Abstract
Synchrony between embryo competency and uterine receptivity is essential for successful implantation. Mice with ablation of chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) in the uterus (PRCre/+;COUP-TFIIflox/flox) exhibit implantation defects and increased estrogen receptor (ER)α activity in the luminal epithelium, suggesting high ERα activity may disrupt the window of uterine receptivity. To determine whether increased ERα activity in the PRCre/+;COUP-TFIIflox/flox uterus is the cause of defective implantation, we assessed whether inhibition of ERα activity could rescue the PRCre/+;COUP-TFIIflox/flox uterine implantation defect. ICI 182,780 (ICI), a pure ERα antagonist, was administered to PRCre/+;COUP-TFIIflox/flox mutant and COUP-TFIIflox/flox control mice during the receptive period, and the number of implantation sites was examined. COUP-TFIIflox/flox control mice treated with oil or ICI showed the normal number of implantation sites. As expected, no implantation sites were observed in PRCre/+;COUP-TFIIflox/flox mutant mice treated with oil, consistent with previous observations. In contrast, implantation sites were greatly increased in ICI-treated PRCre/+;COUP-TFIIflox/flox mutant mice, albeit at a reduced number in comparison with the control mice. ICI treatment was also able to restore the expression of Wnt4 and bone morphogenetic protein 2, important for endometrial decidualization in the PRCre/+;COUP-TFIIflox/flox mutant mice. To confirm that the rescue of embryo attachment and decidualization is a consequence of a reduced ERα activity upon ICI treatment, we showed a reduction of the expression of ERα target genes in PRCre/+;COUP-TFIIflox/flox mutant mice. Because COUP-TFII was also shown in our laboratory to be important for placentation during pregnancy, we asked whether ICI treatment could also rescue the placentation defect to allow full-term pregnancy in these mice. We found that whereas mice were born in COUP-TFIIflox/flox control mice given ICI, no pups were born in the PRCre/+;COUP-TFIIflox/flox mutant mice, suggesting that the increased ERα activity is not the reason for placentation defects. These results demonstrate that during the periimplantation period, COUP-TFII regulates embryo attachment and decidualization through controlling ERα activity. However, COUP-TFII expression is still required in the postimplantation period to facilitate placentation.
Inhibition of ERα activity at the receptive period of uterus largely rescued implantation and decidualization defects displayed by COUP-TFII mutant mice.
The implantation of the blastocyst into the maternal uterus is a crucial step in mammalian reproduction, and it involves an intricate succession of cellular and molecular interactions. The periimplantation period in the mouse has been classified into prereceptive (d 1–3), receptive (d 4), and nonreceptive (refractory) phases (by the afternoon of d 5) (1,2,3). During the prereceptive phase, the uterus is unable to initiate implantation, but the uterine environment is less hostile to blastocyst survival. In contrast, during the refractory phase, the uterine environment is unfavorable to blastocyst survival. For a successful implantation process, synchrony between competent embryo and receptive uterus is essential. Among numerous factors involved in this primary event of pregnancy, two ovarian steroid hormones, estrogen and progesterone (P4), and their cognate receptors, estrogen receptor (ER) and progesterone receptor (PR), respectively, play central roles in this process (1,2,3). The coordinated effects of these steroids render the uterus receptive to blastocyst apposition, attachment, and ultimately implantation (4,5,6,7).
Although estrogen activity is essential for an integrated uterine response, it has been shown that excessive estrogen activity can prematurely close the implantation window (8), suggesting that estrogen activity is tightly controlled during the periimplantation period to allow proper development of the receptive uterus. Ma et al. (8) reported that systemic estrogen must be tightly regulated to avoid a shift of the window of uterine receptivity to either the prereceptive or refractory phase. Using a delayed implantation model, they showed that the minimal dose of 17β-estradiol (E2) that is required to induce uterine sensitivity for implantation was in the range of 1.5–3 ng. They also showed that high E2 levels induced a premature refractory phase, whereas low levels of E2 induced a prereceptive phase. This is associated with aberrant expression of implantation-related genes, including leukemia inhibitory factor, cyclooxygenase 1, and amphiregulin.
Aside from regulating systemic estrogen tightly during the receptive phase, endometrial sensitivity to estrogen must be controlled. During the periimplantation phase, serum P4 levels are increasing. P4 is known to attenuate estrogen-induced gene expression in uterine epithelial cells (9). Intriguingly, this suppression is mediated by stromal PRs (10,11), suggesting that the coordinated action of estrogen and P4 depends on cross talk between the epithelial and stromal compartments of the uterus. Importantly, the orphan nuclear receptor, chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII), has been shown to be a mediator of this stromal epithelial cross talk communication (12).
COUP-TFII belongs to the orphan nuclear receptor superfamily (13,14). COUP-TFII is highly expressed in the mesenchyme and plays critical roles during mouse development. COUP-TFII null mutants die before embryonic d 10.5 (E10.5) due to cardiovascular defects (15). Conditional ablation of COUP-TFII in limbs (16), stomach (17), diaphragm (18), endothelial cells (18), testis (19), brain (20), uterus (12,21), and placenta (21) reveals that COUP-TFII plays a pivotal role in cell growth and differentiation, organ development, and lineage determination. COUP-TFII heterozygous female mice show significantly reduced fecundity, due to both ovarian and uterine defects (22). COUP-TFII is expressed in endometrial stromal cells and the myometrium (12) Using PR-specific Cre to ablate COUP-TFII in the uterus, we found that mutant mice display defects in implantation and decidualization (12,21). Interestingly, the expression of the nuclear receptor, estrogen receptor (ER)α, coregulator [steroid receptor coactivator 1 (SRC-1)], and associated target genes were shown to be elevated in the uterine epithelium of the COUP-TFII mutant, suggesting that ERα activity is increased in the absence of the stromal COUP-TFII. The fact that improper estrogen stimulation of the uterus has been shown to perturb the window of receptivity leads us to hypothesize that the elevated ERα activity resulting from the absence of COUP-TF might underlie the implantation defect in the COUP-TFII mutant (12).
To test this hypothesis, we here used a pure ERα antagonist, ICI 182,780 (ICI) (23), to attenuate ΕRα activity in the uterine luminal epithelium of the COUP-TFII mutant in an attempt to rescue the implantation defect of COUP-TFII mutant mice. Indeed, inhibition of ERα activity at the receptive period largely rescued the implantation and decidualization defects displayed by COUP-TFII mutant mice. These results indicate that stromal and epithelial interactions must tightly couple to control epithelial ERα activity for the implantation process, and COUP-TFII is an essential part of this process.
Results
ICI rescues the implantation defect of COUP-TFII mutant mice
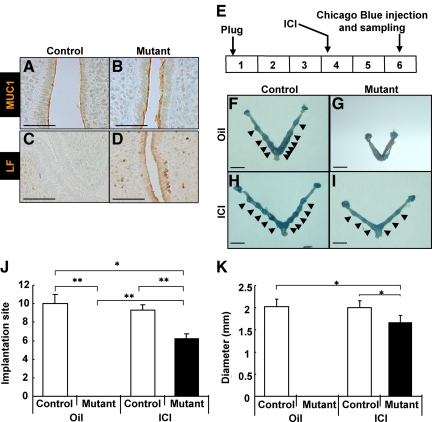
Previously we observed an enhanced expression level of ERα and its coregulator SRC-1 as well as the ERα target genes (Lactoferrin, Mucin-1, and Desmocollin-2) in the uterine luminal epithelium of COUP-TFII-deficient mice, suggesting that dysregulation of ERα activity might contribute to the implantation and decidualization defects exhibited by the COUP-TFII mutant (12). To address this possibility, we administered ICI, a pure ERα antagonist, to inhibit ERα activity during the uterine receptive period and determined the impact of antiestrogen treatment on implantation and pregnancy. Consistent with our previous report, elevated ERα activity, as examined by the expression of ERα targets mucin 1 (MUC1) and lactoferrin (LF), was observed in the uterine luminal epithelium of COUP-TFII mutant mice (Fig. 1, A–D). The strategy for ICI rescue of implantation mice with deletion of COUP-TFII in the uterus is shown in Fig. 1E. Mice were treated with oil or 250 ng/kg of ICI on the morning of d 4 and killed at pregnancy d 6. COUP-TFIIflox/flox control mice treated with oil or ICI showed normal implantation (Fig. 1, F and H). This result suggests that the amount of ICI used in this rescue experiment has no effect on implantation in control mice. Consistent with our published results, PRCre/+;COUP-TFIIflox/flox mutant treated with oil failed to show uterine implantation (Fig. 1G) (12). Interestingly, the implantation defect was rescued in mutant mice treated with ICI (Fig. 1I), because Chicago blue-positive implantation sites were clearly seen in mutant uteri.
Figure 1.
ICI-rescued implantation defect of PRCre/+;COUP-TFIIflox/flox mutant mice. A–D, ERα activity, as examined by the expression of MUC1 and LF, was elevated in the epithelium of COUP-TFII mutant mice. A and B, Expression of MUC1 in the uterine epithelium was elevated in mutant mice at 30 h after eP treatment. C and D, Expression of lactoferrin in the uterine epithelium was elevated in mutant mice at 30 h after eP treatment (scale bar, 100 μm). E, Experimental scheme of rescue experiment for COUP-TFII mutant implantation defect by ICI injection. F–I, Implantation occurred in PRCre/+;COUP-TFIIflox/flox mutant mice treated with ICI. Implantation sites were visualized as blue spots (arrowhead) by iv injection of Chicago blue dye at d 6. F, Control mice treated with oil. G, Mutant mice treated with oil. H, Control mice treated with ICI. I, Mutant mice treated with ICI (scale bar, 5 mm). J, Average number of implantation sites of rescue experiment. ICI rescued implantation defect of mutant mice although the number is slightly reduced. All mutant mice were rescued after ICI treatment (control mice with oil, n = 3; mutant mice with oil, n = 3; control mice with ICI, n =3; mutant mice with ICI, n = 4). K, Implantation sites of ICI-treated mutant mice were only a bit smaller than control mice treated with either oil or ICI. White bars, control; black bars, mutant. *, P < 0.01; **, P < 0.005.
Next, we examined whether the number of implantation sites per mouse and the size of each implantation site are similar in the PRCre/+;COUP-TFIIflox/flox mutants in comparison with the controls upon treatment with ICI. ICI-treated COUP-TFIIflox/flox control mice have the same number of implantation sites per uterus as the oil-treated mice (9.3 vs. 10.0). However, PRCre/+;COUP-TFIIflox/flox mutant mice treated with ICI had increased number of implantation sites from 0 to 6.2 implantation sites on average (Fig. 1J). Similarly, COUP-TFIIflox/flox control mice treated with ICI show comparable size, an average value of 2.0 mm in diameter, of implantation site to control mice treated with oil. Implantation from ICI-treated PRCre/+;COUP-TFIIflox/flox mutant mice were on average of 1.6 mm in diameter in size, which is slightly smaller than the control mice (Fig. 1K). Together these results support our hypothesis that elevated ERα activity in the luminal epithelium of PRCre/+;COUP-TFIIflox/flox uterus is the main reason for implantation failure.
ICI partially rescues the artificial decidualization defects of PRCre/+;COUP-TFIIflox/flox mutant mice
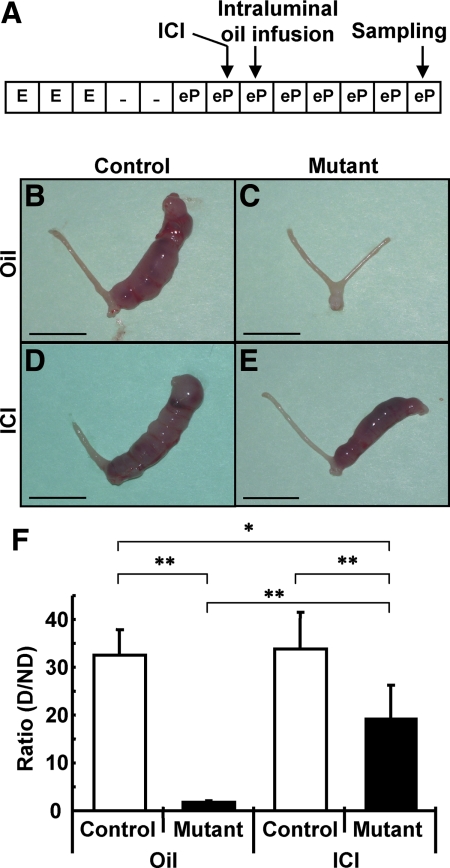
The presence of implantation sites in PRCre/+;COUP-TFIIflox/flox mice treated with ICI prompted us to investigate the effects of ICI on the artificial uterine decidual reaction. Endometrial stromal cells of ovariectomized mice can be induced to undergo an artificial decidual reaction by steroid hormone treatment and infusion of the uterine horn with oil (24). The degree of decidualization and, therefore, the response to steroid hormone induction can be easily quantified by determining the weight gain of the oil-infused horn to the uninfused control horn. As shown in Fig. 2A, ICI was administered 20 h before oil infusion, and the samples were collected at d 5 after stimulation. As in the cases of natural pregnancy, there was no difference between COUP-TFIIflox/flox control mice treated with oil and ICI: both control mice displayed well-developed decidualized uterine horns as compared with unstimulated uterine horns (Fig. 2, B and D). Quantification of the weight increase between these two groups showed no statistical difference (Fig. 2F). This observation confirmed that ICI, at the dose given, exerted little effect on decidualization in control mice. As expected, PRCre/+;COUP-TFIIflox/flox mutant mice treated with oil alone failed to decidualize upon intraluminal oil infusion (Fig. 2C) and displayed no increase in uterine weight compared with the control horn (Fig. 2F). However, decidualization occurred in PRCre/+;COUP-TFIIflox/flox mutant mice treated with ICI (Fig. 2, E and 2F), but the extent of the decidual response only reached about 60% of that of the control mice (Fig. 2F). These data suggest that ICI treatment partially rescued the artificial decidualization defect of PRCre/+;COUP-TFIIflox/flox mutant mice.
Figure 2.
ICI partially rescued decidualization defect of PRCre/+;COUP-TFIIflox/flox mutant mice. A, Experimental scheme of rescue for induced decidualization defect by ICI injection. B–E, Induced decidualization occurred in PRCre/+;COUP-TFIIflox/flox mutant mice treated with ICI. B, Control mice treated with oil 5 d after stimulation by intraluminal oil infusion. The right horn was stimulated, and the left horn was unstimulated. Only the right horn was decidualized. C, Mutant mice treated with oil 5 d after stimulation. No decidualization was observed. D, Control mice treated with ICI 5 d after stimulation by intraluminal oil infusion. The right horn was stimulated, and the left horn was untreated. Only the right horn was decidualized. E, Mutant mice treated with ICI 5 d after stimulation by intraluminal oil infusion. The right horn was stimulated, and the left horn was untreated. Only the right horn was decidualized (scale bar, 10 mm). F, Ratio of right to left horn in weight. Rescue in mutant mice was statistically significant. White bars, control; black bars, mutant (control mice with oil, n = 4; mutant mice with oil, n = 4; control mice with ICI, n = 7; mutant mice with ICI, n = 5; *, P < 0.01; **, P < 0.005). E, E2; eP, E2 + P4; D, decidualized; ND, nondecidualized.
Stromal cells in ICI-treated mutant mice were differentiated into the decidual cells and expressed decidual cell markers, Wnt4 and bone morphogenetic protein 2 (BMP2)
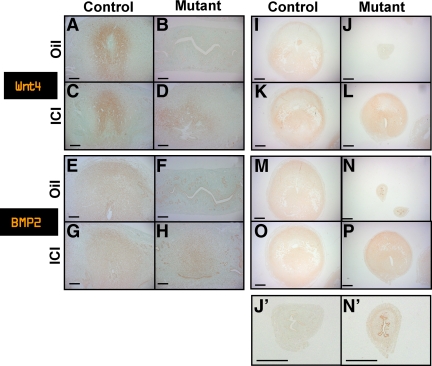
In the murine uterus, stromal cells proliferate and differentiate into decidua after implantation. To ensure that stromal cells differentiated properly to decidual cells in mutant mice after ICI treatment, we performed immunohistochemistry using known decidual cell markers, Wnt4 and BMP2 (25,26), at d 6 uterus. The expression of Wnt4 was detected in decidual cells surrounding the embryo at d 6 in both control mice treated with oil or ICI (Fig. 3, A and C). As shown in Fig. 3B, Wnt4 expression was not detected in mutant mice treated with oil alone. However, Wnt4 expression was clearly seen in decidual cells surrounding the embryo in mutant mice treated with ICI (Fig. 3D). Similarly, the expression of BMP2, another decidual cell marker, was detected in control mice treated with oil or ICI, whereas BMP2 was detected only in the decidual cells of the ICI-treated mutant mice, but not oil-treated mutant mice (Fig. 3, E–H). Similarly, using artificially induced decidualized uterus horns, we found that both Wnt4 and BMP2 were expressed in control mice treated with oil or ICI, whereas only mutant mice treated with ICI, but not mutant mice treated with oil, expressed both decidual cell markers (Fig. 3, I–P). These results indicate that ICI-treated mutant mice exhibit uterine decidual cells that are morphologically and functionally similar to normal decidual cells.
Figure 3.
Decidual cells in ICI-treated mutant mice expressed decidual cell markers, WNTt4 and BMP2. A–D, Decidual cells surrounding implantation sites expressed decidual cell marker, WNT4 at d 6. A, Control mice treated with oil. B, Mutant mice treated with oil. C, Control mice treated with ICI. D, Mutant mice treated with ICI (longitudinal section: scale bar, 200 μm). E–H, Decidual cells surrounding implantation sites expressed decidual cell marker, BMP2 at d 6. E, Control mice treated with oil. F, Mutant mice treated with oil. G, Control mice treated with ICI. H, Mutant mice treated with ICI (longitudinal section: scale bar, 200 μm). I–L, Decidual cells in artificial decidualized uterine horns expressed decidual cell marker, Wnt4. I, Control mice treated with oil. J and J′, Mutant mice treated with oil. K, Control mice treated with ICI. L, Mutant mice treated with ICI (scale bar, 635 μm). M–P, Decidual cells in artificial decidualized uterine horns expressed decidual cell marker, BMP2. M, Control mice treated with oil. N and N′, Mutant mice treated with oil. O, Control mice treated with ICI. P, Mutant mice treated with ICI (scale bar, 635 μm).
ICI decreased ERα activity in the luminal epithelium of the COUP-TFII mutant uterus
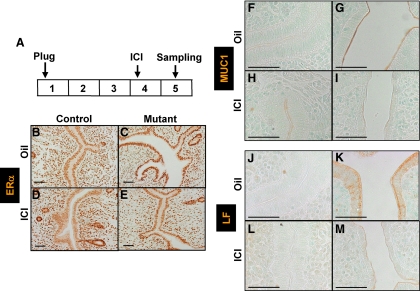
To ascertain whether attenuation of ERα activity by ICI treatment truly accounts for the rescue of defects of embryo attachment and decidualization in PRCre/+;COUP-TFIIflox/flox mice, we examined ERα expression and activity in luminal epithelium of control and mutant mice after ICI treatment. As shown in Fig. 4A, ICI was administered on the morning of d 4, and uterine tissues were isolated at d 5 and used for immunohistological analysis. To examine ERα expression and activity subsequent to ICI treatment, we performed immunohistochemistry against ERα and well-known ERα targets, MUC1 and lactoferrin, in luminal epithelium of the uterus (12,27,28). As shown in Fig. 4, B–E, ERα expression was not affected in control mice treated with either ICI or oil. As expected, ERα expression was enhanced in mutant mice treated with oil as compared with control mice treated with oil. Upon ICI treatment, ERα expression in the uterine luminal epithelium of mutant mice reduced substantially to a level comparable to that of control mice (Fig. 4, B, C, and E). ICI has been shown to inhibit ERα activity by rapid protein degradation, inhibition of nuclear transport, blocking of dimerization, and decreasing binding affinity to DNA (23). These results imply that ICI may inhibit ERα activity by decreasing ERα level in the luminal epithelium. The decrease in ERα activity subsequent to ICI treatment was confirmed by investigating the expression of MUC1 and lactoferrin. The expression of MUC1 was very low in the luminal epithelial cells of control mice treated with either oil or ICI at d 5 (Fig. 4, F and H). As expected, mutant mice treated with oil showed elevated MUC1 expression in luminal epithelium in comparison to control mice treated with either oil or ICI (12). However, MUC1 expression in the luminal epithelium of ICI-treated mutant mice was greatly reduced to a level comparable to control mice treated either with oil or ICI (Fig. 4, F, H, and I). Reduction of MUC1 expression in luminal epithelium during the receptive phase is necessary for embryo attachment in various species including mice (29). The expression of lactoferrin was very low in the uterine luminal epithelial cells of control mice treated with either oil or ICI at d 5 (Fig. 4, J and L). As expected, mutant mice treated with oil showed elevated lactoferrin expression in the uterine luminal epithelial cells in comparison with control mice treated with either oil or ICI (12). However, lactoferrin expression in the uterine luminal epithelial cells of ICI-treated mutant mice was greatly reduced to a level comparable to control mice treated either with oil or ICI (Fig. 4, J, L, and M). These results suggest that ICI efficiently inhibited ERα activity in the uterine luminal epithelium of mutant mice.
Figure 4.
ICI efficiently decreased ERα activity in the luminal epithelial compartment of PRCre/+;COUP-TFIIflox/flox mutant mice. A, Experimental scheme of ICI injection. B–E, Expression of ERα in the uterus epithelium was decreased in mutant mice after ICI treatment at d 5. B, Control mice treated with oil. C, Mutant mice treated with oil. D, Control mice treated with ICI. E, Mutant mice treated with ICI (scale bar, 50 μm). F–I, MUC1 expression in the epithelium of ICI-treated mutant mice was decreased to basal level as control mice at d 5. F, Control mice treated with oil. G, Mutant mice treated with oil. H, Control mice treated with ICI. I, Mutant mice treated with ICI (scale bar, 100 μm). J–M, Lactoferrin (LF) expression in the epithelium of ICI-treated mutant mice was reduced to a basal level as control mice. J, Control mice treated with oil. K, Mutant mice treated with oil. L, Control mice treated with ICI. M, Mutant mice treated with ICI (scale bar, 100 μm).
ERα target genes are decreased by ICI treatment
To validate that rescue of the implantation and decidualization defects are largely due to inhibition of ERα activity by ICI treatment, we performed DNA microarray analysis on uteri from oil- or ICI-treated mutant mice in order to systematically identify the ERα target genes and the signaling pathways that mediate COUP-TFII function. Total RNA extracts were subjected to microarray analysis using the Affymetrix mouse genome 430 2.0 arrays. This analysis revealed 153 and 46 transcripts, the abundance of which was significantly increased or decreased, respectively, in the ICI-treated mutant mice as compared with oil-treated mutant mice. A complete list of the genes, the transcripts of which increase or decrease in abundance, can be found in Supplemental Tables 1 and 2, respectively, published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org. To determine which pathways are regulated by ICI treatment at this time point, we performed pathway analysis using Ingenuity Systems Software. A complete list of the significantly regulated pathways can be found in Supplemental Table 3. The altered pathways include, but are not limited to, those involved in the cell cycle, DNA damage response, pyrimidine metabolism, and aryl hydrocarbon receptor signaling.
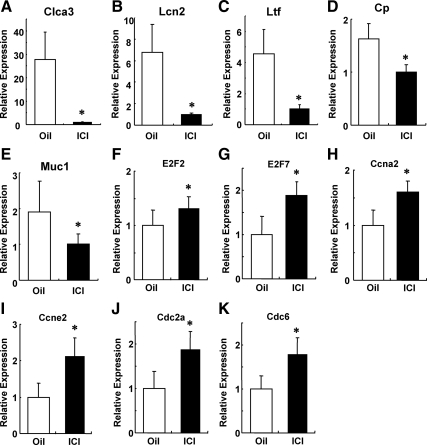
As expected, the expression of ERα target genes, including lactoferrin and Muc1, was significantly decreased in the ICI-treated mutant uterus. These results are consistent with immunohistochemistry data shown in Fig. 4. Among altered genes at 6 h after ICI treatment, chloride channel calcium activated 3 (Clca3), lipocalin 2 (Lcn2), lactoferrin (Lf), and ceruloplasmin (Cp) were significantly decreased (Table 1). These genes have been demonstrated to be ERα target genes (30) that are up-regulated in the mutant uterus during preimplantation period. The mRNA expression of Cacl3, Lcn2, Lf, Cp, and Muc1 were validated by quantitative RT-PCR, and a clear reduction of mRNA levels in the ICI-treated mutant uteri was shown in Fig. 5, A–E.
Table 1.
Summary of selected genes differentially expressed 6 h after ICI treatment
| Gene symbol | Gene title | Fold change |
|---|---|---|
| ERα targets | ||
| Clca3 | Chloride channel calcium-activated 3 | −20.63 |
| Lcn2 | Lipocalin 2 | −4.19 |
| Ltf | Lactoferrin | −3.1 |
| Cp | Ceruloplasmin | −1.92 |
| Cell cycle | ||
| E2f2 | E2F transcription factor 2 | 1.77 |
| E2f7 | E2F transcription factor 7 | 1.90 |
| Mcm4 | Minichromosome maintenance-deficient 4 homolog | 1.30 |
| Mcm5 | Minichromosome maintenance-deficient 5 | 1.38 |
| Mcm6 | Minichromosome maintenance-deficient 6 | 1.74 |
| Mcm7 | Minichromosome maintenance-deficient 7 | 1.30 |
| Ccna2 | Cyclin A2 | 1.53 |
| Ccnb1 | Cyclin B1 | 1.73 |
| Ccnb2 | Cyclin B2 | 1.64 |
| Ccne2 | Cyclin E2 | 1.88 |
| Cdc2a | Cell division cycle 2 homolog A | 1.49 |
| Cdc20 | Cell division cycle 20 homolog | 1.46 |
| Cdc6 | Cell division cycle 6 homolog | 1.65 |
| Cdca3 | Cell division cycle-associated 3 | 1.54 |
| Cdca8 | Cell division cycle-associated 8 | 1.63 |
Figure 5.
mRNA of ERα target genes were decreased 6 h after ICI treatment. Quantitative RT-PCR analysis of selected genes confirmed a decrease in levels of mRNA expression of ERα target genes and an increase in the expression of cell cycle-related genes. A, Quantitation of Cacl3 mRNA expression. B, Quantitation of Lcn2 mRNA expression. C, Quantitation of Lf mRNA expression. D, Quantitation of Cp mRNA expression. E, Quantitation of Muc1 mRNA expression. F, Quantitation of E2F2 mRNA expression. G, Quantitation of E2F7 mRNA expression. H, Quantitation of Ccna2 mRNA expression. I, Quantitation of Ccne2 mRNA expression. J, Quantitation of Cdc2a mRNA expression. K, Quantitation of Cdc6 mRNA expression. *, P < 0.05.
Cell cycle genes are up-regulated after ICI treatment
Using Ingenuity Pathway Analysis, we found 53 genes that are involved in the regulation of the cell cycle were significantly increased after ICI treatment. The E2F family (E2F2 and E2F7), mini-chromosome maintenance (MCM) family (MCM4, MCM5, MCM6, and MCM7), cyclins (Ccna2, Ccnb1, Ccnb2, and Ccne2), cell division cycle (CDC) family (Cdc2a, Cdc20, Cdc6, Cdca3, and Cdca8) were up-regulated in ICI-treated mutant mice (Table 1). The mRNA expression of E2F2, E2F7, Ccna2, Ccne2, Cdc2a, and Cdc6 were validated by quantitative RT-PCR, and an increase in mRNA levels in the ICI-treated mutant uteri was clearly shown in Fig. 5, F–K. Previously, we reported that mutant mice showed reduced proliferation in stromal cells. This result suggests that ICI treatment restored growth potential of the stromal cells of mutant mice and allowed them to proliferate and differentiate and contribute to the decidualization.
ICI failed to rescue placentation defects of COUP-TFII mutant mice
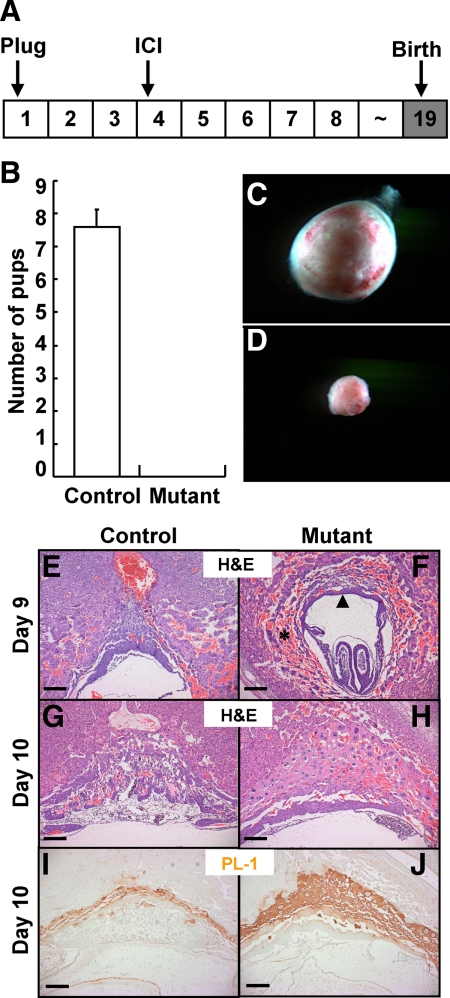
To investigate whether ICI treatment can rescue all of the reproductive defects exhibited by the loss of uterine COUP-TFII, we monitored the delivery of pups in the control and mutant mice treated with ICI (Fig. 6A). COUP-TFIIflox/flox control mice treated with ICI gave birth at the expected due date and had normal litter size; average litter size was 7.6. In contrast, none of the PRCre/+; COUP-TFIIflox/flox mutant mice treated with ICI gave birth (Fig. 6B). Because our earlier paper reported that deletion of COUP-TFII by Amhr-Cre resulted in placentation defect around gestation d 10 (21), we asked whether the inability to deliver pups arises from placenta defects and examined placenta formation at different gestation days. At gestation d 14, control mice treated with ICI had well-developed placentas, and placentas were easily isolated (Fig. 6C), whereas PRCre/+;COUP-TFIIflox/flox mutant mice carried a small embryo ball and placentas were hard to isolate from the uterus. Early reabsorption of embryos was clearly evident, suggesting the development of the defective placenta was an early event in gestation (Fig. 6D). To examine defects of mutant mice, we collected uteri at gestation d 8, d 9, and d 10 and compared their morphology subsequent to hematoxylin and eosin (H&E) staining. There was no apparent abnormality with respect to embryonic development in control and mutant mice at d 8 (data not shown). At d 9, mutant mice had severe hemorrhaging in the decidual tissue surrounding the embryo (asterisk), thin chorionic plate (arrow head), and smaller decidual tissue (Fig. 6, E and F). At d 10, growth of decidual tissue was arrested in mutant mice and major components of placenta, maternal decidua, trophoblast giant cells (TGCs), spongiotrophoblast, and labyrinth layer were not properly formed (31,32) (Fig. 6, G and H). In our previous publication (21) using Amhr2-Cre to ablate COUP-TFII, we showed that COUP-TFII mutant mice displayed placentation defects with an increase in TGC differentiation, a reduction in spongiotrophoblast layer and absence of labyrinth formation, and an improper vascularization of the placenta. Therefore, the enhancement of placental TGCs in the PR-Cre-mediated deletion of COUP-TFII resembles the previous observation and is an expected result (Fig. 6, E–H). To confirm the H&E staining result, we performed immunohistochemistry for TGCs with antiplacenta lactogen I antibody. As shown in Fig. 6, I and J, mutant mice had an increased number of TGCs. These results suggest that rescue of early implantation and decidualization defects with ICI treatment are not sufficient to support whole gestation in mutant mice and COUP-TFII involved in placentation process independent of ERα activity.
Figure 6.
ICI-treated mutant mice had placentation defect. A, Experimental scheme of rescue for placentation defect by ICI treatment. B, No pups were born from PRCre/+;COUP-TFIIflox/flox mutant mice treated with ICI. C and D, Placentas were isolated at d 14 from ICI-treated control and mutant mice. C, Placenta of control mice. D, Placenta of mutant mice. E–H, ICI-treated mutant mice had severe hemorrhage, and placentation was defective. H&E staining of uterus at d 9 and d 10. E, Control mice at d 9. F, Mutant mice at d 9. G, Control mice at d 10. H, Mutant mice at d 10. I and J, Increased trophoblast giant cells were observed in the mutant uterus at d 10. I, Placental lactogen I (PL-1) expression in the control mice at d 10. J, Placental lactogen I expression in the mutant mice at d 10 (scale bar, 200 μm).
Discussion
For a successful pregnancy, the uterus must be prepared to accept the blastocyst and to support further development such as decidualization and placentation. Recent studies using microarray and gene-targeted mouse models revealed that many factors are involved in these processes (33,34,35,36,37). Although individual factors have proven to be essential for uterine receptivity, most of them are directly or indirectly controlled by ovarian steroid hormones, estrogen and/or progesterone.
In our previous report (12), we proposed a model to explain estrogen and progesterone control of uterine implantation. In this model, COUP-TFII mediates progesterone-induced suppression of epithelial estrogen action by means of decreasing epithelial ERα and its coactivator SRC-1 expression and thus inhibiting the epithelial ERα activity during the receptive period of uterus. Importantly, all these effects are likely due to its regulation of stromal PR expression, which was shown to be responsible for the down-regulation of epithelial ERα activity (10,11). Indeed, COUP-TFII mutant mice have lower stromal PR levels (12).
Here we show that treatment with ICI rescued implantation defects exhibited by PRCre/+;COUP-TFIIflox/flox mutant mice. As shown in Fig. 1I, ICI-treated mutant mice had Chicago blue-positive regions, which indicated that the implantation process had been initiated. In addition to the Chicago blue injection experiment, these results indicate that the implantation defect displayed by PRCre/+;COUP-TFIIflox/flox mutant mice has been rescued with ICI treatment. To elucidate whether the rescue of the implantation defect of mutant mice is due to a reduction of ERα activity in the uterine luminal epithelial compartment, we showed that down-regulation of ERα activity in the luminal epithelium is necessary for implantation, and inhibition of ERα activity requires COUP-TFII during the receptive period of the uterus. We further confirmed the decreased expression of ERα target genes subsequent to ICI treatment using microarray analysis. The expression of ERα targets, including Clca3, Lcn2, Lf, and Cp, was efficiently down-regulated in the mutant uteri. These are known ERα target genes, which are expressed in the uterine epithelium; their down-regulation represents a direct action of ICI on inhibiting ERα activity in the epithelium.
Another important new finding is that the robust induction of the expression of many cell cycle-regulated genes in the stromal compartment are up-regulated with ICI treatment, suggesting that the impaired stromal cell growth and thus the decidualization defect of the COUP-TFII-deficient mice has been restored by ICI treatment. However, it is unclear how ICI treatment rescued decidualization of mutant mice. Two key events must take place for successful decidualization. First, proper signals must be derived from luminal epithelial cells to instruct the stromal cells to decidualize upon implantation. Second, the competent stromal cells, in response to the decidualization signals, undergo proliferation and differentiation. One possibility is that rescued decidualization is a consequence of rescued implantation after ICI treatment. Although implantation certainly must play a role in decidualization, the fact that the success of artificial induced decidualization by oil on ICI-treated PRCre/+;COUP-TFIIflox/flox mutants indicates that an additional role for ERα must be to induce stroma decidualization independently of an embryo attachment-induced event. At this time we do not know whether the epithelial ERα or stromal ERα is responsible for the control of this decidualization reaction. The fact that, in contrast to epithelial ERα, stromal ERα level is not affected in PRCre/+;COUP-TFIIflox/flox mutant (12) or upon ICI treatment (Fig. 4, B–E) suggests that epithelial ER is the likely candidate for this regulation.
The reason why ICI treatment cannot fully rescue the implantation defect is unclear at present. A possible explanation is that although we had successfully rescued the implantation defect in PRCre/+;COUP-TFIIflox/flox mutant mice, our experimental condition may not exactly mimic the physiological condition. Another possible explanation is that the uterine horn of the PRCre/+;COUP-TFIIflox/flox mutant mice is shorter than that of the COUP-TFIIflox/flox control mice (Refs. 12 and 21 and our unpublished data). Therefore, the limitation of space for embryo implantation may contribute to the lower number of implantation sites in PRCre/+;COUP-TFIIflox/flox mutant mice.
Furthermore, although implantation defects of the mutant mice were largely rescued, the size of the implantation site was smaller than that of control mice at 5.5 d post coitus. Because uterine stromal cells in mutant mice showed less phosphohistone H3-positive cells at 3.5 d post coitus (12), mutant stromal cells couldn’t proliferate as seen in control mice to develop normal decidual tissue. This notion is supported by microarray analysis in which cell cycle-related genes are up-regulated after ICI treatment. Thus, mutant mice regained proliferation potential after ICI treatment, and this recovery may contribute to rescue of decidualization defect of mutant mice. Proliferation defects of COUP-TFII knockdown cells were also observed in different cell types, HUVEC cells (Chen, X., J. Qin, S. Y. Tsai, and M. J. Tsai, manuscript in preparation). This smaller implantation size thus might be caused by less proliferative stromal cells in mutant mice.
Pregnancy in the mouse is a continuous and sequential process including implantation, decidualization, placentation, and parturition. Because Petit et al. (21) showed that Amhr-Cre mediated COUP-TFII-deficient mice exhibit placentation defect, we examined placentation in ICI-treated mutant mice. Surprisingly, the placentation defect, which was not rescued with ICI treatment, was more severe than Amhr-Cre-mediated COUP-TFII -deficient mice. The less severe defect of Amhr-Cre-mediated COUP-TFII-deficient mice may result from incomplete deletion of COUP-TFII in these mice (21). In any event, this result explains why mutant mice treated with ICI failed to deliver pups even though implantation defects were rescued (Fig. 6B). The inability of ICI to rescue the placentation defect also suggests that uncontrolled ERα activity is not the major cause underlying the placentation defects elicited by COUP-TFII. Instead, placentation defects may likely be explained by the essential role of COUP-TFII in the developmental process of the placenta. Nevertheless, our ICI-treated mutant mice can serve as a useful model for studying maternal factors that regulate placentation and trophoblast growth and differentiation.
In conclusion, we clearly demonstrated that implantation and decidualization defects in the uterus of COUP-TFII-deficient mice result from high ERα activity in the uterine epithelium. Because dysregulated ERα activity in the receptive uterus resulted in failure of implantation, it is reasonable to explain that the implantation defect of COUP-TFII mutant mice is a consequence of high ERα activity in the epithelium. ICI treatment decreased ERα activity in the luminal epithelium and rescued the implantation defect of mutant mice. ICI treatment also partially rescued the decidualization defect of mutant mice independent of embryo attachment. This result clearly indicates that we have defined a new function of ERα in the decidualization reaction. This may arise from rescue of signaling elicited by epithelial cells to stromal cell for decidualization or rescue of the ability of stromal cells to receive signals from epithelial cells for decidualization. We still don’t know how COUP-TFII regulates epithelial ERα activity via paracrine regulation, but these ICI-treated mutant mice could serve as an useful tool for studying paracrine signaling between the epithelial and stromal compartments of the uterus during the decidualization process.
Materials and Methods
Animals and chemicals
COUP-TFIIflox/flox and PRCre/+; COUP-TFIIflox/flox mice, with a 129S genetic background, were housed at the Baylor College of Medicine in accordance with National Institutes of Health standards for the care and use of experimental animals. All procedures for animal study were approved by the institutional committee of animal care guidelines at Baylor College of Medicine. Chemicals used in this study are as follows: 17β-estradiol (Sigma Chemical Co., St. Louis, MO), progesterone (Sigma), and ICI 182,780 (Tocris Bioscience, Ellisville, MO) were dissolved in sesame oil (Sigma). Primary antibodies used in this study are as follows: goat polyclonal anti-Wnt4 (R&D Systems, Minneapolis, MN), goat polyclonal anti-BMP2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit polyclonal antilactoferrin (Upstate Biotechnology, Inc., Billerica, MA), rabbit polyclonal anti-ERα (Santa Cruz), goat polyclonal antiplacental lactogen 1 (Santa Cruz). Biotinylated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
ICI treatment
Control and mutant female mice (7 wk of age) were mated with wild-type male mice. For rescue of implantation, we injected mice sc with ICI (250 ng/kg) on the morning of d 4 (d 1 = vaginal plug formed). Implantation sites were visualized by iv injection of 1% Chicago Sky Blue 6B at d 6 (38). For rescue artificial decidualization, we injected ICI at 20 h before stimulation by intraluminal oil infusion.
Artificial decidualization
Artificial decidualization methods have been previously described (39). Briefly, 2 wk after ovariectomy, we first primed mice with 100 ng of E2 for 3 d by daily injection and then rested for 2 d. We started the daily treatment of 6.7 ng of E2 and 1 mg of P4. We stimulated a left uterine hone using intraluminal oil infusion at 54 h after the first E2-P4 treatment, and mice were dissected 5 d later for decidual response measurement. Isolated tissues were weighed and processed for further analysis.
H&E and immunohistological staining
Isolated uterine tissues were fixed in 4% (wt/vol) paraformaldehyde in PBS, processed for paraffin embedding. All the uterine tissues were sectioned to 5 μm thickness. For H&E staining, the tissues were deparaffinized in xylene and rehydrated through a series of ethanol, after which tissues were stained with H&E. For immunohistochemical staining, rehydrated tissues were treated by heat-mediated antigen retrieval in citrate buffer (Vector Laboratories, Inc., Burlingame, CA). The tissues were treated with 1% hydrogen peroxide to inactivate endogenous peroxidase. Tissues were washed with PBS containing 0.05% Tween 20, and then incubated for 2 h with blocking solution (5% normal goat serum). Primary antibodies were incubated overnight at 4 C, and secondary antibodies were incubated for 1 h at room temperature. Signals were amplified with ABC kit (Vector) and visualized with 3,3′-diaminobenzidine substrate kit (Vector). Methyl green (Vector) was used for counterstaining in immunohistochemistry.
Total RNA isolation, microarray hybridization, and data analysis
For microarray analysis, mutant uteri were isolated at 6 h after oil or ICI treatment using an experimental scheme for artificial decidualization. Total RNA was isolated from uteri using the RNeasy kit (QIAGEN, Chatsworth, CA). The total RNA was pooled from the uteri of three mice per genotype. The quality control and microarray hybridization with Affymetrix mouse genome 430 2.0 were done by Microarray core at Baylor College of Medicine. All experiments were performed in triplicate with independent pools of total RNA.
Microarray data analysis was performed as previously described (40,41). We selected differentially expressed genes in the oil- or ICI-treated PRCre/+; COUP-TFIIflox/flox mice. We selected differentially expressed genes within each time exposure using two sample comparisons according to the following criteria: lower bound of 90% confidence interval of fold change greater than 1.2, and absolute value of difference between groups means greater than 50. After excluding expressed sequence tags with no functional annotation, differentially expressed genes were classified according to Gene Ontology function using Affymetrix annotation and Ingenuity Pathways Analysis (Ingenuity Systems, Mountain View, CA). Raw data can be found in the GEO database as accession number GSE19408.
Quantitative real-time RT-PCR
Total RNA was extracted using an RNeasy Mini kit (QIAGEN) and reverse transcribed using TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). Gene expression assay was performed by running the ABI PRISM 7700 Sequence Detector System (Applied Biosystems). TaqMan Universal Master Mix reagents and inventoried primer/probe mixture (Applied Biosytems) were used for the reaction. The primers/probes used in this study are as follows: Clca3 (Mm00489959_ m1), Lcn2 (Mm01324470_m1), Lf (Mm00434787_m1), Cp (Mm00432654_m1), MUC1 (Mm00449604_m1), E2f2 (Mm00624964_m1), E2f7 (Mm00618098_m1), Ccna2 (Mm00438064_m1), Ccne2 (Mm00438077_m1), Cdc2a (Mm00772471_m1), Cdc6 (Mm00488573_g1). Standard curves were generated by serial dilution of a preparation of total RNA, and mRNA quantities were normalized against 18S rRNA determined by using eukaryotic 18S rRNA endogenous control reagents (Applied Biosystems).
Supplementary Material
Acknowledgments
We thank Wen Chen, Xue-Fei Tong, and Wei Qian (Baylor College of Medison, Houston, TX) for technical support. We also thank Dr. Sang Jun Han (Baylor College of Medicine) for helpful discussions.
Footnotes
This work was supported by grants from the National Institutes of Health (NIH) HL076448 (to S.Y.T.), DK059820 (to S.Y.T. and M.J.T.), DK45641 and HD17379 (to M.J.T.), and CA077530 (to J.P.L.). This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH through Cooperative Agreement U54 HD07495 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to F.J.D.).
Current address for I.K.: Internal Medicine, Keio University School of Medicine, Tokyo 160-8582, Japan.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 10, 2010
Abbreviations: BMP2, Bone morphogenetic protein 2; CDC, cell division cycle; COUP-TFII, chicken ovalbumin upstream promoter-transcription factor II; E2, 17β-estradiol; ERα, estrogen receptor α; H&E, hematoxylin and eosin; ICI, ICI 182,780; LF, lactoferrin; MCM, mini-chromosome maintenance; MUC1, mucin 1; P4, progesterone; PR, progesterone receptor; SRC-1, steroid receptor coactivator 1; TGC, trophoblast giant cell; Wnt4, wingless-type murine mammary tumor virus integration site family, member 4.
References
- Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K 2000 Embryo implantation. Dev Biol 223:217–237 [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H 2004 Molecular cues to implantation. Endocr Rev 25:341–373 [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK 2006 Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 7:185–199 [DOI] [PubMed] [Google Scholar]
- Dey S 1995 Implantation. In: Adasni EY, Rock JA, Rosenwaks Z, eds. Reproductive endocrinology, surgery and technology. New York: Lippincott-Raven; 421–434 [Google Scholar]
- Psychoyos A 1973 Hormonal control of ovoimplantation. Vitam Horm 31:201–256 [DOI] [PubMed] [Google Scholar]
- Yoshinaga K 1988 Uterine receptivity for blastocyst implantation. Ann NY Acad Sci 541:424–431 [DOI] [PubMed] [Google Scholar]
- Paria BC, Huet-Hudson YM, Dey SK 1993 Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci USA 90:10159–10162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WG, Song H, Das SK, Paria BC, Dey SK 2003 Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA 100:2963–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW 2002 Reproductive functions of progesterone receptors. Recent Prog Horm Res 57:339–355 [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Lydon JP, Cunha GR 2000 Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod 62:831–838 [DOI] [PubMed] [Google Scholar]
- Cunha GR, Cooke PS, Kurita T 2004 Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol 67:417–434 [DOI] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY 2007 COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 3:e102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Tsai SY, Cook RG, Beattie WG, Tsai MJ, O'Malley BW 1989 COUP transcription factor is a member of the steroid receptor superfamily. Nature 340:163–166 [DOI] [PubMed] [Google Scholar]
- Tsai SY, Tsai MJ 1997 Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr Rev 18:229–240 [DOI] [PubMed] [Google Scholar]
- Pereira FA, Qiu Y, Zhou G, Tsai MJ, Tsai SY 1999 The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev 13:1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Li L, Takamoto N, Martin JF, Demayo FJ, Tsai MJ, Tsai SY 2004 The nuclear orphan receptor COUP-TFII is required for limb and skeletal muscle development. Mol Cell Biol 24:10835–10843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamoto N, You LR, Moses K, Chiang C, Zimmer WE, Schwartz RJ, DeMayo FJ, Tsai MJ, Tsai SY 2005 COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development 132:2179–2189 [DOI] [PubMed] [Google Scholar]
- You LR, Takamoto N, Yu CT, Tanaka T, Kodama T, Demayo FJ, Tsai SY, Tsai MJ 2005 Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc Natl Acad Sci USA 102:16351–16356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Tsai MJ, Tsai SY 2008 Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS ONE 3:e3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Takamoto N, Yan J, Tsai SY, Tsai MJ 2009 Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII) regulates growth and patterning of the postnatal mouse cerebellum. Dev Biol 326:378–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit FG, Jamin SP, Kurihara I, Behringer RR, DeMayo FJ, Tsai MJ, Tsai SY 2007 Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci USA 104:6293–6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamoto N, Kurihara I, Lee K, Demayo FJ, Tsai MJ, Tsai SY 2005 Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol 19:2299–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A, Osborne CK, Morris C, Wakeling AE 2000 ICI 182,780 (Faslodex): development of a novel, “pure” antiestrogen. Cancer 89:817–825 [DOI] [PubMed] [Google Scholar]
- Ledford BE, Rankin JC, Markwald RR, Baggett B 1976 Biochemical and morphological changes following artificially stimulated decidualization in the mouse uterus. Biol Reprod 15:529–535 [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ 2007 Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27:5468–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC 2007 Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282:31725–31732 [DOI] [PubMed] [Google Scholar]
- Ray S, Das SK 2006 Chromatin immunoprecipitation assay detects ERα recruitment to gene specific promoters in uterus. Biol Proced Online 8:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Tan J, Johnson DC, Dey SK 1998 Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology 139:2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza MM, Mani SK, Julian J, Carson DD 1998 Reduction of mucin-1 expression during the receptive phase in the rat uterus. Biol Reprod 58:1503–1507 [DOI] [PubMed] [Google Scholar]
- Moggs JG, Tinwell H, Spurway T, Chang HS, Pate I, Lim FL, Moore DJ, Soames A, Stuckey R, Currie R, Zhu T, Kimber I, Ashby J, Orphanides G 2004 Phenotypic anchoring of gene expression changes during estrogen-induced uterine growth. Environ Health Perspect 112:1589–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knofler M, Vasicek R, Schreiber M 2001 Key regulatory transcription factors involved in placental trophoblast development—a review. Placenta 22(Suppl A):S83–S92 [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC 2001 Placental development: lessons from mouse mutants. Nat Rev Genet 2:538–548 [DOI] [PubMed] [Google Scholar]
- Borthwick JM, Charnock-Jones DS, Tom BD, Hull ML, Teirney R, Phillips SC, Smith SK 2003 Determination of the transcript profile of human endometrium. Mol Hum Reprod 9:19–33 [DOI] [PubMed] [Google Scholar]
- Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B 2002 Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod 8:871–879 [DOI] [PubMed] [Google Scholar]
- Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A, Osteen K, Taylor RN, Lessey BA, Giudice LC 2002 Global gene profiling in human endometrium during the window of implantation. Endocrinology 143:2119–2138 [DOI] [PubMed] [Google Scholar]
- Riesewijk A, Martín J, van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simón C 2003 Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod 9:253–264 [DOI] [PubMed] [Google Scholar]
- Horcajadas JA, Riesewijk A, Domínguez F, Cervero A, Pellicer A, Simón C 2004 Determinants of endometrial receptivity. Ann NY Acad Sci 1034:166–175 [DOI] [PubMed] [Google Scholar]
- Dey SK 2003 Manipulating the mouse embryo. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Finn CA, Martin L 1972 Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod 7:82–86 [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ 2005 Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 146:3490–3505 [DOI] [PubMed] [Google Scholar]
- Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O'Malley BW, DeMayo FJ 2007 The p160 steroid receptor coactivator 2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology 148:4238–4250 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.