than that documented in Japan and a US study in Alaskan Native American Indians [1, 10–14, 40, 41]. Our RSV rates were higher than the rates reported in retrospective studies performed in Germany and Sweden [15, 16].
The age-specific pattern for hospitalization rates was similarly reflected in the RSV A&E outpatient incidence rates where the burden was also highest in the <2 years age group and particularly exaggerated in those aged <1 year, this is similar to findings in other studies [1, 16, 42, 43]. Our study also documents that influenza hospitalization rates were highest in the <2 years age group and particularly exaggerated in those aged <6 months. In contrast, the influenza A&E outpatient rates were highest in those who were aged 6–59 months and these patterns were similar to those seen in other prospective studies.
Overall RSV and influenza infections in the <2 years age group accounted for almost 14% and 3% of all hospitalizations and 7% and 2% of all A&E outpatient visits, respectively, supporting the important epidemiological burden of both infections on hospital services.
Of note the overall influenza A&E rates were seven times higher than the hospitalization rates [28, 31, 36] and the age-specific A&E outpatient rates peaked in the 2–3 years age group. It has been inferred that pre-school centres play a role in the transmission of influenza. However, in our study population the utilization of these centres was extremely low in the <3 years age group, most children making their first contact with groups at the age of 3–4 years when they first start nursery school. We cannot exclude an element of bias as observed numbers were small and a number of adjustments were made in calculating the incidences but every effort was made to collect robust data.
Interpretation of influenza incidence rates is constrained by under-reporting and this is further inhibited by the impracticability of performing respiratory viral testing on all children presenting with an ILI. Our study suffered to a lesser degree from this problem because of its prospective design and our use of diagnostic knowledge for all those seen in A&E. The criteria for eligible children were based on the fact that most of the specific diagnoses included are associated with influenza. Less common associations such as febrile gastroenteritis were included to enhance the robustness of the results. But the latter did not affect the overall incidence estimates, as the influenza proportion positivity was low in this diagnosis. The recruited children were younger and more likely to be admitted when compared to those unrecruited and therefore the study was open to selection bias in favour of selecting more severe patients because only those admitted to hospital ‘after hours’ were included. Adjustments have been made in the analyses to correct for these biases but an element of bias may remain resulting in an underestimate of the true rate of the diseases as both infections are known to be largely community-based infections.
It has been suggested that ethnicity may play a role in the epidemiology of both influenza and RSV infections. Asian (Bangladeshi) was the most predominant ethnic group in our recruited study population and the highest laboratory-confirmed influenza hospitalization infection proportions were documented in those of Black ethnicity. This was 9·5 and 1·5 times higher than the White and Asian groups, respectively. A study in the United States showed that the influenza hospitalization proportions were higher in Blacks and Hispanics when compared to Whites [28]. Adjustments were not made for ethnicity but our recruited population was a reflection of the Tower Hamlets borough childhood population. Notwithstanding there may be some limitation for generalizability when applied across the United Kingdom, particularly as regards influenza hospitalization rates. One strength of the study was that it was conducted over two seasons. We note the difference in both the length of the study periods and time of commencement and as a result there may have been some limitation in interpretation of the data. We believe this is minimal as time-frames and commencement of the influenza seasons in both study periods are comparable with the national UK data. A caveat was that entry onto the eligible non-recruits' database was based on the attending clinician's clinical diagnosis, as the research team in the hospital performed no independent evaluation for accuracy and consistency of diagnosis. Analyses for discharge diagnosis of those admitted were based on the final diagnosis.
The current recommendation for paediatric influenza immunization in the United Kingdom is to target clinical risk groups in those aged ⩾6 months. These risk groups comprise children with asthma and other chronic respiratory conditions, haemoglobinopathies, the immunosuppressed, children with chronic cardiac, renal and liver disease, and those with diabetes.
In our study 10% of all the children had underlying high-risk conditions of which almost half had asthma. It is to be noted that influenza and RSV incidence rates in children with asthma were extremely low in our study in contrast to healthy children and similar findings have been seen in other studies conducted in the United Kingdom [44]. Therefore it can be assumed that our rates are well-matched with those for healthy children. It is not clear why our findings differ from those seen in the United States. Controversy exists as to whether influenza infections trigger asthma exacerbations in children and a number of studies in the United States have supported the role of influenza in causing substantial burden in these children [1, 2, 45, 46]. Christy et al. and Bueving et al. in the United States and The Netherlands respectively demonstrated that influenza vaccination did not reduce the incidence of asthma exacerbations [47, 48]. These latter findings are supported by a Cochrane review on the benefits of influenza vaccination in asthmatics [49]. Unfortunately there was no background data collected to analyse the overall influenza incidence rates in those with other high-risk conditions in our study.
The United States based the expansion of the influenza vaccination programme to cover children aged 6–23 months on evidence that showed that they had influenza hospitalization and outpatient incidence rates similar to those children with high-risk conditions. More recently this has been further expanded to include all children aged <5 years. This is the first comprehensive prospective study in the United Kingdom on the hospital burden of influenza and RSV in childhood, which clearly details the rates of both viruses in the population during two low-intensity transmission seasons. Further studies are required to document these rates in the community and during varying degrees of intensity using the same methodology.
Expanding the current vaccination programme to include healthy children aged <23 months should be considered as hospitalization rates are highest in those aged <2 years. Furthermore, we recommend that all children aged <2 years be immunized against RSV when the ideal vaccine arrives on the market as RSV creates an important burden in the hospital.
ACKNOWLEDGEMENTS
We acknowledge Dr Stephen Teo, Rebecca I'Anson, Shelley Mieres, Ann Bruce and Lynn Hardie for their assistance in the recruitment of children and reviewing the manuscript. We thank the children and parents immensely for taking part in the study. We are also grateful to the medical and nursing staff of the Royal London Hospital.
DECLARATION OF INTEREST
Dr E. K. Ajayi-Obe received partial funding to attend and present at the International Congress on Pediatrics, Mexico 2004. Dr E. David G. McIntosh is employed by Wyeth, one of the manufacturers of influenza vaccines. Professor R. Booy has been a consultant to GSK and Wyeth. The study was funded by an unrestricted grant from Wyeth.
REFERENCES
- 1.Izurieta H et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. New England Journal Medicine. 2000;342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 2.Neuzil KM et al. The effect of influenza on hospitalization, outpatient visits, and courses of antibiotics in children. New England Journal Medicine. 2000;342:225–231. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 3.Neuzil KM et al. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. Journal Infectious Diseases. 2002;185:147–152. doi: 10.1086/338363. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien MA et al. Incidence of outpatient visits and hospitalization related to influenza in infants and young children. Pediatrics. 2004;113:585–593. doi: 10.1542/peds.113.3.585. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Paediatrics http://www.cispimmunize.org/ 2006. http://www.cispimmunize.org/ . Recommended childhood and adolescent immunization schedule United States July–December 2004. ( ). Accessed 17 May .
- 6.Weigl JA, Puppe W, Schmitt HJ. The incidence of influenza-associated hospitalizations in children in Germany. Epidemiology and Infection. 2002;129:525–533. doi: 10.1017/s0950268802007707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro-Mari JM et al. The impact of influenza viruses on hospitalization in infants younger than two years old during epidemics of respiratory syncytial virus infection. Clinical Microbiology and Infection. 2003;9:959–963. doi: 10.1046/j.1469-0691.2003.00672.x. [DOI] [PubMed] [Google Scholar]
- 8.Ploin D et al. Influenza burden in children newborn to eleven months of age in a paediatric emergency department during the peak of an influenza epidemic. Pediatric Infectious Disease Journal. 2003;22:S218–S222. doi: 10.1097/01.inf.0000092191.78339.1a. (Suppl. 10): [DOI] [PubMed] [Google Scholar]
- 9.Nicholson KG et al. Influenza-related hospitalizations among young children in Leicestershire. Pediatric Infectious Disease Journal. 2003;22:S228–S230. doi: 10.1097/01.inf.0000092193.91306.2f. (Suppl. 10): [DOI] [PubMed] [Google Scholar]
- 10.Muller-Pebody B et al. Contribution of RSV to bronchiolitis and pneumonia associated hospitalizations in English children, April 1995–March 1998. Epidemiology and Infection. 2002;129:99–106. doi: 10.1017/s095026880200729x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristensen K et al. Epidemiology of respiratory syncytial virus infection requiring hospitalizations in East Denmark. Pediatric Infectious Disease Journal. 1998;17:996–1000. doi: 10.1097/00006454-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Shay DK et al. Bronchiolitis associated hospitalizations among US children 1980–1996. Journal of the American Medical Association. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 13.Boyce TG et al. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. Journal of Pediatrics. 2000;137:865–870. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 14.Saijo M et al. The role of respiratory syncytial virus in acute bronchiolitis in small children in Northern Japan. Acta Paediatrica Japonica. 1994;36:371–374. doi: 10.1111/j.1442-200x.1994.tb03203.x. [DOI] [PubMed] [Google Scholar]
- 15.Weigl JAI, Puppe W, Schmidt HJ. Incidence of Respiratory Syncytial Virus-Positive hospitalizations in Germany. European Journal of Clinical Microbiololgy and Infectious Diseases. 2001;20:452–459. doi: 10.1007/s100960100527. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson M et al. Population-based rates of severe respiratory syncytial virus infection in children with and without risk factors, outcome in a tertiary care setting. Acta Paediatrica. 2002;91:593–598. doi: 10.1080/080352502753711740. [DOI] [PubMed] [Google Scholar]
- 17.Principi N, Esposito S. Are we ready for universal influenza vaccination in paediatrics? Lancet Infectious Diseases. 2004;4:75–83. doi: 10.1016/S1473-3099(04)00926-0. [DOI] [PubMed] [Google Scholar]
- 18.2001 Census http://www.statistics.gov.uk/census. 2006. http://www.statistics.gov.uk/census . Key Statistics for Local Authorities. Crown Copyright ( ). Accessed 10 June .
- 19.Poddar SK, Espina R, Schnurr DP. Evaluation of a single step multiplex, RT-PCR for influenza virus type and subtype detection in respiratory samples. Journal of Clinical Laboratory Analysis. 2002;16:163–171. doi: 10.1002/jcla.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osiowy C. Direct detection of respiratory syncytial virus, parainfluenza virus and adenovirus in clinical respiratory specimens by multiplex reverse transcriptase-PCR assay. Journal of Clinical Microbiology. 1998;36:3149–3154. doi: 10.1128/jcm.36.11.3149-3154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen S, Desselberger U, McKee TA. The development of an antigen capture polymerase chain reaction assay to detect and type human enteroviruses. Journal of Virology Methods. 1997;65:139–144. doi: 10.1016/s0166-0934(97)02181-2. [DOI] [PubMed] [Google Scholar]
- 22.Echavarria M et al. Rapid detection of adenovirus in throat swab specimens by PCR during respiratory disease outbreaks among military recruits. Journal of Clinical Microbiology. 2003;41:810–812. doi: 10.1128/JCM.41.2.810-812.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stata Corporation Stata Corporation: College Station; TX: 2003. . STATA 8.0. Stata Statistical Software: Release 8.0, [Google Scholar]
- 24.Gupta R, Strachan D http://www.statistics.gov.uk/children/downloads/asthma.pdf. 2006. http://www.statistics.gov.uk/children/downloads/asthma.pdf . Asthma and allergies. The health of young children National Statistics ( ). Accessed 2 July .
- 25.Zambon MC et al. Contributions of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358:1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- 26.Crofts JP et al. Influenza surveillance in the United Kingdom October 2002 to May 2003. Communicable Disease Reports. 2004;14:1–9. (Suppl. 1): [Google Scholar]
- 27.Crofts JP et al. Influenza surveillance in the United Kingdom October 2001 to May 2002. Communicable Disease Reports. 2002;12:1–7. (Suppl. 1): [Google Scholar]
- 28.Iwane MK et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus and parainfluenza virus among young children. Pediatrics. 2004;113:1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 29.Poehling KA et al. The unrecognised burden of influenza in young children. New England Journal Medicine. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 30.Forster J. Influenza in children: the German perspective. Pediatric Infectious Disease Journal. 2003;22:S215–S217. doi: 10.1097/01.inf.0000092190.43140.f7. (Suppl. 10): [DOI] [PubMed] [Google Scholar]
- 31.Aymard M, Valette M, Luciani J. The sentinel physicians from Grippe and Infections Respiratoires Aigues Pediatriques Network. Burden of influenza in children: preliminary data from a pilot survey network on community diseases. Pediatric Infectious Disease Journal. 2003;22:S211–S214. doi: 10.1097/01.inf.0000092189.42748.cc. (Suppl. 10): [DOI] [PubMed] [Google Scholar]
- 32.Principi N et al. for the Flu Study Group. Burden of influenza in healthy children and their households. Archives of Disease in Childhood. 2004;89:1002–1007. doi: 10.1136/adc.2003.045401. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Kim MR, Lee HR, Lee GM. Epidemiology of acute viral respiratory tract infections in Korean children. Journal of Infection. 2000;41:152–158. doi: 10.1053/jinf.2000.0715. [DOI] [PubMed] [Google Scholar]
- 34.Glezen WP, Couch RB. Interpandemic influenza in the Houston area 1974–76. New England Journal Medicine. 1978;298:587–592. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- 35.Glezen WP et al. Influenza virus infections in infants. Pediatric Infectious Disease Journal. 1997;16:1065–1068. doi: 10.1097/00006454-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Sugaya N et al. The impact of winter epidemics of influenza and respiratory syncytial virus on paediatric admissions to an urban general hospital. Journal of Medical Virology. 2000;60:102–106. [PubMed] [Google Scholar]
- 37.Chiu SS et al. Influenza-related hospitalization among children in Hong Kong. New England Journal Medicine. 2002;347:2097–2103. doi: 10.1056/NEJMoa020546. [DOI] [PubMed] [Google Scholar]
- 38.Martin AJ, Gardner PS, McQuillin J. Epidemiology of respiratory viral infection among paediatric inpatients over a six-year period in North-East England. Lancet. 1978;2:1035–1038. doi: 10.1016/s0140-6736(78)92351-6. [DOI] [PubMed] [Google Scholar]
- 39.Heikkinen T et al. Burden of influenza in children in the community. Journal of Infectious Diseases. 2004;190:1369–1373. doi: 10.1086/424527. [DOI] [PubMed] [Google Scholar]
- 40.Lowther SA et al. Bronchiolitis-associated hospitalizations among American Indian and Alaskan native children. Pediatric Infectious Disease Journal. 2000;19:11–17. doi: 10.1097/00006454-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Clark SJ et al. Respiratory syncytial virus infection in high risk infants and the potential impact of prophylaxis in a United Kingdom cohort. Archives of Diseases in Childhood. 2000;83:313–316. doi: 10.1136/adc.83.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Constantopoulos AG et al. Burden of respiratory syncytial viral infections on paediatric hospitals: a two year prospective epidemiological study. European Journal of Clinical Microbiology and Infectious Diseases. 2002;21:102–107. doi: 10.1007/s10096-001-0668-y. [DOI] [PubMed] [Google Scholar]
- 43.Sims DG et al. Respiratory syncytial virus in north-east England. British Medical Journal. 1976;2:1095–1098. doi: 10.1136/bmj.2.6044.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleming DM et al. Respiratory illness associated with influenza and respiratory syncytial virus infection. Archives of Diseases in Childhood. 2005;90:741–746. doi: 10.1136/adc.2004.063461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuzil KM et al. The burden of influenza illness in children with asthma and other chronic medical conditions. Journal of Pediatrics. 2000;137:856–864. doi: 10.1067/mpd.2000.110445. [DOI] [PubMed] [Google Scholar]
- 46.Mullooly JP, Barker WH. Impact of type A influenza on children: a retrospective study. American Journal of Public Health. 1982;72:1008–1116. doi: 10.2105/ajph.72.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christy C et al. The effectiveness of influenza vaccine prevention of asthma exacerbations. Archives of Diseases in Childhood. 2004;89:734–735. doi: 10.1136/adc.2003.030999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bueving HJ et al. Influenza vaccination in children with asthma Randomised double-blind placebo controlled trial. American Journal of Respiratory and Critical Care Medicine. 2004;169:488–493. doi: 10.1164/rccm.200309-1251OC. [DOI] [PubMed] [Google Scholar]
- 49.Cates CJ et al. Vaccines for preventing influenza in people with asthma. Cochrane Database of Systematic Reviews. 2004 doi: 10.1002/14651858.CD000364. . Issue 2, Art. No. CD000364. [DOI] [PubMed] [Google Scholar]