Abstract
Although rapid, membrane-activated estrogen receptor (ER) signaling is no longer controversial, the biological function of this nongenomic signaling is not fully characterized. We found that rapid signaling from membrane-associated ER regulates the histone methyltransferase enhancer of Zeste homolog 2 (EZH2). In response to both 17β-estradiol (E2) and the xenoestrogen diethylstilbestrol, ER signaling via phosphatidylinositol 3-kinase/protein kinase B phosphorylates EZH2 at S21, reducing levels of trimethylation of lysine 27 on histone H3 in hormone-responsive cells. During windows of uterine development that are susceptible to developmental reprogramming, activation of this ER signaling pathway by diethylstilbestrol resulted in phosphorylation of EZH2 and reduced levels of trimethylation of lysine 27 on histone H3 in chromatin of the developing uterus. Furthermore, activation of nongenomic signaling reprogrammed the expression profile of estrogen-responsive genes in uterine myometrial cells, suggesting this as a potential mechanism for developmental reprogramming caused by early-life exposure to xenoestrogens. These data demonstrate that rapid ER signaling provides a direct linkage between xenoestrogen-induced nuclear hormone receptor signaling and modulation of the epigenetic machinery during tissue development.
Non-genomic estrogen receptor signaling regulates the histone methyltransferases EZH2 in estrogen-responsive cells in vitro and in vivo in the developing rat uterus.
Estrogens regulate key physiological functions in numerous tissues, including the reproductive tract, brain, cardiovascular system, bone, and immune systems. Estrogen action is mediated by the estrogen receptor (ER), which exists as two isoforms, ERα and ERβ, that account for the vast majority of the biological effects of these hormones on development, differentiation, and cell proliferation (1,2,3). The ER is a target of both endogenous estrogens, responsible for the natural functions of this hormone, and xenoestrogens, which are nonsteroidal substances that cause endocrine disruption (4,5). Liganded ERs elicit two types of cellular responses, commonly called genomic and nongenomic or rapid, membrane-activated signaling (6,7). The classical genomic signaling pathway is activated when ligands bind to ER and induce a conformational change that causes the receptor to dissociate from chaperone proteins, dimerize, translocate to the nucleus, and bind estrogen-responsive elements in DNA, enabling the receptor and associated coactivators and corepressors to promote or repress gene transcription (8,9,10). An additional part of the genomic response is ER regulation of gene expression indirectly by interacting with other transcription factors, such as activator protein 1, Sp-1 transcription factor and nuclear factor-κB, regulating genes lacking classical estrogen-responsive elements but containing recognition elements for these transcription factors (11).
Nongenomic responses refer to rapid changes in cellular signaling pathways induced by ligand binding to ER outside the nucleus (12,13). The rapid activation of signaling is thought to be due either to the direct association of ER with growth factor receptors, adaptor proteins at the cell membrane, or interaction of ERs with signaling proteins localized in caveolae. In some cases, these protein complexes include receptor and nonreceptor tyrosine kinases such as IGF receptor-1, epidermal growth factor receptor, the p85 subunit of phosphatidylinositol 3-kinase (PI3K), Src, Shc, and Ras, which activate signaling cascades upon ligand binding (7,14,15,16). Although the biological function of nongenomic ER signaling is not fully characterized, the fact that it engages mitogenic pathways such as PI3K and MAPK suggests it may play a role in cell proliferation and survival (6,17). Indeed, rapid signaling from membrane-associated ER is critical to osteocyte survival and prevention of osteoporosis (18,19,20). In addition, estrogen-induced activation of endothelial nitric oxide synthetase via MAPK protects blood vessels through promotion of vasodilatation and prevention of atherosclerosis (21,22). Similarly, nongenomic signaling appears to prevent neurodegeneration by the promotion of neuronal survival via the MAPK pathway (23,24). Furthermore, recent studies have shown that rapid ER signaling modulates neuroendocrine loops that regulate sexual behavior and reproductive function (25,26,27).
Conversely, inappropriate activation of ER signaling can have a dramatic adverse effect on morphogenesis and programming of estrogen-responsive genes in the developing rodent uterus (28,29). Previous studies from our group and others have demonstrated that inappropriate exposure to xenoestrogens during development reprograms gene expression and gives rise to increased uterine tumor incidence in adulthood (30,31,32,33,34,35,36). The mechanism(s) by which xenoestrogens permanently alter uterine gene expression and modulate cancer risk are largely unknown. Xenoestrogens are poorly mutagenic, and it is thought that the mechanism by which xenoestrogens developmentally reprogram gene expression in the uterus is through epigenetic mechanisms, such as alteration of histone or cytosine methylation, the primary epigenetic modifications of chromatin responsible for regulation of chromatin organization and gene expression (37,38).
Histone methylation has recently been recognized as an important epigenetic modification that is heritable and, in many cases, acts to direct other epigenetic alterations, such as DNA methylation (39,40,41,42,43). Site-specific methylation of histones generates binding sites for various chromatin-remodeling complexes as well as other epigenetic effectors, such as DNA methyltransferases (DNMTs) (39,41,42,44,45). Histone methyltransferases (HMTs) are chromatin-remodeling enzymes that catalyze the mono- and dimethylation of arginine and mono-, di-, and trimethylation of lysine residues on histone proteins H1, H2A, H3, and H4 (46,47,48,49), with histone H3 and H4 containing the majority of characterized histone methylation sites. In contrast to DNA methylation, histone methylation can result in either activation (H3K4 and H3R17) or silencing (H3K9 and H3K27) of transcription (50,51).
Mechanism(s) for regulation of HMTs are not well understood. However, communication between extracellular stimuli and chromatin via signal transduction pathways is one possible mechanism for regulation of these key epigenetic modifiers. For example, IGF induces phosphorylation of the HMT enhancer of Zeste homolog 2 (EZH2) by protein kinase B (AKT), which reduces methyltransfease activity and dissociates EZH2 from chromatin to facilitate gene expression (52). Similarly, coactivator-associated arginine methyltransferase 1 is phosphorylated by an unidentified kinase, which inhibits its enzymatic activity and reduces substrate affinity (53). In addition, noncanonical Wnt5a signaling to Nemo-like kinase causes phosphorylation of SET domain bifurcated 1, forming a repressive complex that silences gene expression (54). Thus, there is an emerging paradigm that cell-signaling pathways regulate HMT activity through posttranslational modification of these enzymes.
The ability of diethylstilbestrol (DES) to reprogram gene expression in the developing uterus led us to investigate whether xenoestrogens could modulate epigenetic regulators, specifically, HMTs and histone methylation via rapid ER signaling. In this report, we demonstrate that ER-mediated signaling via the PI3K/AKT pathway results in the phosphorylation of EZH2, reducing levels of trimethylation of lysine 27 on histone H3 (H3K27Me3) in chromatin and reprogramming the expression profile of genes previously identified as targets of xenoestrogen-induced developmental reprogramming in uterine myometrial cells (34,36). EZH2 phosphorylation and decreased H3K27Me3 were rapid, induced by DES and BSA-conjugated estradiol (E2-BSA) that could not enter cells and was inhibited by pharmacological PI3K inhibitors. ER dependence was demonstrated pharmacologically and genetically by loss of estrogen-induced PI3K/AKT signaling upon pretreatment with ICI 182780 and in ER knockout (ERKO) mice. Importantly, DES activated nongenomic signaling via the PI3K/AKT pathway in vivo, increasing EZH2 phosphorylation and reducing H3K27Me3 in chromatin of the uterus during a window of development susceptible to developmental reprogramming. Taken together, these data identify regulation of the HMT EZH2 as a novel biological function of rapid ER signaling, which is also induced during developmental xenoestrogen exposure.
Results
ER signaling results in rapid phosphorylation of EZH2
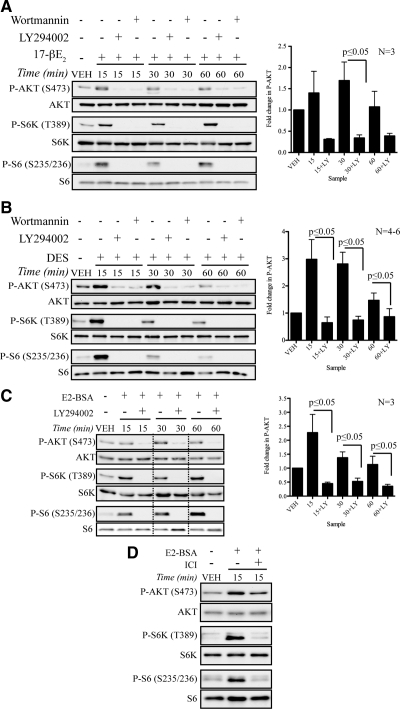
In estrogen-responsive MCF-7 cells depleted of estrogen for 36–48 h, rapid activation of the PI3K-signaling pathway was observed in response to 17β-estradiol (E2) and the xenoestrogen DES, with phosphorylation of AKT and downstream effectors S6K and S6 induced by 15 min (Fig. 1, A and B). The PI3K inhibitors LY294002 and wortmannin effectively blocked AKT, S6K, and S6 phosphorylation, demonstrating the dependence of both E2 and DES-induced ER signaling to AKT on the PI3K pathway (Fig. 1, A and 1B). To establish that the rapid activation of PI3K signaling was due to a nongenomic rather than genomic ER response, E2-BSA, which cannot pass through the plasma membrane and fails to activate genomic responses such as pS2 gene expression (55) (Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), was used to activate nongenomic signaling. Similar to what was observed with E2 and DES, PI3K signaling to AKT was rapidly activated by E2-BSA and ablated by the PI3K inhibitor LY294002 (Fig. 1C). ICI 182780, an ER antagonist (56), also inhibited activation of PI3K/AKT signaling by E2-BSA (Fig. 1D), demonstrating that AKT was activated via nongenomic ER signaling and that activation of this pathway by E2 and DES was ER dependent.
Figure 1.
ER-mediated activation of PI3K/AKT signaling. A, AKT activation by E2 (50 nm) can be inhibited by pretreatment with PI3K antagonists, LY294002 (20 μm) and wortmannin (200 nm), in MCF-7 cells. B, DES-induced activation of AKT pathway is inhibited by PI3K antagonists, LY294002 (20 μm) and wortmannin (200 nm), which were given to some groups of MCF-7 cells before DES (50 nm) treatment. C, MCF-7 cells were pretreated with LY294002 (20 μm) and exposed to E2-BSA (25 nm) for described times. Figure was cropped as indicated by dotted lines. D, E2-BSA (25 nm)-induced nongenomic signaling is inhibited by pretreatment with ICI 182780 (100 nm) in MCF-7 cells. Each panel shows representative Western blots from an independent experiment. Graphs display densitometry analysis of the ratio of phospho-AKT to total AKT protein normalized to vehicle-treated cells (mean ± sem). Statistical significance (*, P ≤ 0.05) was determined by Student’s t test. LY, LY294002.
MCF-7 cells stably expressing myristoylated AKT (57,58) with constitutive AKT activity (CA-AKT) were used to demonstrate that AKT activation was sufficient to induce EZH2 phosphorylation (Supplemental Fig. 2A). EZH2 was immunoprecipitated from isogenic MCF-7 or MCF-7 CA-AKT cells using a phospho-specific antibody directed against the AKT phosphorylation site (S21), and as shown in Supplemental Fig. 2B, MCF-7 cells expressing CA-AKT had dramatically higher levels of phospho-EZH2 than isogenic MCF-7 cells. Concordant with increased levels of EZH2 phosphorylation, MCF-7 CA-AKT cells had significantly reduced levels of H3K27Me3, confirming that AKT-mediated EZH2 phosphorylation decreased HMT activity (Supplemental Fig. 2C). Similarly, we also examined the effect of serum and IGF on the PI3K/AKT pathway and found that these treatments, as expected, activated the PI3K/AKT pathway with kinetics similar to estrogens (Supplemental Fig. 3, A and B) and caused rapid phosphorylation of EZH2 (Supplemental Fig. 3C) (52).
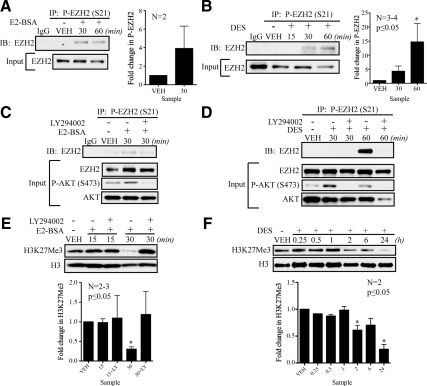
We next investigated whether E2- and DES-induced activation of rapid ER signaling resulted in EZH2 phosphorylation, using MCF-7 cells treated with E2-BSA or DES to activate AKT (Fig. 1, B and C). Rapid activation of AKT was accompanied by increased phosphorylation of endogenous (Fig. 2, A and B) as well as exogenously expressed EZH2 at S21 (Supplemental Fig. 4). In addition to what was observed with 50 nm DES treatment, we also found that a very low concentration of DES (5 nm) caused rapid activation of the PI3K/AKT pathway and subsequent EZH2 phosphorylation, indicating this activation also occurs in response to low levels of ligand (Supplemental Fig. 5). Increased phospho-EZH2 was not a result of increased total levels of EZH2, because EZH2 levels remained unchanged or decreased slightly in response to estrogen as determined by input Western blots that were used as a loading control (Fig. 2, A and B). To confirm that EZH2 phosphorylation was dependent on activation of nongenomic PI3K signaling, cells were pretreated with the PI3K inhibitor LY294002, which inhibited the ability of E2-BSA to induce EZH2 phosphorylation (Fig. 2C). Similar results were observed with LY294002-treated cells exposed to DES (Fig. 2D).
Figure 2.
Nongenomic signaling to PI3K/AKT phosphorylates EZH2 and reduces H3K27Me3 levels. A, Nongenomic ER signaling to the AKT pathway increases P-EZH2 levels in E2-BSA (25 nm)-treated lysates as detected by immunoprecipitation in MCF-7 cells. Western blots of inputs from immunoprecipitation were used to control for variation in total EZH2 in each sample. B, DES (50 or 100 nm) induces phosphorylation of endogenous EZH2 in MCF-7 cells. C, Immunoprecipitation of P-EZH2 from MCF-7 cell-treated E2-BSA (25 nm) with or without LY294002 (20 μm) pretreatment demonstrates dependence of EZH2 phosphorylation on PI3K/AKT pathway. D, DES-induced (50 or 100 nm) phosphorylation of endogenous EZH2 is dependent on the activity of the PI3K/AKT pathway. E, Western blot analysis of MCF-7 cells treated with E2-BSA (25 nm) with or without LY294002 (20 μm) demonstrates dependence of the reduction of H3K27Me3 levels on PI3K-mediated AKT activation. F, Histones were acid precipitated from estrogen-deprived MCF-7 cells that were treated with VEH or DES (50 nm) for 0.25, 0.5, 1, 2, 6, and 24 h and subjected to Western blot analysis, revealing that DES reduces H3K27Me3 levels in chromatin of treated cells. Western blots from independent experiments were analyzed by densitometry, and differences in levels of phosphorylation or methylation relative to VEH are shown as mean ± sem. Statistical significance was determined by Student’s t test or one-way ANOVA (*, P ≤ 0.05). IB, Immunoblotting; IP, immunoprecipitation; LY, LY294002.
Phosphorylation of EZH2 in response to rapid ER signaling reduces H3K27Me3 levels in chromatin
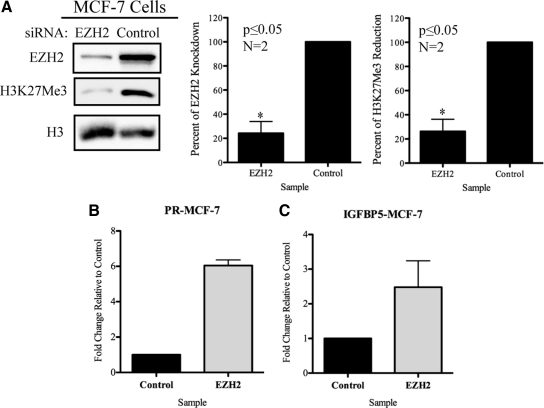
Site-specific histone methylation is often established and maintained by HMTs with redundant activity. Thus, one histone methylation site may be the target of numerous HMTs (59). However, EZH2 is the primary HMT responsible for H3K27Me3 and functions in a nonredundant manner with EZH1, a HMT that catalyzes the mono- and dimethylation of H3K27, to maintain methylation at the K27 site of histone H3 (60,61). Consistent with published data on the specificity of EZH2 and the H3K27Me3 methyl mark (60,61), small interfering RNA (siRNA) knockdown of EZH2 correlated with a dramatic reduction of H3K27Me3 levels in MCF-7 cells (Fig. 3A), confirming EZH2 as the primary HMT for the H3K27Me3 methyl mark.
Figure 3.
EZH2 is the primary HMT responsible for H3K27Me3 and is a regulator of PR and IGFBP5 genes. A, Knockdown of EZH2 protein reduces levels of H3K27Me3 in MCF-7 cells. B, EZH2 is a regulator of PR in MCF-7 cells. RNA was collected from cells treated with EZH2 or control siRNA, and PR expression levels were analyzed by qPCR. C, IGFBP5 is an EZH2 target gene as detected by qPCR analysis of MCF-7 cells where EZH2 protein levels are reduced by siRNA. Panels display a representative Western blot from two independent experiments. Western blot data investigating efficiency of EZH2 knockdown and subsequent reduction in H3K27Me3 levels were analyzed by densitometry. Significance was given to data with P ≤ 0.05 (*) as identified by Student’s t test. For qPCR analysis the calibrator of this experiment was cells treated with control siRNA. Fold change was calculated for each sample in comparison with the calibrator. Graph bars represent mean ± sem obtained from a single experiment read in triplicate.
Examination of H3K27Me3 levels in chromatin of MCF-7 CA-AKT cells revealed that these cells had significantly lower levels of H3K27Me3 than isogenic MCF-7 cells, indicating that constitutive AKT activation and EZH2 phosphorylation correlated with EZH2 activity in estrogen-responsive cells (Supplemental Fig. 2, B and C). Therefore, we next determined whether AKT activation and EZH2 phosphorylation induced by nongenomic signaling modulated EZH2 activity and induced changes in histone methylation. As shown in Fig. 2E, H3K27Me3 levels were reduced by E2-BSA in soluble histones from whole-cell lysates as quickly as 30 min, commensurate with activation of AKT and phosphorylation of EZH2 (Fig. 2, A and C). Reduced H3K27Me3, induced in response to activation of nongenomic signaling by E2-BSA, was inhibited by LY294002, demonstrating the linkage between estrogen-induced activation of PI3K/AKT, EZH2 phosphorylation, and reduced histone methylation (Fig. 2E). Reduction of H3K27Me3 in response to estrogen also occurred in chromatin-associated histones isolated by acid precipitation (Fig. 2F). The decrease in this chromatin-associated methyl mark displayed delayed kinetics, being observed as early as 2 h and continuing to decrease over a 24-h period. By 24 h, levels of chromatin-associated H3K27Me3 levels were 75% less than vehicle (VEH) control (Fig. 2F).
Estrogens modulate EZH2 activity and reprogram gene expression in uterine myometrial cells
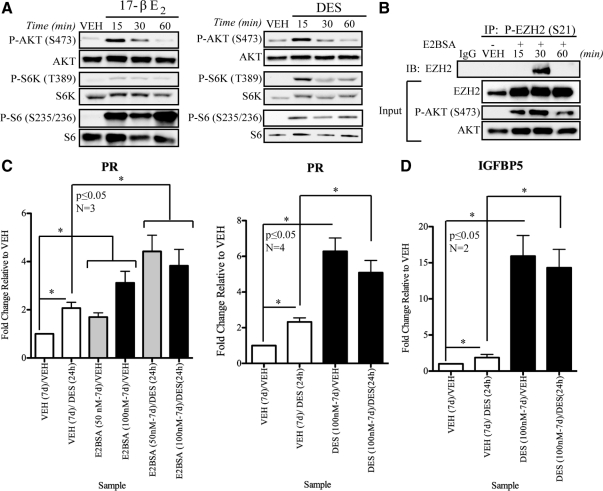
To determine whether nongenomic signaling modulates EZH2 in uterine myometrial cells, the ability of estrogens to induce nongenomic signaling was evaluated using hormone-responsive ELT3 cells (62). Similar to what was observed in MCF-7 cells, E2 and DES both induced rapid activation of PI3K/AKT signaling, indicating that, like breast epithelial cells, the nongenomic PI3K/AKT signaling pathway was intact in uterine myometrial cells (Fig. 4A). E2-BSA also activated the PI3K/AKT pathway and subsequently induced EZH2 phosphorylation as determined by immunoprecipitation of phospho-EZH2 in ELT3 cells (Fig. 4B).
Figure 4.
Estrogens activate nongenomic signaling to phosphorylate EZH2 and regulate gene expression in ELT3 cells. A, ELT3 cells were starved for 48 h and then treated with E2 (10 nm) or DES (10 nm) for indicated times. Activation of PI3K/AKT pathway was determined by Western blot analysis. B, Activation of PI3K/AKT pathway by E2-BSA (50 or 100 nm) induces EZH2 phosphorylation in ELT3 cells, which was detected by immunoprecipitation followed by Western blot analysis. C, ELT3 cells exposed to VEH, E2-BSA (50 or 100 nm), or DES (100 nm) for 7 d. After removal of initial, priming treatments cells were washed with PBS and starved (48 h), cells were reexposed to DES (50 nm), and expression of PR was analyzed by qPCR demonstrating that E2-BSA and DES were capable of reprogramming the basal expression and hormone responsiveness of this gene. D, ELT3 cells were exposed to VEH or DES (100 nm) for 7 d. Treatment media were removed, and cells were washed with PBS and replaced with serum- and estrogen-free medium. After 48 additional hours, cells were rechallenged with DES (50 nm) or VEH (ethanol) for 24 h. Expression of the IGFBP5 gene was analyzed by qPCR, revealing that DES reprogrammed the basal expression level and hormone responsiveness of this gene relative to VEH-primed cells. Bar graphs display mean ± sem gene expression relative to VEH-treated cells from two to four independent experiments. Student’s t test was used to determine statistical significance (*, P ≤ 0.05). IB, Immunoblotting; IP, immunoprecipitation.
We previously identified, by microarray and quantitative real-time PCR (qPCR) analysis, genes that were developmentally reprogrammed in the adult rat myometrium by neonatal DES exposure (34,36). Within this list of reprogrammed genes were several potentially regulated by EZH2, including progesterone receptor (PR) and IGF-binding protein 5 (IGFBP5) (63,64,65,66). Initially, to confirm that PR and IGFBP5 are regulated by EZH2, we knocked down EZH2 with siRNA (Fig. 3A) and analyzed PR and IGFBP5 expression by qPCR. As expected, in the absence of EZH2, H3K27Me3 levels decreased, and both PR and IGFBP5 expression increased (Fig. 3, B and C), consistent with the repressive nature of the H3K27Me3 methyl mark (67). To determine whether activation of rapid ER signaling and modulation of EZH2 and H3K27Me3 by estrogens could modulate gene expression, ELT3 cells were starved for 48 h in serum- and estrogen-free media. ELT3 cells were either maintained in serum- and estrogen-free medium (VEH) or exposed to E2-BSA or DES for 7 d, which functioned as a priming period to promote EZH2 phosphorylation, deplete H3K27Me3, and induce chromatin remodeling (Supplemental Fig. 6). To determine whether EZH2 phosphorylation and depletion of H3K27Me3 had reprogrammed gene expression, qPCR was used to measure basal and estrogen (DES)-induced gene expression. In ELT3 cells cultured in serum- and estrogen-free medium (VEH primed), DES treatment significantly induced PR expression as early as 24 h with maximal expression being attained by 48 h (Fig. 4C, lanes 1 and 2, and data not shown) (68). Priming with E2-BSA and modulation of EZH2 activity via rapid ER signaling resulted in a significant (i.e. 3- to 4-fold), elevation of both basal and estrogen-induced PR expression in these cells (Fig. 4C). Similar to what was observed with E2-BSA, priming with DES also increased both basal and estrogen-induced PR expression relative to cells maintained under serum- and estrogen-free conditions (Fig. 4C). Like PR, expression of IGFBP5 was also reprogrammed in response to rapid ER signaling and inhibition of EZH2, resulting in a more than 15-fold increase in expression relative to serum- and estrogen-starved ELT3 cells (Fig. 4D). Thus, chromatin remodeling in response to rapid, membrane-activated ER signaling can modulate gene expression, revealing that similar to what was previously observed in vivo (34,35,36), the xenoestrogen DES can reprogram gene expression in uterine myometrial cells.
Xenoestrogens regulate EZH2 in vivo in the developing uterus
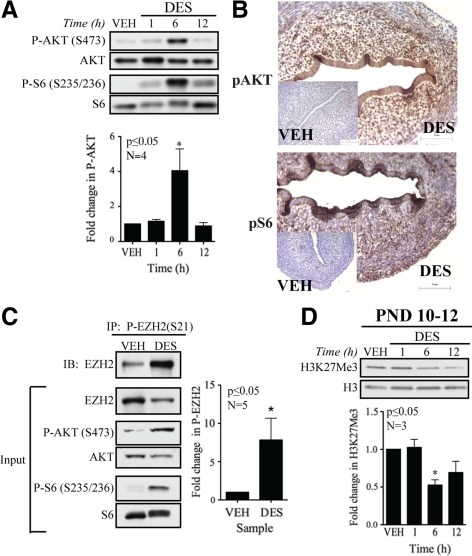
Additionally, to directly demonstrate the link between nongenomic ER signaling and modulation of EZH2 activity and H3K27Me3 levels in the developing uterus, we analyzed nongenomic signaling in neonatal Eker rats exposed to DES (1 mg/kg) on postnatal day (PND) 10–12, a period of uterine development that we previously identified as susceptible to developmental reprogramming (35). As shown in Fig. 5A, AKT and the downstream effector S6 were rapidly phosphorylated in response to DES, indicative of activation of nongenomic ER signaling in DES-exposed uteri. Immunohistochemical analysis of DES and VEH-treated uteri demonstrated increased PI3K/AKT activity as detected by phospho-AKT and phospho-S6 immunoreactivity, confirming nongenomic signaling in both the myometrial and endometrial compartments of DES-exposed uteri (Fig. 5B). Increased PI3K/AKT signaling was consistently seen in all neonates exposed to DES, with AKT phosphorylation generally peaking around 6 h, but observed as early as 1 h after DES injection (Fig. 5, A and B).
Figure 5.
Xenoestrogen-induced modulation of H3K27Me3 in vivo. A, Uteri were pooled from two to three Eker rats injected with VEH or DES. Western blot analysis of PI3K/AKT pathway demonstrated activation of nongenomic signaling in vivo by DES. B, Uteri were collected from Eker rats exposed to DES or VEH for 6 h and stained for P-AKT and P-S6, revealing activation of PI3K/AKT in DES-treated tissue (n = 43 animals; 24 VEH and 19 DES). Scale bar, 75 μm. C, Whole uteri were pooled from one to three Eker rats and homogenized after 6 h DES or VEH exposure. Lysates were immunoprecipitated with anti-P-EZH2 antibody. Immunoprecipitates were analyzed by Western blot for total EZH2, demonstrating enrichment of P-EZH2 in DES-treated tissue. Inputs show activation of nongenomic signaling in treated animals. D, Uteri were pooled from two to three DES- or VEH-treated rats. Histones were acid precipitated. Western blot analysis of histone proteins reveals decreased H3K27Me3 levels after DES (1, 6, and 12 h) exposure relative to VEH-treated animals in animals exposed on PND 10–12, a window of development susceptible to developmental reprogramming. Densitometry analyses of Western blot data show differences in levels of phosphorylation or methylation relative to VEH. The bars in each graph represent mean ± sem. Statistical significance was determined by Student’s t test (*, P ≤ 0.05). IB, Immunoblotting; IP, immunoprecipitation.
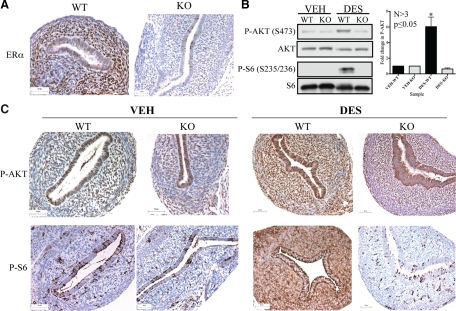
To demonstrate that activation of PI3K/AKT in the developing uterus was ER mediated, ERKO mice were examined for activation of nongenomic signaling by DES. As shown in Fig. 6A, as expected, uteri from ERKO mice lack immunoreactivity to ERα-specific antibodies, when compared with wild-type (WT) mice. DES exposure of ERKO mice on PND 5, a critical window of development for the mouse reproductive tract (33,69), failed to increase PI3K/AKT signaling above VEH controls. Whereas DES failed to activate nongenomic signaling in ERKO mice, it robustly activated this pathway in WT mice expressing ERα, with an increase in both AKT and S6 phosphorylation 6 h after DES exposure as detected by Western blot analysis (Fig. 6B). Similarly, immunohistochemical staining of DES-exposed and VEH control uteri revealed that DES activated nongenomic signaling in WT uteri, whereas ERKO mice remained unresponsive to hormone, demonstrating the dependence of nongenomic signaling on ERα in vivo (Fig. 6C).
Figure 6.
Activation of PI3K/AKT signaling in the uterus is ERα mediated. A, Histological analysis of sections collected from uteri of ERKO or WT mice demonstrate that ERKO animals do not express ERα. Scale bar, 50 μm. B, Western blot analysis of uterine lysates from ERKO mice vs. WT mice treated as described above demonstrates that activation of the PI3K/AKT pathway is ERα dependent in vivo. Each lane represents lysates from one to two pooled uteri from animals of matched genotype. C, Histological analysis of ERKO or WT uteri exposed to VEH or DES (6 h) demonstrates that activation of nongenomic signaling is dependent on ERα. Scale bar, 50 μm. The panels in this figure display representative Western blots. Densitometry analysis of Western blot data presented in bar graph represents mean ± sem. Statistical significance was determined by Student’s t test (*, P ≤ 0.05).
Importantly, we observed that activation of nongenomic signaling in the developing uterus induced EZH2 phosphorylation. As shown in Fig. 5C, EZH2 phosphorylation was induced in response to DES, increasing by greater than 5-fold relative to VEH-exposed uteri. Furthermore, increased EZH2 phosphorylation corresponded to a reduction in H3K27Me3 levels in chromatin, with these levels decreased by approximately 40% 6 h after exposure to DES, demonstrating that decreased H3K27Me3 methylation occurred in vivo in response to AKT activation and phosphorylation of EZH2 (Fig. 5D).
Discussion
We have identified a novel role for rapid ER signaling whereby PI3K/AKT signaling to EZH2 modulates the epigenome through phosphorylation of this HMT and reduction of H3K27Me3 methylation. Identification of ER-induced nongenomic PI3K/AKT signaling to EZH2 reveals a new pathway for nuclear hormone receptor-mediated regulation of chromatin structure. EZH2 is a Polycomb group protein that functions as a lysine methyltransferase for lysine 27 on histone H3 (H3K27) via its highly conserved SET [SU (VAR) 3–9, enhancer of Zeste, trithorax] domain. The methyltransferase activity of EZH2 requires it to complex with suppressor of Zeste 12 and embryonic ectoderm development proteins to form the Polycomb-repressive complex. Methylation at H3K27 is generally associated with transcriptional repression, including X chromosome inactivation, homeotic box (HOX) gene silencing, and maintenance of bivalent chromatin states during development (67,70). However, the function of EZH2 may be more diverse than originally thought as indicated by its role as a transcriptional activator in which H3K27 methylation is associated with some actively transcribed genes (71). Whether phosphorylation of EZH2 mediates an activated or repressed chromatin state in response to ER-mediated nongenomic signaling is not presently known. However, transcriptional profiling indicates that estrogens can both activate and repress gene expression (72,73), suggesting that the effects of modulating H3K27Me3 levels may be gene specific, functioning to activate as well as repress gene expression.
Le Romancer et al. (15) have recently shown that protein arginine methyltransferase 1 (PRMT1) can methylate the ER, and that this methylation is required for assembly of liganded ER with PI3K and subsequent AKT activation. Together, these findings generate a new paradigm for the role of rapid, membrane-activated signaling in regulation of histone modification, with rapid signaling from membrane-associated ER providing a pathway for cross talk between protein methyltransferases (PMTs) whereby PMTs can both prime nongenomic signaling by inducing ER association with PI3K (PRMT1) (15) and respond to activation of this pathway when phosphorylated (EZH2) (this report) to modulate histone methylation and the epigenome. It is intriguing that both genomic (ER nuclear transactivation) and nongenomic (rapid, membrane-activated ER-induced PI3K signaling) ER activities are primed by PRMT1 (15,74). Additionally, it is interesting that nongenomic signaling provides an interface between the activity of PMTs and protein kinases that integrates signaling events in the cytoplasm with regulation of chromatin structure in the nucleus. Taken together, these data demonstrate an unconventional role for rapid ER signaling that links the nucleus and the cytoplasm and provides a mechanism for cross talk between methyltransferases and kinases to regulate chromatin structure.
In the neonatal rodent, the uterus develops in an estrogen-independent manner, such that the developing tissue is protected from both residual maternal hormones and endogenous hormone production by high levels of steroid hormone-binding proteins such as α-feto protein. However, in contrast to endogenous estrogens such as E2, these proteins do not bind xenoestrogens such as DES (75). Thus, exposure to xenoestrogens during development can aberrantly engage ER signaling, giving rise to altered expression of hormone-responsive genes, disruption of tissue morphology and function, and increased risk of tumor development (30,32). We previously demonstrated that neonatal exposure to DES reprograms estrogen-responsive genes in the developing rat uterus, increasing uterine leiomyoma incidence and multiplicity in genetically susceptible animals (34,36). Because this brief exposure to DES can reprogram expression of estrogen-responsive genes in the adult uterus, we hypothesize that inappropriate xenoestrogen-induced ER signaling to HMTs, such as EZH2, may alter chromatin structure in the developing uterus to reprogram gene expression, inducing this developmental reprogramming. Because histone methyl marks such as H3K27 are heritable (76), it is possible that induction of these changes in histone methylation induced during key windows of development persist into adulthood and underlie the observed reprogramming of gene expression. This provides a mechanism whereby xenoestrogens can developmentally reprogram gene expression in the uterus and perhaps other tissues known to be susceptible to xenoestrogen-induced developmental reprogramming such as breast and prostate (77,78).
The exact sequence of molecular events that establish and maintain heritable epigenetic structures in chromatin is not fully characterized (79). Histone methylation appears to function as a precursor for the establishment of transcriptional and cellular memory (80). A number of studies indicate that histone methylation precedes DNA methylation. For example, methylation of histone H3 on lysine 9 (H3K9) by the HMT G9a generates a binding site for heteroprotein-1, which subsequently recruits DNMTs to silence gene expression. H3K9 methylation is a critical precursor to the establishment of heterochromatin as well as Xist-dependent inactivation of the X chromosome (39,40,41,42,43,81). Vire et al. (45) recently demonstrated that EZH2 directly interacts with DNMTs to facilitate DNA methylation and subsequent silencing of EZH2 target genes. Similarly, Leu et al. (64) revealed that the silencing of certain estrogen-responsive genes, including PR, in response to ablation of ER signaling by ERα knockdown, is initiated by changes in histone modifications that are established by EZH2 and histone deacetylase 1 and followed by DNA methylation via recruitment of DNMT1 and DNMT3b. However, Kondo et al. (82) observed that H3K27Me3 silences some tumor-suppressor gene promoters in the absence of DNA methylation, suggesting that enrichment of H3K27Me3 alone may be sufficient to silence expression of some genes. Conversely, several studies report that proteins that bind methylated cytosine, known as methyl-CpG-binding proteins, recruit HMTs to chromatin to facilitate the propagation of histone methylation during cell division, revealing that DNA methylation can direct histone methylation (83,84,85). Clearly, the mechanisms governing xenoestrogen-induced developmental programming are likely to be diverse and the subject of considerable future investigation.
Understanding whether alterations in histone methyl marks induced by xenoestrogen exposures persist over the lifetime of animals, and/or whether initial changes in histone methyl marks induce alterations in DNA methylation to reprogram estrogen-responsive gene expression in the adult uterus has yet to be explored. Furthermore, if chromatin remodeling plays a key role in the early life events that program estrogen-responsiveness in adulthood, it is possible that the cross talk between PI3K/AKT signaling and chromatin-modifying enzymes participates in this process during normal development, as well as in response to adverse environmental exposures. Future studies focused on epigenetic alterations induced by developmental reprogramming will lead to a better understanding of how this and other cell-signaling pathways activated by rapid nuclear hormone receptor signaling contribute to chromatin remodeling and developmental reprogramming.
Materials and Methods
Reagents and antibodies
Diethylstilbestrol (DES), 17β-estradiol (E2), and 17β-estradiol 6-(O-carboxymethyl) oxime: BSA, also called BSA-conjugated estradiol (E2-BSA), wortmannin, and LY294002 were purchased from Sigma Aldrich (St. Louis, MO). ICI 182780 was acquired from Tocris Bioscience (Ellisville, MO). Antibodies recognizing H3K27Me3 and EZH2 were obtained from Active Motif (Carlsbad, CA). Antibodies for phosphorylated AKT (S473), AKT, phosphorylated S6 kinase (T389), S6 kinase, phosphorylated S6 (S235/236), S6, and histone H3 were purchased from Cell Signaling Technology (Danvers, MA). An antibody recognizing phosphorylated EZH2 (S21) was obtained from Bethyl, Inc. (Montgomery, TX). Mouse EZH2 antibody was acquired from BD Biosciences (San Jose, CA).
Cell culture
MCF-7 cells were generously provided by A.M. Soto (Tufts University, Boston, MA). These cells were maintained with DMEM (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS) (86). MCF-7 cells stably transfected with CA-AKT (MCF-7 CA-AKT) were a gift from L. DeGraffenreid (University of Texas, San Antonio, TX) and were maintained as described elsewhere (57). MCF-7 cells acquired from the American Type Culture Collection (Manassas, VA) were used for comparison with CA-AKT MCF-7 cells and were cultured in improved MEM supplemented with 10% FBS (HyClone Laboratories, Logan, UT). The culture conditions for ELT3 cells were previously described (62,87).
Estrogen treatment conditions and use of cell-signaling inhibitors
MCF-7 cells were starved in serum-free, phenol red-free media for 36–48 h before estrogen treatment. After a 48-h starving period in a media free from serum (62,87), ELT3 cells were treated with estrogenic compounds. E2 and DES solutions were prepared in ethanol. To ensure that stock solutions of E2-BSA did not contain free estrogen, crystalline E2-BSA was dissolved in PBS and purified as previously described (55). To inhibit PI3K pathway, cells were pretreated with LY294002 (20 μm) or wortmannin (200 nm) for 30–45 min before estrogen administration. Cells were incubated with ICI 182780 (100 nm) for 24 h before estrogen treatment.
For ELT3 cells exposed for an extended period of time to E2-BSA or DES, cells were plated in 10-cm plates at a density of 300,000 cells per plate. After 24–48 h, complete medium was removed, and cells were washed twice with PBS and basal DF8, an estrogen and serum-free medium, was added to each plate. ELT3 cells were depleted of estrogen and serum for 48 h and separated into four groups, VEH, E2-BSA (50 nm and 100 nm), or DES (100 nm). Media were changed daily along with E2-BSA or DES treatment for a 7-d period of time. To prevent contamination of E2-BSA by free estrogen, E2-BSA stock solution, which had been aliquoted and stored at −20 C, was diluted daily with sterile PBS and purified using a centrifugal filter unit (Millipore Corp., Billerica, MA) (55). The purified stock solution of E2-BSA was diluted to a final concentration of 50 μm and used as a 1000× or 500× stock to treat cell cultures. After a 7-d exposure, E2-BSA- or DES-enriched media were removed, after which cells were washed twice with PBS to remove hormone, and media were replaced with basal DF8 for 48 h. ELT3 cells were then treated for 24 h with 50 nm DES or vehicle (ethanol), after which RNA was collected.
Preparation of cell lysates for Western blot analysis
Cells were lysed via homogenization with an insulin syringe in Cell Signaling Technology (CST) lysis buffer [20 mm Tris-HCl, pH 7.5; 1 mm EGTA; 1 mm Na2EDTA; 150 mm NaCl; 1 mm β-glycerophosphate; 2.5 mm sodium pyrophosphate; 1% Triton; 1 mm Na3VO4; 1 mm phenylmethylsulfonylfluoride; 1 mm NaF; and Complete Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN)]. Lysates were then centrifuged at 13,000 rpm for 10 min at 4 C, and supernatant was collected. Protein concentration of cell lysates was determined by Pierce bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL). Whole-cell lysate was separated by SDS-PAGE (Bio-Rad Laboratories, Inc., Hercules, CA) and transferred to polyvinylidene difluoride membranes. After incubation with primary antibody, membranes were washed with Tris-buffered saline with Tween 20 and exposed to an appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins or posttranslational modifications of interest were visualized using Pierce enhanced chemiluminescence substrate (Pierce Biotechnology) or ECL plus (GE Healthcare Bio-Sciences Corp., Piscataway, NJ).
Preparation of cell lysates for immunoprecipitation
Cell lysates were prepared as described above. Protein concentration of lysates was determined, and equal amounts of lysates were precleared for 1–2 h using rabbit IgG (Millipore Corp.) and protein A sepharose beads (GE Healthcare Bio-Sciences Corp.). After the preclearing step, cell lysates were transferred to an Eppendorf tube with protein A sepharose beads and anti-phospho-EZH2 antibody. After immunoprecipitation, beads were washed five times with CST buffer. Immunoprecipitates were separated by SDS-PAGE and analyzed by Western blot as described above.
Acid precipitation of histone proteins from human cell lines
For histone extraction from MCF-7 cells, monolayers were collected in ice-cold PBS and centrifuged at 5000 rpm for 5 min at 4 C. Cells were then resuspended in RSB buffer (10 mm Tris-HCl, pH 7–7.4; 10 mm NaCl; 3 mm MgCl2; 1 mm phenylmethylsulfonylfluoride; 1 mm Na3VO4; 1 mm NaF; and Complete Protease Inhibitor Cocktail) with 0.5% Nonidet P-40 and homogenized using a Dounce homogenizer and incubated on ice for 10 min. Cell lysates were then centrifuged for 5 min at 10,000 rpm at 4 C. Supernatant was collected and used for Western blot analysis of cytosolic, nongenomic signaling. The pellet from this centrifugation was resuspended in equal volumes (700 μl) of 5 mm MgCl2 and 0.8 m HCl, sonicated at 30% power for 20 sec, and incubated on ice for 1 h. The solution of histone proteins was centrifuged at 14,000 rpm for 10 min at 4 C. Supernatant was transferred to a new tube, and histones were precipitated with a 50% solution of trichloroacetic acid dissolved in deionized water. Precipitated histones were collected via centrifugation for 20 min at 14,000 rpm at 4 C. The resultant pellet was washed with ice-cold acetone, dried, and subsequently resuspended with deionized water (150 μl) and 1.5 m Tris-HCl, pH 8.8 (2 μl). Histone proteins (5–10 μl) were resolved by SDS-PAGE on a 10–20% Tris-tricine gel (Bio-Rad Laboratories), and Coomassie stain was used to correct for variations in histone H3 loading. Then, changes in histone methylation relative to total histone H3 were investigated by Western blot analysis as described above.
Use of siRNA for EZH2 knockdown in human cell lines
ON-TARGET plus siRNA SMARTpool for EZH2 (L-0042198-00-0005) was purchased from Dharmacon (Denver, CO). The siRNA, siCONTROL [RISC-free 1 (D-01220-01)], was used as a negative control. The oligos were resuspended in 1× siRNA buffer to a concentration of 20 nm. MCF-7 cells were plated in six-well plates and allowed to attach and proliferate for 48 h before transfection. siRNA stock solution was diluted to a final concentration of 10 nm in 1× siRNA buffer (1 μl of siRNA stock solution in 99 μl of 1× siRNA buffer). DharmaFECT1 (Dharmacon) was used as a transfection reagent and was diluted by 50-fold in OptiMEM media (Invitrogen). Transfection reagent mixture (200 μl) and diluted siRNA (100 μl) were combined and incubated for 30 min at room temperature. Maintenance media, IMEM (Invitrogen) supplemented with 10% FBS (Hyclone) was removed from cells, which were then washed twice with sterile PBS, and 2 ml of fresh, serum-free media were added to each well. In addition, siRNA in transfection reagent mixture (300 μl) was added to each well. Whole-cell lysates and RNA were collected from cells 48 h after transfection.
RNA isolation and real-time PCR analysis of gene expression in human and rodent cell lines
Media were aspirated from plates and cells were washed twice with ice-cold PBS. RNA was isolated using the RNeasy Mini kit (QIAGEN, Valencia, CA). cDNA was generated using the SuperScriptIII Reverse Transcriptase kit (Invitrogen) and analyzed by qPCR using the 7900T Fast Real-Time detection system from Applied Biosystems (ABI, Foster City, CA). qPCR analysis was conducted using Fast Real-Time Taq Man assays for pS2 (Hs00170216_m1), PR (RN00575662_m1 or Hs0015567_m1), and IGFBP5 (RN0056537_m1 or Hs00181213_m1). All qPCRs were performed in triplicate using the Universal Fast Real-Time Master Mix from ABI along with the gene-specific assay mix using 2 μl of sample cDNA (25 μl reaction). Glyceraldehyde-3-phosphate dehydrogenase was used as a reference gene for each reaction. Conditions for Fast qPCR were 95 C for 10 min, 40 cycles for 1 sec at 95 C and 20 sec at 60 C. qPCRs were quantified by the ΔΔCt method. The average Ct of the reference gene was subtracted from the target gene(s) to obtain an average ΔCt. The calibrator for the qPCR experiments was a VEH sample, in the case of treated cells, or control siRNA, in the case of EZH2 knockdown studies. The calibrator sample was used to subtract all other sample ΔCt values to obtain the ΔΔCt values. Fold change was calculated for each sample in comparison with the calibrator using 2−ΔΔCt. To control for efficiency within the reaction each experiment contained a standard curve from which the slope and efficiency of the reaction was calculated.
Animal care and treatment
Eker rats and αERKO mice were maintained in a colony at M.D. Anderson Cancer Center in accordance with guidelines established by the Association for the Accreditation of Laboratory Animal Care. Additionally, all protocols utilizing these rodents were reviewed and accepted by the M.D. Anderson Animal Care and Use Committee. To investigate the activation of nongenomic signaling and subsequent downstream effects in vivo, neonatal female Eker rats were injected with 1 mg/kg DES, a dose that we previously established to be estrogenic (34,35,36), dissolved in sesame oil (Sigma Aldrich). Animals were euthanized 1, 6, or 12 h after injection, and uterine tissue was collected and immediately placed on ice for homogenization in lysis buffer or fixed in 10% neutral buffered formalin. Tissues fixed with neutral buffered formalin and 70% ethanol were paraffin embedded, sectioned, and subjected to immunohistochemical staining. αERKO +/− breeders were mated to generate WT and KO pups for DES or VEH injections at a dose of 1 mg/kg. Injections were given subcutaneously on PND 5 using an insulin syringe. After exposure, mice were euthanized and uteri were harvested and either frozen in liquid N2 or fixed in 10% neutral buffered formalin (24 h) for immunohistochemistry.
Genotyping
Genomic DNA from αERKO mice was genotyped by PCR using primers designed to amplify a WT band at 281 bp and a KO band at 760 bp. The following reaction protocol was used for amplification of 200 ng of genomic DNA in a 25-μl vol: 12.5 μl GoTaq master mix (Promega, Madison, WI), 0.5 μl NeoF primer (10 μm) (GCTGACCGCTTCCTCGTGCTTTAC), and 0.5 μl of ER primer (10 μm) (CGGTCTACGGCCAGTCGGGCAT) and 1 μl mER primer (10 μm) (CAGGCCTTACACAGC GGCCACCC) at 95 C for 1 min 30 sec (one cycle), 95 C for 45 sec, 62 C for 45 sec, and 72 C for 1 min 30 sec (35 cycles), followed by 4 C for 10 min (one cycle) (88).
Tissue processing for Western blot analysis or immunoprecipitation
Uterine tissue from VEH- or DES-treated rats was homogenized in ice-cold RSB buffer that was supplemented with protease inhibitors. The cytosol from this preparation was used to assess activation of nongenomic signaling, whereas the nuclei from this preparation were used as a source of histone proteins in the preparation described above. To investigate DES-induced activation of nongenomic signaling, tissue lysate were analyzed by Western blot as described above. For immunoprecipitation of P-EZH2, whole uteri were lysed in CST buffer enriched with protease inhibitors by Dounce homogenization. Equal amounts of uterine lysate were precleared with beads and IgG and subsequently incubated with precipitation antibody overnight at 4 C. Immunoprecipitates were washed five times with lysis buffer and subjected to Western blot and visualized as described above.
Statistical analysis
Student’s t test or one-way ANOVA (Graph Pad Prism, Graphpad Software, Inc., La Jolla, CA) was used to determine whether differences among groups of data were statistically significant. Statistical significance was granted to P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank members of the Walker laboratory, particularly Dr. Jaeho Lee, Tia Berry, Sean Hensley, and Amanda Martin for technical assistance with this work. We thank Aimee Iberg and Dr. Donghang Cheng at the Science Park Research Division (University of Texas, M.D. Anderson Cancer Center, Smithville, TX) for their valuable input regarding these studies. In addition, we thank the Histology Core, Molecular Biology Core, and Animal Care Facilities at the Science Park Research Division (University of Texas, M.D. Anderson Cancer Center, Smithville, TX) for assistance with preparation of tissues for histological evaluation, immunohistochemistry, nucleic acid isolation, genotyping, and animal care.
Footnotes
This work was supported by grants from the National Institute of Environmental Health Sciences (ES008263 and P30-ES007784) and National Cancer Institute (P30-CA016672). T.B. is supported by a grant from the National Institute of Environmental Health Sciences (F32-ES016509).
Disclosure Summary: T.B., L.G., S.S., M.C.H., M.B., and C.W. have no conflicts of interest that they wish to declare in relation to the submitted work.
First Published Online March 29, 2010
Abbreviations: AKT, Protein kinase B; CA-AKT, constitutive AKT activity; CST, Cell Signaling Technology; DES, diethylstilbestrol; DNMT, DNA methyltransferase; ER, estrogen receptor; E2, 17β-estradiol; E2-BSA, BSA-conjugated estradiol; ERKO, ER knockout; EZH2, enhancer of Zeste homolog 2; FBS, fetal bovine serum; H3K27Me3, trimethylation of lysine 27 on histone H3; H3K9, histone H3 on lysine 9; HMT, histone methyltransferase; IGFBP5, IGF-binding protein 5; IMEM, improved MEM; PI3K, phosphatidylinositol 3-kinase; PMT, protein methyltransferase; PND, postnatal day; PR, progesterone receptor; PRMT1, protein arginine methyltransferase 1; qPCR, quantitative real-time PCR; siRNA, small interfering RNA; VEH, vehicle; WT, wild type.
References
- Acconcia F, Kumar R 2006 Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett 238:1–14 [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC 2002 Production and actions of estrogens. N Engl J Med 346:340–352 [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Korach KS 2006 Estrogen receptors and human disease. J Clin Invest 116:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel SC, vom Saal FS, Welshons WV 1999 Developmental effects of estrogenic chemicals are predicted by an in vitro assay incorporating modification of cell uptake by serum. J Steroid Biochem Mol Biol 69:343–357 [DOI] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM 1993 Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect 101:378–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Galluzzo P, Ascenzi P 2006 Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics 7:497–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnström L, Sjöberg M 2005 Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19:833–842 [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS 2001 The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276: 36869–36872 [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP 2005 Coregulators in nuclear estrogen receptor action: from concept to therapeutic targeting. Mol Interv 5:343–357 [DOI] [PubMed] [Google Scholar]
- Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA 2001 Mechanisms of estrogen action. Physiol Rev 81:1535–1565 [DOI] [PubMed] [Google Scholar]
- Safe S, Kim K 2008 Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways. J Mol Endocrinol 41:263–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER 2002 Cellular functions of plasma membrane estrogen receptors. Steroids 67:471–475 [DOI] [PubMed] [Google Scholar]
- Levin ER 2001 Cell localization, physiology, and nongenomic actions of estrogen receptors. J Appl Physiol 91:1860–1867 [DOI] [PubMed] [Google Scholar]
- Pietras RJ, Levin ER, Szego CM 2005 Estrogen receptors and cell signaling. Science 310:51–53 (author reply) [DOI] [PubMed] [Google Scholar]
- Le Romancer M, Treilleux I, Leconte N, Robin-Lespinasse Y, Sentis S, Bouchekioua-Bouzaghou K, Goddard S, Gobert-Gosse S, Corbo L 2008 Regulation of estrogen rapid signaling through arginine methylation by PRMT1. Mol Cell 31:212–221 [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK 2000 Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407:538–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang S, Safe S 2006 Activation of kinase pathways in MCF-7 cells by 17β-estradiol and structurally diverse estrogenic compounds. J Steroid Biochem Mol Biol 98:122–132 [DOI] [PubMed] [Google Scholar]
- Chen JR, Plotkin LI, Aguirre JI, Han L, Jilka RL, Kousteni S, Bellido T, Manolagas SC 2005 Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J Biol Chem 280:4632–4638 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC 2002 Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298:843–846 [DOI] [PubMed] [Google Scholar]
- Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T 2005 Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J Biol Chem 280:7317–7325 [DOI] [PubMed] [Google Scholar]
- Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW 1999 Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest 103:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul PW 1999 Rapid activation of endothelial nitric oxide synthase by estrogen. Steroids 64:28–34 [DOI] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD 2005 17β-Estradiol-induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience 135:59–72 [DOI] [PubMed] [Google Scholar]
- Numakawa Y, Matsumoto T, Yokomaku D, Taguchi T, Niki E, Hatanaka H, Kunugi H, Numakawa T 2007 17β-Estradiol protects cortical neurons against oxidative stress-induced cell death through reduction in the activity of mitogen-activated protein kinase and in the accumulation of intracellular calcium. Endocrinology 148:627–637 [DOI] [PubMed] [Google Scholar]
- Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW 2008 Rapid estrogen signaling in the brain. Neurosignals 16:140–153 [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL 2007 Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE 2007 Estrogen response element-independent estrogen receptor (ER)-α signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERα knockout mice. Endocrinology 148:5288–5294 [DOI] [PubMed] [Google Scholar]
- Korach KS 1994 Insights from the study of animals lacking functional estrogen receptor. Science 266:1524–1527 [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS 2003 Oestrogen receptor knockout mice: roles for oestrogen receptors α and β in reproductive tissues. Reproduction 125:143–149 [DOI] [PubMed] [Google Scholar]
- Newbold RR, Moore AB, Dixon D 2002 Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES). Toxicol Pathol 30:611–616 [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Banks E, Haseman J 2004 Developmental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: low versus high dose effects. Reprod Toxicol 18:399–406 [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Grissom SF, Padilla-Banks E, Snyder RJ, Lobenhofer EK 2007 Developmental exposure to diethylstilbestrol alters uterine gene expression that may be associated with uterine neoplasia later in life. Mol Carcinog 46:783–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Hansman R, Newbold R, Davis B, McLachlan JA, Barrett JC 2003 Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol Carcinog 38:78–84 [DOI] [PubMed] [Google Scholar]
- Greathouse KL, Cook JD, Lin K, Davis BJ, Berry TD, Bredfeldt TG, Walker CL 2008 Identification of uterine leiomyoma genes developmentally reprogrammed by neonatal exposure to diethylstilbestrol. Reprod Sci 15:765–778 [DOI] [PubMed] [Google Scholar]
- Cook JD, Davis BJ, Goewey JA, Berry TD, Walker CL 2007 Identification of a sensitive period for developmental programming that increases risk for uterine leiomyoma in Eker rats. Reprod Sci 14:121–136 [DOI] [PubMed] [Google Scholar]
- Cook JD, Davis BJ, Cai SL, Barrett JC, Conti CJ, Walker CL 2005 Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. Proc Natl Acad Sci USA 102:8644–8649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen GH, Metzler M 1987 Sex hormones and neoplasia: genotoxic effects in short term assays. Arch Toxicol Suppl 10:264–278 [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Burow M, Chiang TC, Li SF 2001 Gene imprinting in developmental toxicology: a possible interface between physiology and pathology. Toxicol Lett 120:161–164 [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T 2001 Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410:120–124 [DOI] [PubMed] [Google Scholar]
- Jackson JP, Lindroth AM, Cao X, Jacobsen SE 2002 Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416:556–560 [DOI] [PubMed] [Google Scholar]
- Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T 2001 Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410:116–120 [DOI] [PubMed] [Google Scholar]
- Smallwood A, Estève PO, Pradhan S, Carey M 2007 Functional cooperation between HP1 and DNMT1 mediates gene silencing. Genes Dev 21:1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaru H, Selker EU 2001 A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414:277–283 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Rank G, Tan YT, Li H, Moritz RL, Simpson RJ, Cerruti L, Curtis DJ, Patel DJ, Allis CD, Cunningham JM, Jane SM 2009 PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol 16:304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F 2006 The Polycomb group protein EZH2 directly controls DNA methylation. Nature 439:871–874 [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Schneider R, Kouzarides T 2002 Histone methylation: dynamic or static? Cell 109:801–806 [DOI] [PubMed] [Google Scholar]
- Lachner M, O'Sullivan RJ, Jenuwein T 2003 An epigenetic road map for histone lysine methylation. J Cell Sci 116:2117–2124 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Reinberg D 2001 Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev 15:2343–2360 [DOI] [PubMed] [Google Scholar]
- Jenuwein T 2006 The epigenetic magic of histone lysine methylation. FEBS J 273:3121–3135 [DOI] [PubMed] [Google Scholar]
- Berger SL 2007 The complex language of chromatin regulation during transcription. Nature 447:407–412 [DOI] [PubMed] [Google Scholar]
- Lee DY, Teyssier C, Strahl BD, Stallcup MR 2005 Role of protein methylation in regulation of transcription. Endocr Rev 26:147–170 [DOI] [PubMed] [Google Scholar]
- Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, Ping B, Otte AP, Hung MC 2005 Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science 310:306–310 [DOI] [PubMed] [Google Scholar]
- Higashimoto K, Kuhn P, Desai D, Cheng X, Xu W 2007 Phosphorylation-mediated inactivation of coactivator-associated arginine methyltransferase 1. Proc Natl Acad Sci USA 104:12318–12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S 2007 A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-γ transactivation. Nat Cell Biol 9:1273–1285 [DOI] [PubMed] [Google Scholar]
- Stevis PE, Deecher DC, Suhadolnik L, Mallis LM, Frail DE 1999 Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology 140:5455–5458 [DOI] [PubMed] [Google Scholar]
- Hermenegildo C, Cano A 2000 Pure anti-oestrogens. Hum Reprod Update 6:237–243 [DOI] [PubMed] [Google Scholar]
- DeGraffenried LA, Friedrichs WE, Fulcher L, Fernandes G, Silva JM, Peralba JM, Hidalgo M 2003 Eicosapentaenoic acid restores tamoxifen sensitivity in breast cancer cells with high Akt activity. Ann Oncol 14:1051–1056 [DOI] [PubMed] [Google Scholar]
- Kohn AD, Takeuchi F, Roth RA 1996 Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem 271:21920–21926 [DOI] [PubMed] [Google Scholar]
- Martin C, Zhang Y 2005 The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 6:838–849 [DOI] [PubMed] [Google Scholar]
- Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, Dynlacht BD, Reinberg D 2008 Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell 32:503–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Liu Y, Hsu YJ, Fujiwara Y, Kim J, Mao X, Yuan GC, Orkin SH 2008 EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell 32:491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SR, Everitt JI, Gottardis MM, Walker C 1995 Estrogen/antiestrogen responsiveness in an in vivo/in vitro model for myometrial tumorigenesis. Ann NY Acad Sci 761:373–375 [DOI] [PubMed] [Google Scholar]
- Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K 2006 Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev 20:1123–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu YW, Yan PS, Fan M, Jin VX, Liu JC, Curran EM, Welshons WV, Wei SH, Davuluri RV, Plass C, Nephew KP, Huang TH 2004 Loss of estrogen receptor signaling triggers epigenetic silencing of downstream targets in breast cancer. Cancer Res 64:8184–8192 [DOI] [PubMed] [Google Scholar]
- Kondo Y, Shen L, Suzuki S, Kurokawa T, Masuko K, Tanaka Y, Kato H, Mizuno Y, Yokoe M, Sugauchi F, Hirashima N, Orito E, Osada H, Ueda R, Guo Y, Chen X, Issa JP, Sekido Y 2007 Alterations of DNA methylation and histone modifications contribute to gene silencing in hepatocellular carcinomas. Hepatol Res 37:974–983 [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall E, O'Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE 2007 Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448:553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Zhang Y 2004 The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev 14:155–164 [DOI] [PubMed] [Google Scholar]
- Hodges LC, Houston KD, Hunter DS, Fuchs-Young R, Zhang Z, Wineker RC, Walker CL 2002 Transdominant suppression of estrogen receptor signaling by progesterone receptor ligands in uterine leiomyoma cells. Mol Cell Endocrinol 196:11–20 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 2004 Estrogen receptor-α mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract. Toxicology 205:55–63 [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES 2006 A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125:315–326 [DOI] [PubMed] [Google Scholar]
- Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K 2003 EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 22:5323–5335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Yoshida N, Omoto Y, Oguchi S, Yamori T, Kiyama R, Hayashi S 2002 Development of cDNA microarray for expression profiling of estrogen-responsive genes. J Mol Endocrinol 29:175–192 [DOI] [PubMed] [Google Scholar]
- Soulez M, Parker MG 2001 Identification of novel oestrogen receptor target genes in human ZR75–1 breast cancer cells by expression profiling. J Mol Endocrinol 27:259–274 [DOI] [PubMed] [Google Scholar]
- Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F 2003 Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751–763 [DOI] [PubMed] [Google Scholar]
- Sheehan DM, Young M 1979 Diethylstilbestrol and estradiol binding to serum albumin and pregnancy plasma of rat and human. Endocrinology 104:1442–1446 [DOI] [PubMed] [Google Scholar]
- Hansen KH, Bracken AP, Pasini D, Dietrich N, Gehani SS, Monrad A, Rappsilber J, Lerdrup M, Helin K 2008 A model for transmission of the H3K27me3 epigenetic mark. Nat Cell Biol 10:1291–1300 [DOI] [PubMed] [Google Scholar]
- Prins GS, Huang L, Birch L, Pu Y 2006 The role of estrogens in normal and abnormal development of the prostate gland. Ann NY Acad Sci 1089:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto AM, Maffini MV, Sonnenschein C 2008 Neoplasia as development gone awry: the role of endocrine disruptors. Int J Androl 31:288–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Zhang Y 2007 Mechanisms of epigenetic inheritance. Curr Opin Cell Biol 19:266–272 [DOI] [PubMed] [Google Scholar]
- Ringrose L, Paro R 2004 Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet 38:413–443 [DOI] [PubMed] [Google Scholar]
- Heard E, Rougeulle C, Arnaud D, Avner P, Allis CD, Spector DL 2001 Methylation of histone H3 at Lys-9 is an early mark on the X chromosome during X inactivation. Cell 107:727–738 [DOI] [PubMed] [Google Scholar]
- Kondo Y, Shen L, Cheng AS, Ahmed S, Boumber Y, Charo C, Yamochi T, Urano T, Furukawa K, Kwabi-Addo B, Gold DL, Sekido Y, Huang TH, Issa JP 2008 Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat Genet 40:741–750 [DOI] [PubMed] [Google Scholar]
- Sarraf SA, Stancheva I 2004 Methyl-CpG binding protein MBD1 couples histone H3 methylation at lysine 9 by SETDB1 to DNA replication and chromatin assembly. Mol Cell 15:595–605 [DOI] [PubMed] [Google Scholar]
- Fuks F, Hurd PJ, Wolf D, Nan X, Bird AP, Kouzarides T 2003 The methyl-CpG-binding protein MeCP2 links DNA methylation to histone methylation. J Biol Chem 278:4035–4040 [DOI] [PubMed] [Google Scholar]
- Estève PO, Chin HG, Smallwood A, Feehery GR, Gangisetty O, Karpf AR, Carey MF, Pradhan S 2006 Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication. Genes Dev 20:3089–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO 1995 The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect 103(Suppl 7):113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SR, Gottardis MM, Everitt JI, Walker C 1995 Estrogen stimulation and tamoxifen inhibition of leiomyoma cell growth in vitro and in vivo. Endocrinology 136:4996–5003 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.