Abstract
In selected tissues and cell lines, 17β-estradiol (E2) regulates the expression of estrogen-related receptor α (ERRα), a member of the orphan nuclear receptor family. This effect is thought to be mediated by the estrogen receptor α (ERα). However in the ERα- and ERβ-negative SKBR3 breast cancer cell line, physiological levels of E2 also stimulate ERRα expression. Here, we explored the molecular mechanism that mediates estrogen action in ER-negative breast cancer cells. We observed that E2, the ERα agonist, as well as the ERα antagonists ICI 182,780 and tamoxifen (TAM), a selective ER modulator, stimulate the transcriptional activity of the ERRα gene and increase the production of ERRα protein in SKBR3 cells. Moreover, the ERRα downstream target genes expression and cellular proliferation are also increased. We show further that the G protein-coupled receptor GPR30/GPER-1 (GPER-1) mediates these effects. The GPER-1 specific ligand G-1 mimics the actions of E2, ICI 182,780, and TAM on ERRα expression, and changing the levels of GPER-1 mRNA by overexpression or small interfering RNA knockdown affected the expression of ERRα accordingly. Utilizing inhibitors, we delineate a different downstream pathway for ER agonist and ER antagonist-triggered signaling through GPER-1. We also find differential histone acetylation and transcription factor recruitment at distinct nucleosomes of the ERRα promoter, depending on whether the cells are activated with E2 or with ER antagonists. These findings provide insight into the molecular mechanisms of GPER-1/ERRα-mediated signaling and may be relevant to what happens in breast cancer cells escaping inhibitory control by TAM.
GPER-1 mediates the actions of 17beta-estradiol, G-1, ICI 182 780, and tamoxifen, transactivates ERRα expression through both common and different signaling pathways in the ER-null SKBR3 cells.
Estrogen is required for normal development and function of various physiological systems. However, it has also been implicated in a range of pathological conditions in mammals (see Refs. 1 and 2 and references therein). Therefore, understanding estrogen signaling pathways is essential for drug development and treatment of estrogen-related diseases. Classically, estrogen action is mediated by two genetically distinct nuclear estrogen receptors (ERs), ERα and ERβ (3), that interact either directly or indirectly in a ligand-dependent manner with estrogen response elements in the regulatory sequences of estrogen target genes (4,5,6,7). By activating or repressing its target genes, this molecular mechanism of estrogen action leads to a long-term genomic effect. Ligand-dependent ER action also elicits rapid nongenomic effects such as the generation of second messengers and activation of the MAPK system, which is traditionally considered to be mediated by receptors with tyrosine kinase activity and by G protein-coupled receptors (GPCRs) (see review in Refs. 8,9,10 and references therein). Recently, an orphan GPCR, GPR30 (rename by Receptor Nomenclature Committee of the International Union of Pharmacologists as GPER-1) was identified as a new member of the ER family which binds both ER agonists and antagonists (11,12,13,14), as well as a specific ligand G-1 (15). In contrast to the majority of GPCRs that reside in the plasma membrane (16), GPER-1 is located in the endoplasmic reticulum membrane (13), and mediates estrogen- and phytoestrogen-dependent activation of c-fos gene expression in breast cancer cells (17).
The estrogen-related receptors (ERRs) α, β, and γ are orphan nuclear receptors of the NR3B subfamily of the nuclear receptor superfamily (18). The ERRs share a high degree of sequence identity to ERs but do not bind estrogens or any other known natural ligand (19). ERRα is ubiquitous, expressed in all tissues examined, and is involved in many physiological processes (see review in Ref. 20 and references therein). It is highly expressed in metabolically active tissues, including heart, kidney, liver, and skeletal muscle, and regulates genes that participate in mitochondrial biogenesis and oxidative metabolism, thus suggesting the involvement of ERRα in an energy homeostasis program. In agreement with this view, ERRα has recently been demonstrated to be a key target of peroxisome proliferator-activated receptor γ coactivator-1α (20,21,22), a critical regulator that controls the network of energy balance program (23,24). As a constitutive activator (25,26), the functional activity of ERRα may be controlled by its expression level. The known regulators for ERRα expression are peroxisome proliferator-activated receptor γ coactivator-1α (20,21), estrogen (7,27,28), and cAMP (29). Deregulation of ERRα expression could be linked to various pathological conditions involved in energy imbalance and leads to cancer, osteoporosis, and metabolic disorders.
Due to the close structural similarity of ERs and ERRs, the functional relationship between these two groups of receptors was explored. ERRα binds a variety of estrogen response elements and its own unique response element (30,31,32) in the absence of a known ligand and recruits coregulators similar to those recruited by the ERs, thereby mimicking ER-mediated gene expression (26,33,34,35). Furthermore, we have previously demonstrated that the ERRα gene, ESRRA, is an ER-dependent estrogen target in certain estrogen responsive tissues and cell lines (7,27,28). Recent clinical studies implicated ERRα as a negative diagnostic marker for breast cancer progression (36,37,38). Its expression is significantly increased in ERα-negative tumors and correlates with the expression of ErbB2, a known marker of aggressive tumors (39). ERRα may promote the growth of breast cancers by stimulating the expression of the key enzymes, CYP19A1 (P450 aromatase) (40) and dehydroepiandrosterone sulfotransferase (41), in estrogen biosynthesis pathways, thus increasing the local production of estrogen. To understand the transcriptional activation of ERRα by estrogen, we mapped the nucleosomal positions in an area of the ERRα gene that is known to harbor a multihormone response element (MHRE) (27). We studied the effect of 17β-estradiol (E2) on the local chromatin architecture, as well as changes in histone acetylation levels. The recruitment of coregulators and RNA polymerase II around the MHRE region in both ER-positive (ER+) and ER-negative (ER−) breast cancer cells was also investigated (7). Surprisingly, a similar chromatin modification was found in both ER+ MCF7 and ER− SKBR3 breast cancer cells after E2 treatment. Although we have investigated the molecular mechanism of E2 action on the ERRα gene in the ER+ MCF7 cells, the transducer for E2 in ER− SKBR3 cells was not identified. The present study investigates the potential transducer that mediates E2 action in the SKBR3 cells, and we found GPER-1, the newly discovered and somewhat controversial membrane ER (see review Refs. 42,43,44 and references therein), to be a viable candidate. The ER antagonist, ICI 182,780 (ICI), and the selective ER modulator (SERM), tamoxifen (TAM), also stimulate ERRα expression through this receptor. Interestingly, the downstream signaling pathways of GPER-1 induced by ER agonist in comparison with those induced by the ER antagonist and the SERM TAM are dissimilar, which leads to differential histone acetylation and transcription factor recruitment to distinct targeted nucleosomes. This study provides insight into the mechanism by which ERRα is overexpressed in aggressive breast cancers and into the potential development of therapeutic intervention for breast cancer progression.
Results
E2, G-1, ICI, and TAM induce ERRα and its downstream target genes mRNA expressions and enhance SKBR3 cell proliferation
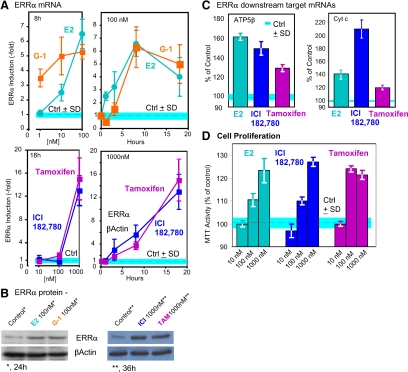
We have previously shown that E2 stimulates ERRα gene expression in the absence of classical ERs (ERα and ERβ) in SKBR3 cells (7). Recent emerging evidences demonstrated that a GPCR GPER-1 abundant in the SKBR3 cells could be a potential transducer of estrogen in SKBR3 cells that activates ERRα gene expression because it binds the ER agonist E2 and the ER antagonists, ICI and the selective ER modulator TAM, with high affinity (14). In addition, a recently identified specific ligand for GPER-1, G-1, which binds the receptor at nanomolar level (15) was included as the positive control in the study. We used real-time RT-PCR and Western blotting to determine the response of ERRα expression to E2, G-1, TAM. and ICI. The top panels of Fig. 1A illustrate the dependence on time and ligand concentration of ERRα mRNA induction by the ER agonist E2 and the GPER-1 agonist G-1 in SKBR3 cells. Surprisingly (Fig. 1A, bottom panels), the ERα antagonist ICI and the SERM TAM also induced ERRα mRNA. Synthesis of ERRα mRNA was accompanied by increases in ERRα protein as seen in Western blottings with ERRα-specific antibody (Fig. 1B) (28). Whereas all four ligands increased both mRNA and ERRα protein in SKBR3 cells, the kinetics of the responses differed between E2 and G-1 on one hand, and ICI and TAM, on the other hand, raising the possibility that the mechanisms by which the two types of ligands acted were similar but not identical. In addition to the ERRα induction, downstream target genes of ERRα such as ATP5β and cytochrome c (Fig. 1C) (22) and the cellular proliferation (Fig. 1D) were also induced.
Figure 1.
E2, G-1, ICI, and TAM induce the expression of ERRα in ER negative SKBR3 breasts cancer cells. A, Dependence of mRNA induction on compound concentration and time of incubation. The experiments were repeated three times, and results are presented as fold induction ± sd. B, mRNA induction correlates with protein accumulation. C, E2, ICI, and TAM induce the expression of ATP5β and Cytc. D, E2, ICI, and TAM induce SKBR3 cell proliferation. The experiments were repeated three times and results are presented as induction ± sd. *, P < 0.05.
E2, G-1, ICI, and TAM stimulate ERRα expression via GPER-1
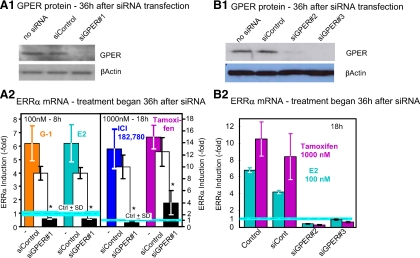
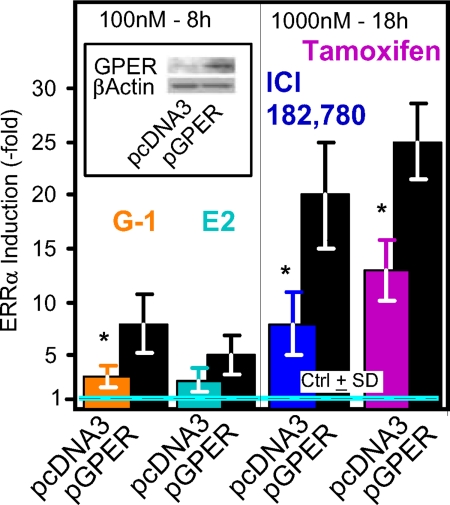
Using 8-h incubations with 100 nm to test for effects of E2 and G-1 and 18-h incubations and 1000 nm concentrations to test for effects of ICI and TAM, we next sought further evidence for participation of GPER-1 in the effects of not only the GPER-1 agonist, but also the other ligands, by down-regulating the levels of the GPER-1 receptor (Fig. 2). Indeed, the effects of all four ligands were severely blunted by treatment of SKBR3 cells with small interfering (si)GPER#1 (Fig. 2A, 1 and 2). To verify the specificity of the siRNA knockdown, we use two additional oligos, siGPER#2 and siGPER#3 (Fig. 2B, 1 and 2) of the GPER-1. The effectiveness of the siRNA used was confirmed by Western blotting with a GPER-1 specific antibody (Fig. 2, A1 and B1). Consistent with the involvement of GPER-1 in the action of the four ligands, overexpression of the receptor by transfection of an expression plasmid, pGPER-1, GPER-1 cDNA under the control of the cytomegalovirus (CMV) promoter of pcDNA3 (Invitrogen, Carlsbad, CA), augmented the induction of ERRα mRNA in response to the four ligands (Fig. 3). The effectiveness of the transfection process was confirmed by Western blotting (Fig. 3, inset).
Figure 2.
GPER-1 mediates the effect of E2, G-1, ICI, and TAM on ERRα expression in SKBR3 cells. A1, GPER-1 protein is lowered by GPER-1 targeted siRNA (siGPER#1). β-Actin was used as a loading control. A2, Knock down of GPER-1 mRNA by siGPER#1 oligonucleotides blocks the effect of E2, G-1, ICI, and TAM on ERRα mRNA expression. B1, GPER-1 protein is lowered by GPER-1 targeted siRNA (siGPER#2 and siGPER#3). β-Actin was used as a loading control. B2, Knock down of GPER-1 mRNA by siGPER#2 and #3 oligonucleotides blocks the effect of E2 and TAM on ERRα mRNA expression. The experiments were repeated three times and results are presented as induction ± sd. *, P < 0.05.
Figure 3.
Ectopic expression of GPER-1 potentiates the ERRα mRNA inducing effects of E2, G-1, ICI, and TAM. SKBR3 cells were transfected with control plasmid (pcDNA3) or the plamsid pcDNA3 directing the expression of the GPER-1 cDNA under the control of the cytomegalovirus promoter (Western blotting; inset). The treatment with E2, G-1, ICI, or TAM was initiated 24 h after the transfection. Except for E2, the experiments were repeated three times, and results are presented as induction ± sd. The experiment with E2 was repeated two times, and the results are reported as fold induction ± 1/2 the range. *, P < 0.05.
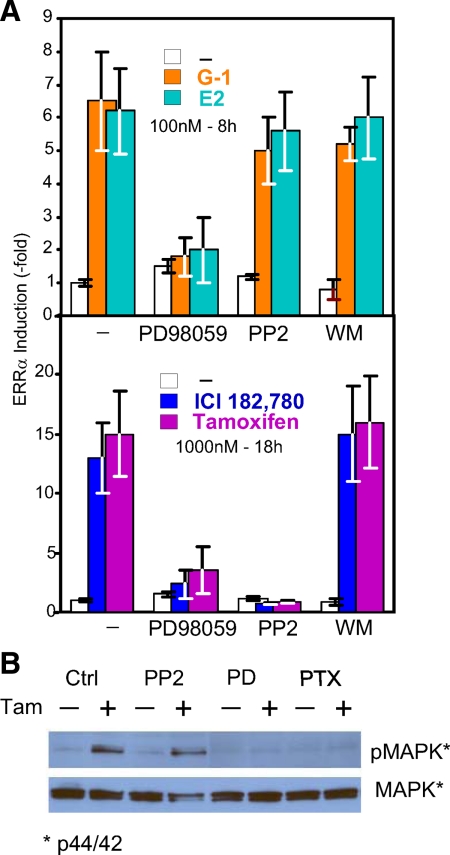
Identification with inhibitors of the signaling pathway that increases ERRα in response to E2, G-1, ICI, and TAM treatment
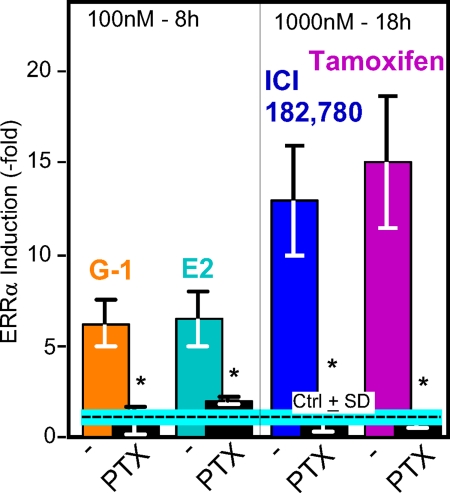
The GPER-1 was demonstrated to be a GPCR (12). Inhibition of the effects of the four ligands by pretreating the cells with pertussis toxin (PTX) (Fig. 4) not only supported the concept that GPER-1 is indeed a GPCR, but also indicated that the four ligands act by a common pathway involving one receptor and one subfamily of heterotrimeric G proteins (Gi/o). Given that Go is expressed mostly in neuronal and cardiac cells, the likely G proteins are of the Gi type, i.e. Gi1, Gi2, and/or Gi3. This question was not explored further. Instead, we determined which of the several classical downstream pathways is activated by GPER-1 in SKBR3 breast cancer cells (Fig. 5).
Figure 4.
PTX treatment, which inhibits the activation of the Gi/Go class of G proteins, blocks the induction of ERRα mRNA expression by E2, G-1, ICI, and TAM. SKBR3 cells were first incubated with 100 ng/ml PTX (Biological Laboratories, Inc.) and then treated with the indicated compounds for 8 or 18 h, followed by mRNA analysis by real-time PCR. The experiments were repeated three times, and results are presented as induction ± sd. *, P < 0.05.
Figure 5.
Effects of signaling pathway inhibitors on the induction of ERRα mRNA expression and MAPK phosphorylation. A, Effect on ERRα mRNA induction by E2, G-1, ICI, and TAM. The Erk1/2 MAPK inhibitor PD (10 μm), the src family tyrosine kinase inhibitor PP2 (10 μm) and the PI3K inhibitor WM (0.5 μm) were added to SKBR3 cells 1 h before initiation of treatments with the indicated compounds. The experiments were repeated three times, and results are presented as induction ± sd. *, P < 0.05. B, Effect on activation of MAPK (ERK1/2) phosphorylation by TAM. Cells were cultured and lysed under the conditions described in Materials and Methods. Protein level of phospho-p44/42 and total p44/42 were detected with specific antibodies.
The role and signaling pathways used by GPER-1 in cells in which both E2 and the active metabolite of TAM, 4-hydroxytamoxifen, have proproliferative actions accompanied by induction of the early response gene c-fos, e.g. endometrial Ishikawa cells, has been found to involve activation of several kinase pathways, including src related tyrosine kinase(s), the MAPKs Erk1/2, the EGF receptor kinase and the phosphatidylinositol kinase (PI3K) Akt (serine-threonine kinase). As is the case in the SKBR3 cells reported here, induction of c-fos by E2 and 4-hydroxytamoxifen (4-OHT) is independent of ERα (45). We thus tested for involvement of kinase pathways using the selective inhibitors that proved useful in Ishikawa cells. Figure 5A, top and bottom panels, shows that although the effects of all four ligands depended on activation of the MAPKs Erk1/Erk2, the effects of E2 and G-1 did not involve the nonreceptor tyrosine kinase src, whereas those of the ERα antagonist ICI and the TAM did depend on src, because they were inhibited by the src selective inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine(PP2). Further support for the dichotomy in E2/G-1 vs. TAM/ICI downstream signal of MAPK was presented by Western blotting (Fig. 5B), showing that TAM activates MAPK (phosphorylated ERK) in a PTX- and PD 98059 (PD)-sensitive, but PP2-insensitive manner. The induction of ERRα by all four ligands occurred independently of the PI3K/Akt signaling pathway as they were not affected by Wortmannin (WM), an inhibitor of the Akt signaling pathway. Both src-independent and src-mediated activation in response to Gi protein activation have been shown to be mediated by the G protein’s Gβγ signaling arm (46), which, like GPER-1 activation, is capable of stimulating types 2, 4, and 7 adenylyl cyclases (see review in Refs. 47 and 48). Indeed, we found that SKBR3 cells expresses mRNA encoding adenylyl cyclases 3, 6, 7, and 9 plus minor amounts of mRNA coding for adenylyl cyclases 4 and 5 (data not shown). The presence of adenyly cyclase 3 therefore constitutes a rational explanation for the fact that stimulation of the GPER-1 leads to increases in cAMP that can be blocked by PTX. In addition, adenylyl cyclase 2, 4, and 7 are activated by Gβγ, offering a rationale for possible additional increase in cAMP triggered by activation of a PTX sensitive Gi in response to GPER-1 activation.
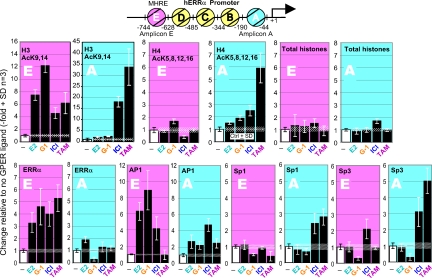
E2, G-1, ICI, and TAM induce histone acetylation and transcription factor recruitment at distinct nucleosomes of the ERRα promoter
Two distinct events associated with transcriptional activation are recruitment of transcription factors to promoter nucleosomes and acetylation of their histones, whereas the modification of histone acetylation status of the gene promoter is the earliest step in transcriptional activation (49,50). We thus investigated whether the G protein-dependent signaling pathways delineated above lead to differential histone modification of the ERRα promoter nucleosomes (Fig. 6). The locations of nucleosome positions at the ERRα promoter have been previously mapped (7,22). Further, we have demonstrated that a MHRE of the ERRα gene resides at nucleosome E and that a GC-rich region of the promoter is present at nucleosome A (Fig. 6, diagram). By using the native chromatin immunoprecipitation (N-ChIP) approach, we analyzed the histone acetylation patterns at nucleosomes A and E as described (22). We treated SKBR3 cells with E2 and G-1 for 3 h and with ICI and TAM for 6 h. This was followed by isolation of nuclei, partial digestion with micrococcal nuclease (MNase) to degrade chromatin to mono-, di-, and tri-nucleosomes, immunoprecipitation with antibodies specific for acetylated histone H3 and H4, and analysis the ERRα promoter sequences in the immunoprecipitates by real-time quantitative PCR with oligonucleotide probes specific for nucleosomes E and A. As can be seen from inspection of the changes in the ERRα promoter DNA recovered after the different exposures to ligands, these treatments led to patterns that were more similar to each other when comparing E2 with G-1 and ICI with TAM than when E2 or G-1 are compared with ICI or TAM. However, each ligand also promoted changes that were unique, not shared with any of the other ligands (Fig. 6, top panels). As expected, the levels of total histones at either nucleosome E or A were not changed (Fig. 6, total histone panels). These ChIP results demonstrated that exposure to E2, G-1, ICI, and TAM leads to modifications of chromatin structure at distinct nucleosomes of the ERRα gene promoter. Whether differential histone acetylation correlates with differential transcription factor recruitment was next examined. We selected to study recruitment of activator protein-1 (AP-1), ERRα, Sp1, and Sp3 to these nucleosomes based on the presence of known response elements in the DNA residing at these nucleosomes. ERRα and AP-1 binding elements are located at nucleosome E, and multiple Sp1 and Sp3 binding elements are present at nucleosome A (7,22,51). By using standard ChIP assays, we found that ERRα is actively recruited by all four compounds to the nucleosome E but not to nucleosome A (Fig. 6, ERRα panels), whereas AP-1 is mainly recruited by E2 and G-1 to the nucleosome E (Fig. 6, AP-1 panels). Similar experiments were conducted with antibodies to Sp1 and Sp3 in the ChIP assays (Fig. 6, Sp1 and Sp3 panels). Significant increases of Sp1 and Sp3 occupancy of nucleosomes A and E were only found upon treatment of the cells with the ER antagonist ICI and the SERM TAM. Treatment with E2 and G-1 did not recruit Sp1 or Sp3 to either nucleosome. These results, together with the results obtained with the various types of inhibitors (Fig. 5), demonstrate that E2, G-1, ICI, and TAM induce ERRα expression through common as well as distinct signaling pathways downstream of the GPER-1 receptor. These signaling pathways lead to modifications of chromatin structure and recruitment of transcription factors to different nucleosomes of the ERRα promoter, which translates into stimulated mRNA transcription and accumulation of ERRα protein.
Figure 6.
Effects of E2, G-1, ICI, and TAM on histone acetylation and transcription factor recruitment at the ERRα gene promoter. Diagram, Representation of the nucleosome positions at the ERRα promoter. Regions spanning DNA of nucleosome A and E were analyzed for presence of acetylated histones and the indicated transcription factors. Cells were treated with vehicle (control) or 100 nm of E2 or G-1 for 3 h or 1000 nm ICI or TAM for 6 h, followed by isolation of nuclei and either analysis of histones by native ChIP (upper panels) or of transcription factor association by conventional ChIP (lower panels). The experiments were repeated three times, and the results are presented as change in amount of nucleosomal DNA recovered in the immunoprecipitates relative to control ± sd.
Discussion
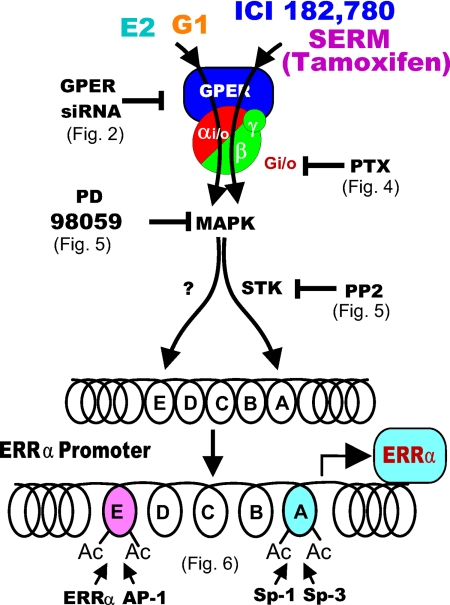
As summarized in Fig. 7, this study presents several novel findings: 1) GPER-1, an orphan GPCR, mediates the actions of E2, G-1, ICI, and TAM and transactivates ERRα gene expression in the classical ER-null SKBR3 cell line; 2) E2, G-1, ICI, and TAM activate common as well as different signaling pathways downstream of GPER-1; 3) E2, G-1, ICI, and TAM induce ERRα gene expression by modifying the chromatin structure at different nucleosomes of the ERRα promoter and recruit different transcription factors to the corresponding nucleosomes; and 4) the ability of GPER-1 to trigger cellular responses that differ with the nature of the ligand.
Figure 7.
A model of the organization of the signaling pathways that were addressed in the present experiments. The results delineate the existence of two signaling pathways down stream of the GPER-1. One, activated by E2 and G-1, does not depend of activation of a member of the src family of tyrosine kinases; the other, activated by the ERα antagonist ICI and the inhibitory SERM TAM, depends on activation of a PP2-sensitive tyrosine kinase. Activation of the Erk1/2 MAPKs is required for the action of all four GPER-1 ligands.
Nucleosome assembly and the remodeling of chromatin architecture at the gene promoter are critical initial steps in the regulation of gene expression (50). We have previously shown that E2 induces restriction enzyme hypersensitivity and histone H3 and H4 acetylation at the well-characterized MHRE in nucleosome E of the ERRα gene in both ER+ and ER− cells. Changes of the local chromatin structure leads to the recruitment of coregulators and RNA polymerase II, as well as increased ERRα gene expression (7). We have further demonstrated that ERα in ER+ cells that is tethered to the MHRE by DNA binding transcription factor, AP-1, and ERRα at the MHRE is responsible for the estrogen stimulation of ERRα expression. However, the transducer of estrogen action in the ER− cells remained unidentified. Here, we present evidences to demonstrate that GPER-1 is the mediator of estrogen (E2), antiestrogen (ICI), SERM (TAM), and a GPER-1 specific ligand (G-1) stimulation of ERRα expression in cells lacking the classical ERs. It has been shown that GPER-1 binds estrogen, ICI, TAM, and DDE (an endocrine disruptor) with high affinity while not binding to progestogen, testosterone, and cortisol (12,14). Recently, through a combination of virtual and biomolecular screening approaches, a specific ligand to GPER-1 (G-1) was isolated (15). Although E2, G-1, ICI, and TAM all bind GPER-1, E2, and G-1 bind in the nanomolar range, whereas ICI and TAM bind in the micromolar range (14) consistent with the ligand concentrations required to activate ERRα expression (Fig. 1, A and B). The unusual pharmacology of GPER-1 is distinct from the classical ER, and the recent characterization of GPER-1-null mouse revealed no obvious defects in the reproductive organs. This raised concern as to whether or not GPER-1 is a bona fide ER. However, some laboratories did show alterations in glucose tolerance, bone growth, blood pressure, and serum insulin-like growth factor 1 levels in GPER-1-null mouse (see review in Refs. 42,43,44 and references therein). Although the physiological function of GPER-1 in normal and diseased state has yet to be established, our current studies demonstrated that ERRα expression in SKBR3 cells is stimulated by E2, G-1, ICI, and TAM at the dose- and time-dependent manner. Although the different pattern of induction suggests that there may be mechanistic differences among these compounds in transactivation of the ERRα gene expression via GPER-1, the fact that ERRα and its downstream target gene expression were up-regulated and the cell proliferation increased by these ligands in the absence of classical ERs underscore the biological significance of the ERRα/GPER-1 signaling pathway.
Recent studies revealed that binding of estrogen and related compounds to GPER-1 activated multiple intracellular signaling pathways, including the MAPK, adenylyl cyclase, and PI3K signaling pathways through the transactivation of epidermal growth factor receptor (EGFR) (12,13,14). EGFR has been identified as a key element in the complex signaling network and served as the signal information convergence point for membrane receptors (52). Cross talk between the estrogen and EGFR signaling pathways has been well documented (9,53). Because of the cross talk, estrogen can initiate rapid MAPK signaling in an ER-independent manner while maintaining the estrogen responsiveness of ER− breast cancer cells. Whether EGFR is involved in the expression of ERRα has yet to be examined. The ability of GPER-1 to activate downstream signaling pathways that differ with the ligand is intriguing. This GPCR is unusual not only in that it is activated by estrogen but also in that it is primarily if not exclusively located in internal membranes as opposed to the plasma membrane (13). Plasma membrane GPCRs have been shown to have the ability to signal through a G protein-dependent as well as a G protein-independent pathway, the independent pathway being triggered by agonist-induced association of arrestins to the GPCR (54). Although not rigorously excluded, this second mechanism does not appear to be in play with GPER-1, as G protein ADP-ribosylation with PTX, which prevents interaction of the receptor with the G protein, effectively blocked the effects of the four ligands (Fig. 4). GPER-1 is not only unique among GPCRs in its intercellular location, but also in its ability to promote rapid and sustained in intercellular Ca2+ in response to E2. This increase was not inhibited by the phospholipase C inhibitor U73122, and hence to be independent of phospholipase C suggested a divergent pathway for E2 and GPER-1 in mobilization of the Ca2+ (13). It remains to be determined whether TAM and ICI also promote elevation of Ca2+ and hence whether the differences in their way of signaling through GPER-1 from the way E2 and G-1 signal may have its root in differential ability to trigger Ca2+ changes. Perhaps the most surprising, as well as the most striking, of these findings is that a clinically used SERM that is an inhibitor at the level of the classical ERs in normal and cancerous breast epithelial cell is a GPER-1 receptor agonist, and that by promoting the synthesis of ERRα, the GPER-1/ERRα signaling pathway so formed converts TAM from an agent that checks tumor growth into an agent that promotes tumor growth as we demonstrated in Fig. 1D that TAM enhances SKBR3 cell proliferation. There was apparent discordance between the TAM dose response for ERRα mRNA induction (Fig. 1A) and cell proliferation (Fig. 1D). A possible explanation is that the expression of mRNA after 18 h of treatment may require a higher concentration (1 μm) of TAM to produce enough detectable messages. Lower concentrations (100 nm), however, could enhance cell proliferation to its full level 4 d later (96 h), when the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) activity assays were performed. Nonetheless, the possibility that low dose TAM induces proliferation through a non-GPER mechanism cannot be excluded.
Mounting clinical evidence has demonstrated that ERRα expression is up-regulated in aggressive breast cancer cells and correlates with a poor prognosis of the disease (36,37,55,56). The association of ERRα expression in breast cancer and other aggressive tumors (57,58) suggests that ERRα may have roles in tumor development and progression. Because of the close relationship between ERs and ERRs, the presence of ERRα in breast cancer cells could potentially induce ER target gene expression with or without the presence of classical ERs. Importantly, ERRα itself is estrogen responsive, and the estrogen action can be mediated by either ER (7,27,28) or GPER-1 as demonstrated in this study. It has been shown that the expression of aromatase is regulated by ERRα (59) and its mRNA level is closely associated with ERRα mRNA levels in breast carcinoma cells (60). Additionally, the dehydroepiandrosterone sulfotransferase, which is critical in increasing the pool of aromatase substrates, is also the downstream target of ERRα (41). Taken together, ERRα contributes to the increased local production of estrogen and ERRα itself is estrogen responsive in breast cancer cells, thus presenting a feed-forward loop model in aggressive breast cancer. Of note, ERRα is also stimulated by ER antagonists and the clinically used SERM TAM through GPER-1. Recently, GPER-1 was also implicated in carcinogenesis and cancer progression (42,43). These observations have important clinical implications because TAM resistance has been well documented among breast cancer patients who have been on hormone therapy for a number of years (61). The activation of ERRα by ICI and TAM in breast cancer cells as seen in the present study provides an important link between the pharmacology of estrogen and breast cancer, thus suggesting that ERRα as well as GPER-1 can be a critical targets for drug development.
The potential proproliferative effect of TAM reported here for ER− breast tumor cells adds to the complexity of the actions of this SERM. While this manuscript was in preparation, TAM was reported to be able to have positive effects also in ER+ cells in which the TAM-ER complex, through recruitment of amplified in breast cancer-1, may suppress an inhibitory control of the proproliferative ERBB2-HER-2 gene product (62). In addition, a novel TAM analog, STX, increases nuclear receptor steroidogenic factor-1 transcription and promotes endometrial cell proliferation via GPER-1 receptor in ERs-negative primary human endometriotic H-38 cells (63). STX treatment stimulates PI3K and MAPK pathway in a GPER-1-dependent manner, an observation consistent with our current findings.
Materials and Methods
Materials
17β-E2 and TAM citrate were purchased from Sigma-Aldrich (St. Louis, MO), G-1, (1-[4-(6-bromobenzo[1, 3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c] quinolin-8-yl]-ethanone) was from Cayman Chemical (Ann Arbor, MI) and ICI was from Tocris Bioscience (Ellisville, MO). PTX was obtained from List Biological Laboratories, Inc. (Campbell, CA). The Erk1/2 MAPK inhibitor PD and src family tyrosine kinase inhibitor PP2 were from Calbiochem (San Diego, CA). The PI3K inhibitor WM was obtained from Sigma-Aldrich.
Antibodies
Anti-ERRα polyclonal antibody (clone: P3-84) was generated from the C terminus peptide of ERRα (SVHIEDAEAVEQLREALHEALLEYEA) as previously described (28). Anti-β-actin monoclonal antibody (clone: AC-74) was from Sigma-Aldrich, anti-GPER-1 polyclonal antibody (catalog no. LS-A4272) was from MBL International Corp. (Woburn, MA). The phosphor-p44/42 MAPK (Thr202/tyr204) antibody (no. 9101) and p44/42 MAPK (Erk1/2) antibody (no. 9102) were from Cell Signaling Technology (Danvers, MA). The antiacetyl-H3 histone antibody (antiacetyl-H3K9,14] antibody; catalog no. 06-599), antiacetyl-H4 histone antibody (antiacetyl H4K5,8,12,16 antibody; catalog no 06-866), anti-C terminus of histone H3, pan (clone: A3S; catalog no. 05-928) antibody, and normal rabbit IgG (catalog no. 12-370) were from Upstate, Millipore Corp. (Temecula, CA). Anti-Sp1 (clone: H-225) and anti-Sp3 (clone: D-20) were purchased from Santa Cruz Biotechnology, Inc. (San Diego, CA).
Cell line and tissue culture
ER− and GPER-1-positive SKBR3 human breast cancer cells (HTB-30) were obtained from ATCC (Manassas, VA) and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and 4 mml-glutamine. For the experiments, cells were seeded in six-well plates at 8 × 105 cells per well and cultured overnight. Before treatment, culture medium was switched to phenol red-free RPMI 1640 without FBS for 1 d. Cells were then treated with E2, G-1, ICI, or TAM for different times at various concentrations as indicated in the individual figures and figure legends.
Cell proliferation assay
Cells were seeded in 96-well plates at 5–6 × 103 cells per well and cultured with phenol red-free RPMI 1640 medium with 10% FBS overnight. Before treatment, culture medium was switched into phenol red/FBS-free RPMI 1640 for 1 d. Cells were treated with E2, ICI, or TAM for 1 d and then switched back to phenol red-free RPMI 1640 with 10% FBS for additional 3 d (total 4-d treatments) at various concentration as indicated in the figures and figure legends. Cell proliferation assay was performed by TACS 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay kit according to the supplier’s protocol (catalog no. TA5355; R&D Systems, Inc., Minneapolis, MN). The viability of the cells was verified by 4′,6-diamidino-2-phenylindole (DAPI) staining of the apoptotic cells. Cells treated with 100 nm E2, 1000 nm ICI or TAM for 3 d, and no detectable cell death were found.
Cell culture and sample preparation for MAPK (ERK1/2) analysis
Cells were seeded in 60-mm dishes, cultured overnight in phenol red-free RPMI 1640 medium with 10% FBS, and then starved in phenol red-free RPMI 1640 with 1% charcoal-stripped FBS for 2–3 d. Cells were pretreated without or with inhibitors (10 μm PP2 or PD, 100 ng/ml PTX) for 1 h followed by the addition of 1000 nm TAM for 20 min. Cells were washed twice with cold PBS and then lysed in ice-cold lysis buffer [1% Nonidet P-40 (no. 56741; Sigma), 0.5% sodium deoxycholate, 0.1% SDS, 1× complete minitab (no. 11-836-170-001; Roche, Indianapolis, IN), 10 mm sodium fluoride, and 1 mm sodium orthovanadate] for 1 h followed by sonication with probe sonicator for 15–20 sec on ice. The supernatant was used for Western blot analysis. Commercial antibodies of phospho-p44/42 MAPK and total p44/42 MAPK were diluted 1:500 before used in Western blotting.
RNA extraction and real-time PCR
Total RNA was extracted by using QIAGEN RNeasy Mini kit (QIAGEN, Valencia, CA). First-strand cDNA synthesis was performed using superscript reverse transcriptase (Invitrogen). The mRNA levels of ERRα and β2-microglobulin were measured using TaqMan gene expression assays (Applied Biosystems, Foster City, CA), whereas SYBR green assays (Applied Biosystems) were used for ATP5β (ATP synthase H+ transporting mitochondrial F1 complex β subunit) and cytochrome C (Cytc). The sequences of primers used in real-time PCR were as follows: for human ERRα, forward primer 5′-GGCCCTTGCCAATTCAGA-3′ and reverse primer 5′-GGCCTCGTGCAGAGCTTCT-3′, the TaqMan probe was TGCACATCGAAGATGCCGAGGCT; for human β2-microgloblin, forward primer 5′-TGCTCGCGCTACTCTCTCTTT-3′ and reverse primer 5′-TCTGCTGGATGACGTGAGTAAAC-3′, the TaqMan probe was 5′-CCTGGAGGCTATCCAGCGTACTCCAAA; for ATP5β (β-subunit of the F1/Fo mitochondrial proton pump ATPase), forward primer 5′-GCAAGGCAGGGAGACCAGA-3′ and reverse primer 5′-CCCAAAGTCTCAGGACCAACA-3′; and for Cytc (somatic mitochondrial cytochrome C), forward primer 5′-CCAGTGCCACACCGTTGAA-3′ and reverse primer 5′-TCCCCAGATGATGCCTTTGTT-3′. Each sample was quantified against its β2-microglobulin transcript content, and then normalized with respect to the control group. The experiments were repeated three times and results are presented as fold increase ± sd. See Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org.
Protein extraction and Western blot analysis
Whole cell lysates were prepared by using BD TransFactor Extraction kits according to the supplier’s protocol (BD Biosciences, Palo Alto, CA). The protein concentrations were determined using Bio-Rad Protein assay reagents (Pierce, Rockford, IL). After heating the samples at 95 C for 5 min, they were loaded on a freshly made 10% SDS-PAGE gel at 40 μg/lane and separated by electrophoresis. The proteins were then electrotransferred onto polyvinylidene difluoride membranes followed blocking in PBS containing 0.05% Tween 20 (PBS-T) and 5% nonfat milk for 2 h. The blots were incubated with primary antibodies in PBS-T at 4 C overnight, rinsed with PBS-T, and then incubated with appropriate horseradish peroxidase conjugated secondary antibodies at a dilution of 1:5000 in PBS-T/milk at room temperature for 1 h. The immunoreactive products were detected by the enhanced chemiluminescence (ECL) system (Amersham Pharmacia, Piscataway, NJ). Presence of β-actin was used as a loading control.
Transfection of siRNA and GPER-1 expression plasmid
The siRNA oligonucleotides (siGPER#1) were purchased from QIAGEN. The sequences were: 5′-AAUUGGGAAGUCACAUCCAU-3′ for GPER-1 and 5′-AAGAUCUCAGCACGGCAAAU-3′ for the scrambled control (17). The siGPER#2: CCATCGGCTTTGTGGGCAA and siGPER#3: GGATGAGCTTCGACCGCTA were purchased from Dharmacon, Inc. (Chicago, IL).
The mouse GPER-1 cDNA was cloned by the RT-PCR procedure using total RNA extracted from 129SvEv mouse fibroblasts as template, 5′-CATCAAGCTTATGGATGCGACTACTCCAGCCCAAAC as the forward primer, and 5′-ACTAGAATTCACACAGCACTGCTGAACCTGACCTC as the reverse primer. The reaction product of 1146 bp was digested with HindIII and EcoRI, cloned into HindIII and EcoRI, digested pcDNA3 plasmid, and verified by sequencing. The nucleotide sequence and open reading frame of the cloned GPER-1 was found to be as described in GenBank accession no. NM_029771, except for codon 314, which was found to be TTC (Phe) instead of ATC (Ile). The sequence of the newly cloned cDNA is in agreement with the CDS sequence predicted by genomic DNA (GenBank accession no. NT_039316, nucleotides 740253-744862). For transient transfections, 500 ng of siControl or siGPER-1 duplexes were transfected using RNAiFect transfection reagent (QIAGEN) for 36 h, whereas 4 μg of expression plasmids, pcDNA3-vector, or pcDNA-GPER-1 were transfected using the Effectene transfection reagent (QIAGEN) for 8 h. At the end of transfections, cells were reseeded into the 60-mm dishes. After overnight culture, the culture medium was changed to phenol red-free RPMI 1640 without FBS; 24 h later, the cells were treated as described in the figures and figure legends.
Isolation of nuclei, MNase digestion, and ChIP analyses
Nuclei were prepared, and chromatin was fragmented by digestion with MNase (Worthington, Lakewood, NJ) until it became mono-, di-, and tri-nucleosomes in size (7). N-ChIP (for acetylated histone analysis) and standard ChIP (for recruitment of transcription factors) analyses were performed as described (22). The amounts of ERRα promoter nucleosome A and E DNA recovered in the immunoprecipitates were quantified by real-time PCR and compared with the amounts of nucleosome A and E DNA recovered from control immunoprecipitates performed with chromatin from SKBR3 cells treated identically but without addition of inducers of ERRα mRNA. The sequences of primers used in N-ChIP and ChIP assays were as follows: nucleosome A DNA (amplicon A), forward primer 5′-CGCCCCGCTGGTCTGATTAGC-3′ and reverse primer 5′-GCCGTGCGCATGCAACAC-3′; nucleosome E DNA (amplicon E), forward primer 5′-TCAGTGCAGGACAGCCCGCGTGAG-3′ and reverse primer 5′-GATAGGGCCCGGACGGAGAAAGC-3′. The results are expressed as increases in DNA recovered from treated cells relative to DNA recovered from control cells.
Supplementary Material
Acknowledgments
We thank Dr. Joel Abramowitz for designing the cloning strategy used to obtain the mouse GPER-1 cDNA and Mr. Dagoberto Grenet for executing the cloning strategy; and the critical reading and valuable comments of Dr. Peggy Kaminski, Dr. Paul Wade, Dr. Anton Jetten, Dr. Trevor Archer, and Dr. Joel Abramowitz.
Footnotes
This work was supported by the Intramural Research Program of National Institutes of Health and by the National Institute of Environmental Health Sciences Projects Z01-ES-070067 (to C.T.T.) and Z01-ES-101643 (to L.B.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 8, 2010
Abbreviations: Akt, Serine-threonine kinase; AP-1, activator protein-1; Cytc, cytochrome C; E2, estradiol; EGFR, epidermal growth factor receptor; ER, estrogen receptor; ER−, ER-negative; ER+, ER-positive; ERR, estrogen-related receptor; FBS, fetal bovine serum; GPCR, G protein-coupled receptor; GPER-1, GPCR GPR30; ICI, ICI 182,780; MHRE, multihormone response element; MNase, micrococcal nuclease; N-ChIP, native chromatin immunoprecipitation; PD, PD 98059; PI3K, phosphatidylinositol kinase; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyramidine; PTX, pertussis toxin; SERM, selective ER modulator; si, small interfering; TAM, tamoxifen; WM, Wortmannin.
References
- Deroo BJ, Korach KS 2006 Estrogen receptors and human disease. J Clin Invest 116:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager JD, Davidson NE 2006 Estrogen carcinogenesis in breast cancer. N Engl J Med 354:270–282 [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA 2003 Estrogen signaling: a subtle balance between ERα and ERβ. Mol Interv 3:281–292 [DOI] [PubMed] [Google Scholar]
- Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J 2004 Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors α and β by coactivators and corepressors. J Mol Endocrinol 33:387–410 [DOI] [PubMed] [Google Scholar]
- Safe S 2001 Transcriptional activation of genes by 17β-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm 62:231–252 [DOI] [PubMed] [Google Scholar]
- Umayahara Y, Kawamori R, Watada H, Imano E, Iwama N, Morishima T, Yamasaki Y, Kajimoto Y, Kamada T 1994 Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. J Biol Chem 269:16433–16442 [PubMed] [Google Scholar]
- Hu P, Kinyamu HK, Wang L, Martin J, Archer TK, Teng C 2008 Estrogen induces estrogen-related receptor α gene expression and chromatin structural changes in estrogen receptor (ER)-positive and ER-negative breast cancer cells. J Biol Chem 283:6752–6763 [DOI] [PubMed] [Google Scholar]
- Björnström L, Sjöberg M 2005 Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19:833–842 [DOI] [PubMed] [Google Scholar]
- Levin ER 2005 Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW 2007 Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev 28:1–19 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton Jr AR, Bland KI 2002 Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P 2005 GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab 16:362–367 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER 2005 A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J 2005 Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–632 [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER 2006 Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212 [DOI] [PubMed] [Google Scholar]
- Kroeze WK, Sheffler DJ, Roth BL 2003 G-protein-coupled receptors at a glance. J Cell Sci 116:4867–4869 [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Andò S 2004 The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem 279:27008–27016 [DOI] [PubMed] [Google Scholar]
- Committee NRN 1999 A unified nomenclature system for the nuclear receptor superfamily. Cell 97:161–163 [DOI] [PubMed] [Google Scholar]
- Giguère V, Yang N, Segui P, Evans RM 1988 Identification of a new class of steroid hormone receptors. Nature 331:91–94 [DOI] [PubMed] [Google Scholar]
- Villena JA, Kralli A 2008 ERRα: a metabolic function for the oldest orphan. Trends Endocrinol Metab 19:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM 2004 Errα and Gabpa/b specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA 101:6570–6575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li Y, Hu P, Teng CT 2008 PGC-1α induces dynamic protein interactions on the ERRα gene multi-hormone response element nucleosome in kidney cells. Biochem J 416:407–419 [DOI] [PubMed] [Google Scholar]
- Knutti D, Kralli A 2001 PGC-1, a versatile coactivator. Trends Endocrinol Metab 12:360–365 [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM 2003 Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): transcriptional coactivator and metabolic regulator. Endocr Rev 24:78–90 [DOI] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Filipuzzi I, Schilb A, Riou V, Graham A, Strauss A, Geiser M, Fournier B 2004 Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor α (ERRα): crystal structure of ERRα ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1α. J Biol Chem 279:49330–49337 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Teng CT 2000 Estrogen receptor-related receptor α 1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem 275:20837–20846 [DOI] [PubMed] [Google Scholar]
- Liu D, Zhang Z, Gladwell W, Teng CT 2003 Estrogen stimulates estrogen-related receptor α gene expression through conserved hormone response elements. Endocrinology 144:4894–4904 [DOI] [PubMed] [Google Scholar]
- Shigeta H, Zuo W, Yang N, DiAugustine R, Teng CT 1997 The mouse estrogen receptor-related orphan receptor α1: molecular cloning and estrogen responsiveness. J Mol Endocrinol 19:299–309 [DOI] [PubMed] [Google Scholar]
- Liu D, Benlhabib H, Mendelson CR 2009 cAMP Enhances estrogen-related receptor α (ERRα) transcriptional activity at the SP-A promoter by increasing its interaction with protein kinase A and steroid receptor coactivator 2 (SRC-2). Mol Endocrinol 23:772–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JB, Laganière J, Giguère V 2006 A single nucleotide in an estrogen-related receptor a site can dictate mode of binding and peroxisome proliferator-activated receptor G coactivator 1a activation of target promoters. Mol Endocrinol 20:302–310 [DOI] [PubMed] [Google Scholar]
- Johnston SD, Liu X, Zuo F, Eisenbraun TL, Wiley SR, Kraus RJ, Mertz JE 1997 Estrogen-related receptor α1 functionally binds as a monomer to extended half-site sequences including ones contained within estrogen-response elements. Mol Endocrinol 11:342–352 [DOI] [PubMed] [Google Scholar]
- Yang N, Shigeta H, Shi H, Teng CT 1996 Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. J Biol Chem 271:5795–5804 [DOI] [PubMed] [Google Scholar]
- Vanacker JM, Pettersson K, Gustafsson JA, Laudet V 1999 Transcriptional targets shared by estrogen receptor-related receptors (ERRs) and estrogen receptor (ER)α, but not by ERβ. EMBO J 18:4270–4279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganière J, Tremblay GB, Dufour CR, Giroux S, Rousseau F, Giguère V 2004 A polymorphic autoregulatory hormone response element in the human estrogen-related receptor α (ERRα) promoter dictates peroxisome proliferator-activated receptor γ coactivator-1α control of ERRα expression. J Biol Chem 279:18504–18510 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen K, Shih JC, Teng CT 2006 Estrogen-related receptors-stimulated monoamine oxidase B promoter activity is down-regulated by estrogen receptors. Mol Endocrinol 20:1547–1561 [DOI] [PubMed] [Google Scholar]
- Ariazi EA, Clark GM, Mertz JE 2002 Estrogen-related receptor α and estrogen-related receptor γ associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res 62:6510–6518 [PubMed] [Google Scholar]
- Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, Ohuchi N, Sasano H 2004 Estrogen-related receptor α in human breast carcinoma as a potent prognostic factor. Can Res 64:4670–4676 [DOI] [PubMed] [Google Scholar]
- Stein RA, Chang CY, Kazmin DA, Way J, Schroeder T, Wergin M, Dewhirst MW, McDonnell DP 2008 Estrogen-related receptor α is critical for the growth of estrogen receptor-negative breast cancer. Cancer Res 68:8805–8812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes NE, Stern DF 1994 The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta 1198:165–184 [DOI] [PubMed] [Google Scholar]
- Chen S, Zhou D, Okubo T, Kao YC, Yang C 1999 Breast tumor aromatase: functional role and transcriptional regulation. Endocr Relat Cancer 6:149–156 [DOI] [PubMed] [Google Scholar]
- Seely J, Amigh KS, Suzuki T, Mayhew B, Sasano H, Giguere V, Laganière J, Carr BR, Rainey WE 2005 Transcriptional regulation of dehydroepiandrosterone sulfotransferase (SULT2A1) by estrogen-related receptor α (ERRα). Endocrinology 146:3605–3613 [DOI] [PubMed] [Google Scholar]
- Maggiolini M, Picard D 2010 The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol 204:105–114 [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M 2009 Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat 89:89–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Maggiolini M 2009 Mechanisms of estrogen signaling and gene expression via GPR30. Mol Cell Endocrinol 308:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Andò S, Maggiolini M 2006 The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17β-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol 20:631–646 [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Hawes BE, van Biesen T, Luttrell DK, Lansing TJ, Lefkowitz RJ 1996 Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J Biol Chem 271:19443–19450 [DOI] [PubMed] [Google Scholar]
- Birnbaumer L 2007 Expansion of signal transduction by G proteins. The second 15 years or so: from 3 to 16 α subunits plus βγ dimers. Biochim Biophys Acta 1768:772–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG 1996 Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36:461–480 [DOI] [PubMed] [Google Scholar]
- Kouzarides T 2007 Chromatin modifications and their function. Cell 128:693–705 [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL 2007 The role of chromatin during transcription. Cell 128:707–719 [DOI] [PubMed] [Google Scholar]
- Shi H, Shigeta H, Yang N, Fu K, O'Brian G, Teng CT 1997 Human estrogen receptor-like 1 (ESRL1) gene: genomic organization, chromosomal localization, and promoter characterization. Genomics 44:52–60 [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Leserer M, Ullrich A 2000 Tyrosine kinase signalling in breast cancer. Epidermal growth factor receptor: convergence point for signal integration and diversification. Breast Cancer Res 2:184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER 2003 Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol 17:309–317 [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ 2006 β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem 281:1261–1273 [DOI] [PubMed] [Google Scholar]
- Deblois G, Hall JA, Perry MC, Laganière J, Ghahremani M, Park M, Hallett M, Giguère V 2009 Genome-wide identification of direct target genes implicates estrogen-related receptor α as a determinant of breast cancer heterogeneity. Cancer Res 69:6149–6157 [DOI] [PubMed] [Google Scholar]
- Stein RA, McDonnell DP 2006 Estrogen-related receptor α as a therapeutic target in cancer. Endocr-Relat Cancer 13(Suppl 1):S25–S32 [DOI] [PubMed] [Google Scholar]
- Cavallini A, Notarnicola M, Giannini R, Montemurro S, Lorusso D, Visconti A, Minervini F, Caruso MG 2005 Oestrogen receptor-related receptor α (ERRα) and oestrogen receptors (ERα and ERβ) exhibit different gene expression in human colorectal tumour progression. Eur J Cancer 41:1487–1494 [DOI] [PubMed] [Google Scholar]
- Fujimura T, Takahashi S, Urano T, Kumagai J, Ogushi T, Horie-Inoue K, Ouchi Y, Kitamura T, Muramatsu M, Inoue S 2007 Increased expression of estrogen-related receptor α (ERRα) is a negative prognostic predictor in human prostate cancer. Int J Cancer 120:2325–2330 [DOI] [PubMed] [Google Scholar]
- Yang C, Zhou D, Chen S 1998 Modulation of aromatase expression in the breast tissue by ERRα-1 orphan receptor. Cancer Res 58:5695–5700 [PubMed] [Google Scholar]
- Miki Y, Suzuki T, Tazawa C, Yamaguchi Y, Kitada K, Honma S, Moriya T, Hirakawa H, Evans DB, Hayashi S, Ohuchi N, Sasano H 2007 Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer Res 67:3945–3954 [DOI] [PubMed] [Google Scholar]
- Group EBCTC 2005 Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717 [DOI] [PubMed] [Google Scholar]
- Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS 2008 Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature 456:663–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin BC, Suzawa M, Blind RD, Tobias SC, Bulun SE, Scanlan TS, Ingraham HA 2009 Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res 69:5415–5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.