SUMMARY
The first epidemic of sporotrichosis in humans as a result of zoonotic transmission was identified in Rio de Janeiro, Brazil, in 1998. A cross-sectional study was conducted applying questionnaires to patients seen in 2002 at Evandro Chagas Clinical Research Institute, Fiocruz, with a confirmed diagnosis of sporotrichsosis. A total of 73 dwellings were studied, where 255 individuals, including 94 patients and 161 healthy household contacts, lived with 133 cats with sporotrichosis. Most dwellings were houses with 83% having complete basic sanitation. Among patients, there was a predominance of women with a median age of 41 years who were engaged in domestic activities. These women contracted the disease twice more often than men. The prevalence of sporotrichosis was four times higher among patients caring for animals, irrespective of gender. In the current epidemic of sporotrichosis, taking care of sick cats was the main factor associated with transmission of the disease to humans.
Sporotrichosis caused by the dimorphic fungus Sporothrix schenckii is widely distributed throughout the world, especially in tropical and subtropical regions. Classically, infection is caused by traumatic inoculation of soil, plants and organic matter contaminated with the fungus. Some leisure and occupational activities such as agriculture and floriculture have been associated with transmission of the disease [1]. To date, the largest epidemic of sporotrichosis occurred in Witwatersrand, South Africa, in the 1940s when about 3000 miners were infected from wood timbers in the mines [2]. However, the literature about epidemics is scant and usually related to a common source of infection [3, 4].
Singer & Muncie [5] reported the first case of naturally acquired feline sporotrichosis. Until the 1980s, feline sporotrichosis was rare and its transmission to humans was eventually described in isolated cases or in small outbreaks among veterinarians, technicians, caretakers and owners of cats with the disease [4, 6–10]. Most cats with sporotrichosis present lesions rich in parasites, often accompanied by systemic involvement, and eventually die [11]. Other animals have been reported to be possible transmitters of the disease, although without a significant zoonotic potential [12].
Since 1998, the Infectious Diseases and Zoonosis Services of the Evandro Chagas Clinical Research Institute (IPEC), Oswaldo Cruz Foundation (Fiocruz), Brazil, have been diagnosing a growing number of cases of human and feline sporotrichosis emanating from the city of Rio de Janeiro and itssurroundings.
Although the main clinical characteristics of human and feline sporotrichosis have been described elsewhere [10, 13], many questions related to the mechanism of zoonotic transmission and to the context in which this transmission occurs remain unanswered.
Between January and December 2002, a structured questionnaire was applied to each patient with sporotrichosis seen at IPEC. The criterion for inclusion in the study was a diagnosis of sporotrichosis established by isolation of S. schenckii in culture and a history of cohabiting with cats with the disease.
Simple frequencies of the household variables and of the variables related to the behaviour of the cats were described. Personal characteristics and the type of contact with cats were compared between patients and healthy subjects. Prevalences were compared by the χ2 test and means were compared by Student's t test. The strength of association was measured by the prevalence ratio, with the respective 95% confidence interval (95% CI).
Data were collected from 255 subjects: 94 patients and 161 family members living in 73 dwellings (1·29 patients/dwelling). The total number of cats living with the individuals was 280, including 133 sick animals.
Most dwellings were located in the municipalities of Rio de Janeiro (n=34, 46·6%) and Duque de Caxias (n=25, 34·2%). Except for one flat, all dwellings were houses (n=72, 98·6%). Most of them were built of bricks (n=67, 91·8%), had a cover slab under the roof (n=55, 75·3%), had well-ventilated rooms (n=61, 83·6%), and a bathroom (n=69, 94·5%). These houses were supplied by the public energy networks (n=73, 100%) and most were connected to waste collection (n=68, 93·2%), water supply (n=67, 91·8%) and the sanitary sewer system (n=60, 82·8%). The number of rooms per dwelling ranged from 1 to 4 (median=2). The streets were paved with asphalt in 75·4% (n=55) of cases or with paving stone in 12·3% (n=9). The mean family income was 5·1±5·0 minimum wages/month, with a median of 3·3 (one minimal wage=US$ 90·00). The length of schooling was <8 years in 61 (64·9%) of the patients and in 88 (54·7%) of the family members. The main occupation of the patients was domestic service (n=30, 31·9%), and most family members were students (n=51, 31·7%).
The municipality of Rio de Janeiro, the city with the largest number of sporotrichosis cases, comprises an area of 1182 km2 with a population of 5 857 904 inhabitants (2002). The sanitation infrastructure provides a public network water supply, a sanitary sewer system and waste collection from 78·2% to 98·9% of dwellings. However, there is a wide diversity of social classes living in this geographic area. The municipality of Duque de Caxias, with 465 km2 and 775 456 inhabitants (2002), provides public network water, a sanitary sewer system and waste collection for 57·0% to 88·9% of the dwellings. The region is characterized by large areas of poverty. The other municipalities affected (Nova Iguaçú, Nilópolis and São João de Meriti) present characteristics similar to Duque de Caxias.
The fact that sporotrichosis is not subject to compulsory notification in the State of Rio de Janeiro makes it difficult to know the exact geographic distribution of the disease. However, the identified areas are urban with a sanitation infrastructure equal to or above the mean of the affected municipalities. The need for repeated expenses with urban transportation and the difficulty of transporting animals in collective transport systems are important obstacles for the population of lower socioeconomic level to gain access to medical services, veterinarians and medications. The sample studied, which consisted of individuals with sufficient resources to overcome these obstacles, may present a selection bias towards individuals with some purchasing power in these communities. However, indicators such as a family income of five minimum wages and the level of schooling demonstrate the underprivileged socioeconomic level of this group in comparison with other populations.
Patients of 27·4% (n=20) of the dwellings had neighbours with sporotrichosis and 47·9% (n=35) had sick cats in the neighborhood, with 32·9% (n=24) having sick animals circulating freely in the surroundings.
The presence of rats in the backyard or house was reported for 54·8% (n=40) of the dwellings and in half of these the cat was used as a measure of controlling the rodents.
The environment of the backyards of the dwellings studied seems to favour the saprophytic growth of S. schenckii: they consisted of soil in 58·9% (n=43) of the dwellings, construction material or rubbish in 43·8% (n=32), and trees, plants, flower-garden or kitchen-garden in 83·5% (n=61), half of them containing thorns.
The number of individuals per dwelling ranged from 1 to 7 (median=4). About two-thirds (n=159, 62·4%) were women. The frequency of sporotrichosis was twice as high in women (n=73, 45·9%) than in men (n=21, 21·9%). The prevalence ratio was 2·09 (95% CI 1·38–3·17, P=0·00). The mean (± standard deviation) age of these patients was 41·9±19·4 years and was higher than the mean age of family members of the same sex (32·8±19·3 years, P=0·000). The prevalence of sporotrichosis was four times higher in subjects taking care of animals (79·8%) than in those who did not (20·2%) (prevalence ratio=5·6, 95% CI 3·5–8·6, P=0·00). The strength of the association between taking care of animals and contracting sporotrichosis did not differ between genders (homogeneity test, P=0·33). The Table summarizes the main characteristics of this population.
Table.
Distribution of the clinical and epidemiological characteristics of patients with sporotrichosis (n=94) and their healthy household contacts (n=161), Rio de Janeiro, 2002
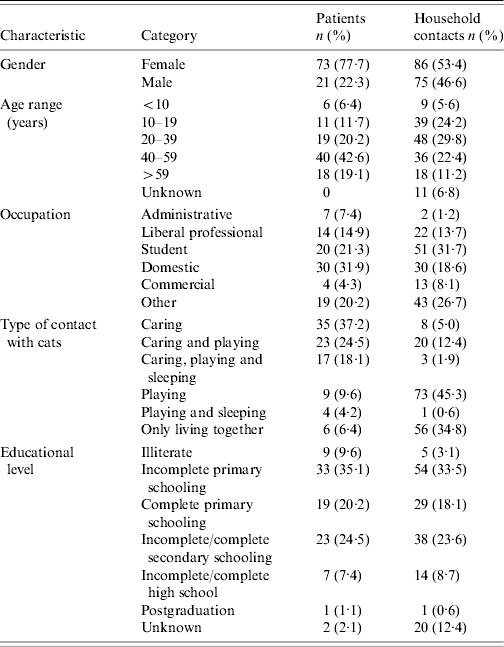
The proportion of children aged <10 years (5·9%) was small and lower than the mean for the same age range observed in the municipalities of Rio de Janeiro (15%) and Duque de Caxias (19·6%). This finding alone does not permit any discussion about the age composition of the dwellings with cats. The results show that, irrespective of sex, it is while taking care of cats with sporotrichosis that individuals come in contact with the lesions rich in parasites and are at risk of suffering scratches and bites. Among healthy individuals, the most frequent type of contact with the animals was playing or domestic social contact without involvement in the care of the animal.
The most frequent clinical presentation was the cutaneous-lymphatic form (n=42, 44·7%), followed by the localized cutaneous (n=29, 30·8%), disseminated cutaneous (n=22, 23·4%) and palpebral form with involvement of the conjunctiva (n=1, 1·1%). The high percentage of the disseminated cutaneous forms was probably due to multiple inoculations related to small skin traumas to which patients with cat-transmitted disease are subjected [14]. According to Vismer & Hull [15], exposure to small numbers of conidia over long periods of time may gradually confer immunity. Individuals living in endemic areas may become resistant to sporotrichosis or develop the localized form of cutaneous disease. As observed for several other dimorphic pathogenic fungi, the size of the in vivo inoculum plays an important role, with larger and deeper inocula resulting in the localized or cutaneous-lymphatic form of the disease depending on previous contact with the fungus. These arguments may explain, in part, the higher frequency of contracting the disease among individuals taking care of cats with sporotrichosis compared to those only living and playing with the animals. In this respect, it would be relevant to perform a survey, including a skin reaction test to sporotrichin, in order to evaluate subclinical infection among the household contacts of these patients.
The number of cats per dwelling ranged from 1 to 30 (median=2), for a total of 280 animals. Of these, 47·5% (n=133) had sporotrichosis. The number of sick cats per dwelling ranged from 1 to 14 (median=1). Most cats were males (n=100, 78·1%), with 12·5% (n=16) being neutered, compared to 2·3% (n=3) spayed females.
Among the 133 sick cats, 80·5% (n=107) circulated freely throughout the house and 78·9% (n=105) circulated throughout the neighborhood; 64·7% (n=86) slept inside the house, 88·7% (n=118) had contact with other animals, 66·9% (n=89) were engaged in fights, and 45·1% (n=60) hunted rats. A total of 104 (78·2%) cats had contact with soil and plants, 113 (85%) played with people, and 89 (66·9%) had scratched or bitten someone in the dwelling. During the period of disease, 64 (48·1%) cats were confined and 71 (53·4%) died from the disease or were euthanized.
The mobility of the cats in the dwellings and surroundings and their involvement in fights, mainly disputes for females, facilitate dispersal of the fungus in the environment and may explain the higher prevalence of the disease in adult male animals [10].
Although S. schenckii is a fungus widely found in nature, especially in soil [12], and zoonotic transmission of sporotrichosis by cats has been increasingly reported [7, 16], there are no reports of other similar epidemics. The method used here (application of questionnaires to patients seeking care at a referral centre) may lead to memory bias or poor classification of the categories of variables because the patient is unaware of the particularities of household contacts, limiting the internal validity and external generalization of the results. The importance of the present study resides in the fact that no such epidemic has been previously reported and in the lack of epidemiological data. Moreover, it is intriguing to ask which conditions may have led to the occurrence of this epidemic. Environmental questions and the increase of the feline population in the metropolitan region of Rio de Janeiro may have favoured this situation, although this assumption cannot be confirmed due to the lack of environmental mycological and animal census studies. The lack of public veterinary services and the lack of knowledge about the environmental sources of infection and transmission mechanisms, together with the lack of public health actions interrupting the chain of animal transmission, may be contributing to the growing number of human and feline sporotrichosis cases. Although IPEC offers veterinary care and medications free of charge, the percentage of abandonment and requests for euthanasia on the part of owners is high, demonstrating the difficulty in handling cats with sporotrichosis [10]. Field epidemiological studies, educational actions for the population, control of the feline population, and measures of treatment or elimination of cats with sporotrichosis will be necessary to better understand and control this epidemic.
ACKNOWLEDGEMENTS
The authors thank Laís de Lima Barros Fraga for technical support. This study was partially supported by Papes III, CNPq and Faperj.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the management of patients with sporotrichosis. For the Mycoses Study Group. Infectious Diseases Society of America. Clinical Infectious Diseases. 2000;30:684–687. doi: 10.1086/313751. [DOI] [PubMed] [Google Scholar]
- 2.Helm M, Berman C. The clinical, therapeutic and epidemiological features of sporotrichosis infection of the mines. In: Proceedings of the Transvaal Mine Medical Officers' Association Sporotrichosis Infection on Mines of the WitwatersrandJohannesburg: The Transvaal Chamber of Mines; 1947 [Google Scholar]
- 3.Dixon DM et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest U.S. epidemic of sporotrichosis. Journal of Clinical Microbiology. 1991;29:1106–1113. doi: 10.1128/jcm.29.6.1106-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kauffman CA. Sporotrichosis. Clinical Infectious Diseases. 1999;29:231–236. doi: 10.1086/520190. ; quiz 7. [DOI] [PubMed] [Google Scholar]
- 5.Singer J, Muncie J. Sporotrichosis. Etiologic considerations and report of additional cases from New York. New York State Journal of Medicine. 1952;52:2147–2153. [PubMed] [Google Scholar]
- 6.Dunstan RW et al. Feline sporotrichosis: a report of five cases with transmission to humans. Journal of the American Academy of Dermatology. 1986;15:37–45. doi: 10.1016/s0190-9622(86)70139-4. [DOI] [PubMed] [Google Scholar]
- 7.Marques SA et al. Sporotrichosis of the domestic cat (Felis catus): human transmission. Revista do Instituto de Medicina Tropical de Sao Paulo. 1993;35:327–330. [PubMed] [Google Scholar]
- 8.Reed KD et al. Zoonotic transmission of sporotrichosis: case report and review. Clinical Infectious Diseases. 1993;16:384–387. doi: 10.1093/clind/16.3.384. [DOI] [PubMed] [Google Scholar]
- 9.Smilack JD. Zoonotic transmission of sporotrichosis. Clinical Infectious Diseases. 1993;17:1075. doi: 10.1093/clinids/17.6.1075. [DOI] [PubMed] [Google Scholar]
- 10.Schubach TM et al. Evaluation of an epidemic of sporotrichosis in cats: 347 cases (1998–2001. Journal of the American Veterinary Medical Association. 2004;224:1623–1629. doi: 10.2460/javma.2004.224.1623. [DOI] [PubMed] [Google Scholar]
- 11.Schubach TM et al. Pathology of sporotrichosis in 10 cats in Rio de Janeiro. Veterinary Record. 2003;152:172–175. doi: 10.1136/vr.152.6.172. [DOI] [PubMed] [Google Scholar]
- 12.Rippon J, Rippon J. Medical Mycology – The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd edn. Philadelphia: W. B. Saunders Company; 1988. Sporotrichosis; pp. 325–352. , pp. [Google Scholar]
- 13.Barros MBL et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clinical Infectious Diseases. 2004;38:529–535. doi: 10.1086/381200. [DOI] [PubMed] [Google Scholar]
- 14.Barros MBL et al. Sporotrichosis with widespread cutaneous lesions – a report of 24 cases related to transmission by domestic cats in Rio de Janeiro, Brazil. International Journal of Dermatology. 2003;42:677–681. doi: 10.1046/j.1365-4362.2003.01813.x. [DOI] [PubMed] [Google Scholar]
- 15.Vismer HF, Hull PR. Prevalence, epidemiology and geographical distribution of Sporothrix schenckii infections in Gauteng, South Africa. Mycopathologia. 1997;137:137–143. doi: 10.1023/a:1006830131173. [DOI] [PubMed] [Google Scholar]
- 16.Fleury RN et al. Zoonotic sporotrichosis. Transmission to humans by infected domestic cat scratching: report of four cases in Sao Paulo, Brazil. International Journal of Dermatology. 2001;40:318–322. [PubMed] [Google Scholar]


