SUMMARY
We studied the role of host genetics in the susceptibility to severe Salmonella and Campylobacter infections and chronic sequelae of these infections. Participants of a previous case-control study were sent a buccal swab kit and a questionnaire about occurrence of chronic sequelae. Single nucleotide polymorphisms (SNPs) in the TLR4 (rs4986790), IFNG (rs2430561 and rs1861493), STAT1 (rs1914408), IL1B (rs16944), NRAMP (SLC11A1 rs2276631), JUN (rs11688) and VDR (rs10735810) genes were determined. In total, 687 controls, 457 Campylobacter cases and 193 Salmonella cases participated. None of the SNPs were associated with Campylobacter or Salmonella infections. None of the participants developed Guillain–Barré, Miller–Fisher or Reiter's syndrome. Reactive arthritis occurred in 5% and 2% of cases and controls, respectively. Campylobacter cases more frequently experienced gastroenteritis episodes than controls. Campylobacter or Salmonella infection in women, use of proton pump inhibitors and an SNP in the IFNG gene were independent risk factors for reactive arthritis. Another SNP in the IFNG gene and use of proton pump inhibitors were risk factors for recurrent episodes of gastroenteritis. In conclusion, reactive arthritis and recurrent gastroenteritis episodes are common after infection and host genetic factors play a role in susceptibility to these long-term health effects.
INTRODUCTION
Campylobacter and Salmonella are the most common causes of bacterial gastroenteritis in The Netherlands with around 100 000 and 50 000 cases per year, respectively [1]. Some of these cases consult a general practitioner and from these cases samples are collected for diagnostics. In 2005, about 6200 Campylobacter cases and 2100 Salmonella cases were laboratory-confirmed [2], which represent the more severe cases of gastroenteritis caused by these agents.
Campylobacter and Salmonella infections occasionally lead to chronic sequelae of infection. The association between Campylobacter jejuni infection and the Guillain–Barré and Miller–Fisher syndromes has been well-documented [3, 4]. Other well-known sequelae of both Campylobacter and Salmonella infections are reactive arthritis and Reiter's syndrome [5, 6]. Other studies suggested that Campylobacter and Salmonella infections cause chronic gastrointestinal diseases, such as irritable bowel syndrome (IBS), dyspepsia and inflammatory bowel disease (IBD) [7–9].
Case-control studies on risk factors for campylobacteriosis and salmonellosis have shown that multiple sources, transmission routes and risk factors are associated with illness [10–13]. For instance, our previous Dutch case-control study on campylobacteriosis (Y. Doorduyn, W. E. van den Brandhoff, Y. T. H. P. van Duynhoven, J. A. Wagenaar & W. van Pelt, unpublished data) and salmonellosis [14] revealed that besides exposure to the pathogen, factors that influence the host's gastrointestinal environment such as use of gastric acid inhibitors and antibiotics were associated with the development of both diseases. However, the risk and the severity of Salmonella and Campylobacter infections may also depend on host susceptibility. In addition, it is still unknown if host susceptibility plays a role in the development of chronic sequelae after a Campylobacter or Salmonella infection.
To study whether genetic factors influence host susceptibility to Campylobacter and Salmonella infections and chronic sequelae of these infections, we selected seven candidate genes involved in innate and adaptive immunity to infection. Since the host's defence against Campylobacter is still poorly understood, we focused on genes involved in the response to Salmonella.
The innate immune response to Salmonella is mainly triggered by the recognition of lipopolysaccharide on the outer membrane of the bacteria by the host cell receptor complex [15]. This is followed by an influx of phagocytes that engulf the pathogen and eliminate it. However, Salmonella is a facultative intracellular pathogen and is able to defend itself against intracellular killing [16, 17]. In a later stage, the adaptive immune response is triggered, and Th1 cytokine interferon-γ (IFN-γ) is one of the key cytokines involved in elimination of intracellular Salmonella [18, 19].
The importance of Th1 immunity in the host's defence against Salmonella is highlighted by the fact that deleterious mutations in genes involved in the Th1 pathway are linked to severe human infections due to intracellular pathogens, including Salmonella, that are normally only weakly pathogenic [20]. These mutations are rare and therefore unlikely to contribute much to the incidence of Salmonella infections in the general population. Therefore, the present study focused on more frequently occurring subtle genetic variations, single nucleotide polymorphisms (SNPs).
Participants of our previous case-control study on risk factors for campylobacteriosis and salmonellosis [14], the CaSa study, were re-contacted to ascertain whether SNPs in several immunomodulatory genes (shown in Table 1) influence the host's susceptibility to Salmonella and Campylobacter infection. In addition, we evaluated the occurrence of chronic sequelae in this study population. We also studied the influence of the determined SNPs (and previously registered risk factors) on the development of these chronic sequelae.
Table 1.
Immunomodulatory genes involved in the host's immune response to Salmonella that are of interest in the present study

METHODS
Study design
Subjects were selected from participants of a previous Dutch case-control study [14], the CaSa study. In the CaSa study, questionnaires about risk factors were obtained from 1446 laboratory-confirmed cases with campylobacteriosis and 573 laboratory-confirmed cases with salmonellosis for the period April 2002 to April 2003. In addition, 3409 frequency-matched community controls (according to age, sex, degree of urbanization and season) completed the questionnaire. Participants in the CaSa study who did not object to being contacted for future studies, who were born in The Netherlands and whose parents were born in The Netherlands were selected for inclusion in the present study, to avoid population admixture. A total of 3706 participants were selected for the present study: 2114 former controls, 1143 former Campylobacter cases and 449 former Salmonella cases. In November 2005, these subjects were sent a self-administrable buccal swab kit, a questionnaire about chronic sequelae and an accompanying letter with instructions about the swab kit and information about the study. Informed consent was obtained from each subject in accordance with the guidelines of the medical ethics committee of the University Medical Centre, Utrecht.
SNP selection
To study the role of host-genetic factors in Salmonella and Campylobacter infection, SNPs in seven candidate genes were studied: IFNG, STAT1, IL1B, TLR4, NRAMP, JUN and VDR (see Table 1). SNPs in these genes were selected based on previously published associations with various diseases [21–27], thus increasing the chance of selecting SNPs with functional consequences.
DNA isolation and genotyping
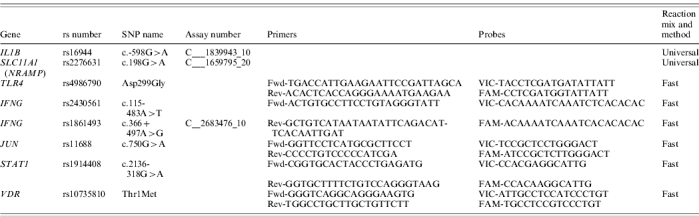
DNA was isolated from buccal swabs using the QIAamp DNA Blood Mini kit (Qiagen NV, Venlo, The Netherlands). Polymorphisms in IL1B and SLC11A1 (the gene encoding NRAMP) were genotyped using pre-designed TaqMan SNP genotyping assays (Applied Biosystems, Nieuwerkerk a/d IJssel, The Netherlands). For each sample 2·5 μl TaqMan universal PCR master mix (Applied Biosystems) and 10 ng genomic DNA were used in a total volume of 5 μl. The reaction was run according to the following protocol: 10 min at 95°C, 40× (15 s, 92°C; 1 min, 60°C), 4°C. Polymorphisms in IFNG, JUN, STAT1, TLR4 and VDR were genotyped using pre-designed or custom TaqMan SNP genotyping assays (Applied Biosystems). For each sample 2·5 μl TaqMan fast universal PCR master mix (Applied Biosystems) and 10 ng genomic DNA were used in a total volume of 5 μl. The reaction was run according to the following protocol: 20 s at 95°C, 40× (3 s, 95°C; 3 s, 60°C), 4°C. Primer and probe sequences and assay numbers where appropriate are given in Table 2. All genotyping assays were performed on a 7500 Fast Real Time PCR system (Applied Biosystems).
Table 2.
PCR primer and probe sequences, or assay numbers used to determine polymorphisms in IL1B, SLC11A1 (NRAMP), TLR4, IFNG, JUN STAT1 and VDR genes
Questionnaire design
To study the occurrence of chronic sequelae in the 3 years after infection, a questionnaire was used. The questionnaire included questions regarding occurrence of gastroenteritis episodes, development of joint or back problems, reactive arthritis, Guillain–Barré, Miller–Fisher or Reiter's syndrome since the Campylobacter or Salmonella infection in 2002–2003 (for cases), or participation in the CaSa study in 2002–2003 (for controls). Cases and controls were also asked about visits to the general practitioner, hospital admission and use of medication for the above-mentioned conditions and about the presence of other chronic illnesses. We also asked them the native country of their grandparents. Parents were asked to complete questionnaires for their children.
Statistical analysis
In the analysis, only subjects whose grandparents were also born in The Netherlands were included. Genotype distributions of each determined SNP among Campylobacter cases, Salmonella cases and controls were studied using cross-tabulations, χ2 tests and ‘single’ variable logistic regression models (which also included age, sex and degree of urbanization) for significance testing. In the same way, the occurrence of chronic sequelae (Guillain–Barré syndrome, Miller–Fisher syndrome, reactive arthritis or Reiter's syndrome) and other health outcomes after follow-up (occurrence of gastroenteritis episodes, development of joint or back problems, presence of other chronic illnesses) were compared between Campylobacter cases, Salmonella cases, and controls. To study the influence of the determined SNPs on the development of chronic sequelae, the genotype distributions of each SNP in subjects who developed chronic sequelae were compared with the genotype distributions in subjects who did not develop chronic sequelae using cross-tabulations, χ2 tests and single variable logistic regression models. If the occurrence of chronic sequelae differed between the type of participant (Campylobacter case, Salmonella case or control), the type of participant was added to the logistic regression model. In the same way, the effect of factors previously registered in the CaSa study on the occurrence of chronic sequelae were studied. Finally, a multivariate logistic regression model was obtained by forward selection of SNPs and risk factors that showed a P value of ⩽0·10 in the single variable analysis. Factors that multivariably showed a P value of ⩽0·05 were retained in the model. All analyses were performed using SAS statistical software version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Questionnaires and buccal swabs were received from 457 native Dutch Campylobacter cases (response 40%), 193 Salmonella cases (43%) and 687 controls (32%). The response for both cases and controls was lower among subjects in the 18–29 years age group (response respectively 29%, 27%, and 20%) and higher among subjects who used proton pump inhibitors in the CaSa study (respectively 48%, 57%, and 51%). Questionnaires were completed at a median of 3·2 years (range 2·4–3·8 years) after the CaSa study.
Role of host genetics in Salmonella or Campylobacter infection
We studied four SNPs in four genes involved in innate immunity (TLR4, NRAMP, VDR, JUN), and three SNPs in two genes involved in Th1 immunity (IFNG, STAT1) to Salmonella infection. In addition, we selected a SNP in IL1B as a candidate for susceptibility to both Campylobacter and Salmonella infection, because of its modifying effect on gastric acid production. No association was found between the determined SNPs and Campylobacter or Salmonella infection, although for the selected SNP in the STAT1 gene a marginally significant difference in genotype distribution was found between Salmonella cases and controls (Table 3).
Table 3.
Genotype distribution of SNPs in IFNG, STAT1, IL1B, NRAMP, TLR4, JUN and VDR genes among Campylobacter cases, Salmonella cases and controls

OR, Odds ratio; CI, confidence interval.
χ2 test.
Adjusted for age, sex and degree of urbanization.
P=0·06.
Chronic sequelae after a Campylobacter or Salmonella infection
The occurrence of chronic sequelae and other health outcomes after follow-up among cases and controls are shown in Table 4. None of the study subjects developed Guillain–Barré syndrome, Miller–Fisher syndrome or Reiter's syndrome since the CaSa study. Joint or back problems occurred in respectively 22% and 10% of the study subjects and these were as common among cases as among controls. Since the CaSa study, more (former) Campylobacter cases experienced gastroenteritis episodes than controls (Table 4).
Table 4.
Occurrence of illnesses about 3 years after Campylobacter cases, Salmonella cases and controls participated in a case-control study in The Netherlands in 2002–2003

OR, Odds ratio; CI, confidence interval.
Adjusted for age, sex, degree of urbanization and level of education.
It is unknown whether these illnesses developed before or after participation in the CaSa study.
Reactive arthritis occurred more frequently among Campylobacter and Salmonella cases than among controls (OR 1·9 and 2·3, respectively). Women especially have an elevated risk of developing reactive arthritis after a Campylobacter or Salmonella infection, compared to controls (OR 6·8 and 6·1, respectively, Table 4). Reactive arthritis occurred only in adults aged ⩾23 years. The median age of reactive arthritis cases was 60 years.
Chronic disease and susceptibility to Salmonella or Campylobacter infection
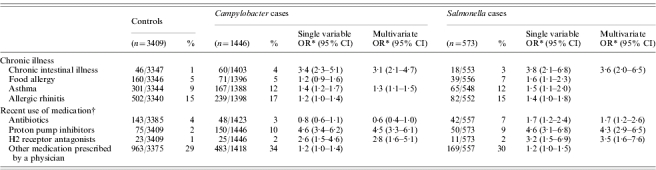
In the present study, Campylobacter and Salmonella cases were more likely to have chronic diseases than controls, especially chronic intestinal diseases (IBS, IBD, coeliac disease), asthma and rheumatism (Table 4). The association between infection with Salmonella or Campylobacter and the occurrence of these chronic diseases may suggest that there is a causal link between the two. However, from the follow-up questionnaire it was unclear whether these chronic diseases developed before or after the infection and enrolment in the CaSa study.
To further investigate this link, data from the CaSa study were analysed in which participants were asked if, at that time, they had chronic intestinal illness, asthma, food allergy or allergic rhinitis. When adjusted for use of medication, these analyses showed that having a chronic intestinal illness was significantly associated with both Campylobacter and Salmonella infections. In addition, having asthma was associated with Campylobacter infections (Table 5). These data suggest that patients with these chronic diseases are more susceptible to Campylobacter or Salmonella infections.
Table 5.
Association between chronic illness and use of medication with campylobacteriosis and salmonellosis based on data from the CaSa study in The Netherlands, 2002–2003
OR, Odds ratio; CI, confidence interval.
Adjusted for age, sex, degree of urbanization and level of education.
Use of medication in the 4 weeks prior to infection (cases) or in the previous 4 weeks (controls).
Role of host genetics in chronic sequelae of infection
Genetic factors and registered risk factors from the CaSa study that were associated with reactive arthritis and recurrent gastroenteritis episodes are summarized in Table 6. For reactive arthritis, having had a Campylobacter or Salmonella infection was only a risk factor in women. In addition, use of proton pump inhibitors and a SNP in the IFNG gene (rs2430561) were independent risk factors for reactive arthritis. In a multivariate model including the use of proton pump inhibitors, Campylobacter infection was no longer associated with gastroenteritis episodes. Of the determined SNPs, a SNP in the IFNG gene (rs1861493) was associated with gastroenteritis episodes (Table 6).
Table 6.
Risk factors for reactive arthritis and gastroenteritis episodes developed within 3 years after controls, Campylobacter cases and Salmonella cases participated in the CaSa study in The Netherlands, 2002–2003

OR, odds ratio; CI, confidence interval.
Adjusted for age, sex, degree of urbanization.
Only adults were included, because reactive arthritis was only observed among subjects aged ⩾23 years.
DISCUSSION
Participants of a previous case-control study on Campylobacter and Salmonella infections 3 years ago, the CaSa study, were re-contacted. SNPs in genes involved in the host's immune response to infection were determined in DNA obtained from buccal swabs of these cases and controls to study the influence of these genes in the susceptibility to Campylobacter and Salmonella infections. In addition, participants were asked to complete a questionnaire in order to study whether they developed chronic sequelae after infection. We also studied the influence of the determined SNPs on the development of chronic sequelae after infection.
None of the determined SNPs were associated with Campylobacter and Salmonella infections. However, a marginal effect was observed between a candidate, Salmonella susceptibility SNP in STAT1 and Salmonella infection. STAT1 is involved in IFN-γ mediated signal transduction. The genotype distribution of this polymorphism was different for Salmonella cases compared to controls, but due to the relatively low number of Salmonella cases, the present study lacked the power to statistically demonstrate this difference. The present study would have the power to indicate an OR of at least 1·6, but the power was too limited to indicate an OR of 1·4, as was found for the association between a SNP in STAT1 and Salmonella infection. Therefore, the possible association between this SNP in STAT1 and Salmonella infection should in future be studied using larger numbers of Salmonella cases.
None of the study subjects developed Guillain–Barré, Miller–Fisher or Reiter's syndrome, which confirms the low incidences of these illnesses after a Campylobacter or Salmonella infection [4, 28]. This study showed a high association between Campylobacter and Salmonella infections and reactive arthritis. Interestingly, we found that women especially were at increased risk of developing reactive arthritis after a Campylobacter or Salmonella infection. Of the Campylobacter and Salmonella cases in our study, about 5% developed reactive arthritis. This is relatively low compared to outbreaks where proportions from 3% to 18% have been reported [6, 29, 30]. We observed that reactive arthritis only occurred in adults and the median age of these reactive arthritis cases was high. This may explain (part of) the high incidence of reactive arthritis found in outbreak settings where most of the cases were adults. In two other population-based studies, 7% of Campylobacter cases and 6% of Salmonella cases developed reactive arthritis [5, 31], which is close to our estimate. Among Campylobacter cases, reactive arthritis occurred mainly in women, just as we observed in our study [5].
Both Campylobacter and Salmonella cases were more likely to have asthma and chronic intestinal illnesses (IBD, IBS, coeliac disease). From data of the CaSa study it appeared that these chronic illnesses were already present before infection, which suggests that patients with these chronic diseases are more susceptible to infection. This contradicts the suggestion of others that gastrointestinal infections may be a cause of chronic intestinal illnesses [7–9]. Patients with IBD or IBS have a disturbed intestinal function and it is conceivable that this may facilitate enteric pathogens to cause infection. It has been shown that patients with asthma may have oesophageal dysmotility or gastroesophageal reflux [32]. However, even when adjusted for gastric acid inhibitors, the association between asthma and Campylobacter infection remained significant.
Recurrent episodes of gastroenteritis were common among Campylobacter cases, but this was mainly due to the use of proton pump inhibitors by these cases. It is well known that use of these gastric acid inhibitors increases the susceptibility to gastrointestinal infections [33, 34]. A SNP in the IFNG (rs1861493) gene was also independently associated with gastroenteritis episodes. As IFN-γ is a crucial cytokine in the immune response against enteric infections, it is conceivable that changes in the expression of this protein would increase the susceptibility to gastroenteritis. However, it is unknown if this SNP results in such a functional alteration.
Use of proton pump inhibitors, Campylobacter and Salmonella infections and a SNP in the IFNG (rs24305621) gene were independently associated with reactive arthritis. So far, except for HLA-B27 no other genetic factors have previously been described that may increase the susceptibility to reactive arthritis. We hypothesize that the effect of both proton pump inhibitors and SNPs in the IFNG gene on reactive arthritis susceptibility is mediated by prolonged or more frequently occurring gastrointestinal infections, because of the gastric acid inhibitory effect of proton pump inhibitors and the crucial role of IFN-γ in the defence against gastrointestinal pathogens.
Limitations of the present study are the retrospective design and the reliance on subjective information about chronic sequelae of infection. In the questionnaire, we asked the participants if a physician ever diagnosed reactive arthritis. Participants reporting reactive arthritis or joint symptoms were not examined by a rheumatologist to confirm the diagnosis. This may have resulted in misclassification of reactive arthritis cases. However, the occurrence of reactive arthritis among Campylobacter and Salmonella cases in our study was comparable to the incidence found in two other population-based studies where participants with joint symptoms were examined by a rheumatologist [5, 31].
Furthermore, we conducted our study in a selected group of Campylobacter and Salmonella cases and controls. In the CaSa study, 33% of the approached controls and 47% of all laboratory-confirmed Campylobacter and Salmonella cases in 2002–2003 were enrolled. In the present study, another selection of these cases and controls participated. Therefore, the study population in the present study may not have been fully representative of all initially approached cases and controls.
In conclusion, our study revealed a clear association between Campylobacter and Salmonella infections and reactive arthritis. Women are especially at increased risk of developing reactive arthritis after infection. However, such an association was not found for chronic intestinal diseases like IBD and IBS. In fact, our study showed that people who have chronic intestinal diseases and also people with asthma are at increased risk of acquiring Campylobacter or Salmonella infections. We did not find an association between the determined SNPs and Campylobacter or Salmonella infections. The results of our study suggest that polymorphisms in IFNG genes are involved in long-term health effects of Salmonella or Campylobacter infections, such as reactive arthritis and recurrent episodes of gastroenteritis. It is not yet clear whether these gene variants are indeed the causal variants in the development of human disease. Future research is needed to confirm these associations as well as to elucidate the functionality of these SNPs.
ACKNOWLEDGEMENTS
We thank Carlo Strien and Bert Verlaan for isolating the DNA. We are also grateful to Natasja Kragten and Hennie Hodemaekers for their help in polymorphism genotyping.
DECLARATION OF INTEREST
This investigation has been performed by and for the account of the National Institute of Public Health and the Environment within the framework of project S340210 ‘From gene to function; Genetic susceptibility for Salmonella and Campylobacter infections: the role of the host’.
REFERENCES
- 1.Wit MA de et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. American Journalof Epidemiology. 2001;154:666–674. doi: 10.1093/aje/154.7.666. [DOI] [PubMed] [Google Scholar]
- 2.Pelt W van et al. Trends in gastroenteritis in the Netherlands, 1996–2005. Increase of hospital uptakes and death between 2003 and 2005: an increase in the role of viral infections? Infectieziekten Bulletin. 2006;17:364–370. [Google Scholar]
- 3.Yuki N, Koga M. Bacterial infections in Guillain–Barré and Fisher syndromes. Current Opinion in Neurology. 2006;19:451–457. doi: 10.1097/01.wco.0000245367.36576.e9. [DOI] [PubMed] [Google Scholar]
- 4.Tam CC et al. Incidence of Guillain–Barré syndrome among patients with Campylobacter infection: a general practice research database study. Journal of Infectious Diseases. 2006;194:95–97. doi: 10.1086/504294. [DOI] [PubMed] [Google Scholar]
- 5.Hannu T et al. Campylobacter-triggered reactive arthritis: a population-based study. Rheumatology (Oxford) 2002;41:312–318. doi: 10.1093/rheumatology/41.3.312. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin MS et al. Reactive arthritis and Reiter's syndrome following an outbreak of gastroenteritis caused by Salmonella enteritidis. Clinical Infectious Diseases. 2001;33:1010–1014. doi: 10.1086/322644. [DOI] [PubMed] [Google Scholar]
- 7.Marshall JK et al. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology. 2006;131:445–450. doi: 10.1053/j.gastro.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 8.Mearin F et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. doi: 10.1053/j.gastro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Helms M, Simonsen J, Molbak K. Foodborne bacterial infection and hospitalization: a registry-based study. Clinical Infectious Diseases. 2006;42:498–506. doi: 10.1086/499813. [DOI] [PubMed] [Google Scholar]
- 10.El-Omar EM et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 11.Friis LM et al. In vitro cell culture methods for investigating Campylobacter invasion mechanisms. Journal of Microbiological Methods. 2005;61:145–160. doi: 10.1016/j.mimet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Geboes K. Crohn's disease, ulcerative colitis or indeterminate colitis – how important is it to differentiate? Acta Gastroenterologica Belgica. 2001;64:197–200. [PubMed] [Google Scholar]
- 13.Janssen R, Jonge R de, Hoebee B Bilthoven: RIVM; 2006. . Genetic susceptibility to Campylobacter infection. ; Report No.: Rapport 340210002. [Google Scholar]
- 14.Doorduyn Y et al. Risk factors for Salmonella Enteritidis and Typhimurium (DT104 and non-DT104) infections in The Netherlands: predominant roles for raw eggs in Enteritidis and sandboxes in Typhimurium infections. Epidemiology and Infection. 2006;134:617–626. doi: 10.1017/S0950268805005406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freudenberg MA et al. Role of lipopolysaccharide susceptibility in the innate immune response to Salmonella typhimurium infection: LPS, a primary target for recognition of Gram-negative bacteria. Microbes and Infection. 2001;3:1213–1222. doi: 10.1016/s1286-4579(01)01481-2. [DOI] [PubMed] [Google Scholar]
- 16.Lindgren SW, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proceedings of the National Academy of Sciences USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazquez-Torres A et al. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 18.Eckmann L, Kagnoff MF. Cytokines in host defense against Salmonella. Microbes and Infection. 2001;3:1191–1200. doi: 10.1016/s1286-4579(01)01479-4. [DOI] [PubMed] [Google Scholar]
- 19.Lalmanach AC, Lantier F. Host cytokine response and resistance to Salmonella infection. Microbes and Infection. 1999;1:719–726. doi: 10.1016/s1286-4579(99)80073-2. [DOI] [PubMed] [Google Scholar]
- 20.Ottenhoff TH et al. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nature Genetics. 2002;32:97–105. doi: 10.1038/ng0902-97. [DOI] [PubMed] [Google Scholar]
- 21.Rossouw M et al. Association between tuberculosis and a polymorphic NFkappaB binding site in the interferon gamma gene. Lancet. 2003;361:1871–1872. doi: 10.1016/S0140-6736(03)13491-5. [DOI] [PubMed] [Google Scholar]
- 22.Taverna MJ, Selam JL, Slama G. Association between a protein polymorphism in the start codon of the vitamin D receptor gene and severe diabetic retinopathy in C-peptide-negative type 1 diabetes. Journal of Clinical Endocrinology and Metabolism. 2005;90:4803–4808. doi: 10.1210/jc.2004-2407. [DOI] [PubMed] [Google Scholar]
- 23.Tal G et al. Association between common Toll-like receptor 4 mutations and severe respiratory syncytial virus disease. Journal of Infectious Diseases. 2004;189:2057–2063. doi: 10.1086/420830. [DOI] [PubMed] [Google Scholar]
- 24.Furuta T et al. Polymorphism of interleukin-1beta affects the eradication rates of Helicobacter pylori by triple therapy. Clinical Gastroenterology and Hepatology. 2004;2:22–30. doi: 10.1016/s1542-3565(03)00288-x. [DOI] [PubMed] [Google Scholar]
- 25.Dunstan SJ et al. Typhoid fever and genetic polymorphisms at the natural resistance-associated macrophage protein 1. Journal of Infectious Diseases. 2001;183:1156–1160. doi: 10.1086/319289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sigurdsson S et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. American Journal of Human Genetics. 2005;76:528–537. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen R et al. Genetic susceptibility to respiratory syncytial virus bronchiolitis is predominantly associated with innate immune genes. Journal of Infectious Diseases. 2007;196:826–834. doi: 10.1086/520886. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy N, Giesecke J. Incidence of Guillain–Barré syndrome following infection with Campylobacter jejuni. American Journal of Epidemiology. 2001;153:610–614. doi: 10.1093/aje/153.6.610. [DOI] [PubMed] [Google Scholar]
- 29.Hannu T et al. Reactive arthritis following an outbreak of Campylobacter jejuni infection. Journal of Rheumatology. 2004;31:528–530. [PubMed] [Google Scholar]
- 30.Locht H, Molbak K, Krogfelt KA. High frequency of reactive joint symptoms after an outbreak of Salmonella enteritidis. Journal of Rheumatology. 2002;29:767–771. [PubMed] [Google Scholar]
- 31.Buxton JA et al. Reactive arthritis and other sequelae following sporadic Salmonella typhimurium infection in British Columbia, Canada: a case control study. Journal of Rheumatology. 2002;29:2154–2158. [PubMed] [Google Scholar]
- 32.Campo S et al. Esophageal dysmotility and gastroesophageal reflux in intrinsic asthma. Digestive Disease and Science. 1997;42:1184–1188. doi: 10.1023/a:1018841704897. [DOI] [PubMed] [Google Scholar]
- 33.Amsterdam JGC Bilthoven: RIVM; 2004. Genetic susceptibility for Salmonella infections. ; Report No.: Rapport 340210001. [Google Scholar]
- 34.Taylor DN et al. Influence of strain characteristics and immunity on the epidemiology of Campylobacter infections in Thailand. Journal of Clinical Microbiology. 1988;26:863–868. doi: 10.1128/jcm.26.5.863-868.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agnese DM et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. Journal of Infectious Diseases. 2002;186:1522–1525. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 36.Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends in Microbiology. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- 37.Canonne-Hergaux F et al. The Nramp1 protein and its role in resistance to infection and macrophage function. Proceedings of the Association of American Physicians. 1999;111:283–289. doi: 10.1046/j.1525-1381.1999.99236.x. [DOI] [PubMed] [Google Scholar]
- 38.Adcock IM. Transcription factors as activators of gene transcription: AP-1 and NF-kappa B. Monaldi Archives of Chest Disease. 1997;52:178–186. [PubMed] [Google Scholar]
- 39.Selvaraj P et al. Regulatory role of vitamin D receptor gene variants of Bsm I, Apa I, Taq I, and Fok I polymorphisms on macrophage phagocytosis and lymphoproliferative response to mycobacterium tuberculosis antigen in pulmonary tuberculosis. Journal of Clinical Immunology. 2004;24:523–532. doi: 10.1023/B:JOCI.0000040923.07879.31. [DOI] [PubMed] [Google Scholar]
- 40.Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227–234. doi: 10.1136/gut.42.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]


