Abstract
Activin is a major physiological regulator of FSH. We identify FoxL2 as a critical component in activin induction of FSHβ, both for the mouse gene, induction of which is Sma- and Mad-related protein (Smad) dependent, and for the human gene that is Smad independent. FoxL2 has been shown to regulate gonadotrope gene expression (GnRH receptor, α-glycoprotein subunit, porcine FSHβ, and follistatin), but the mechanisms of action are not well understood. We identify novel sites required for activin action in both the mouse and human FSHβ promoters, some of which bind FoxL2, and show that the FoxL2-binding element encompasses a larger region (12 bp) than the previously identified forkhead-binding consensus (7 bp). Remarkably, although required for activin induction, FoxL2 sites neither contribute to basal FSHβ promoter activity nor confer activin response to a heterologous promoter; thus, they are neither classical activin-response elements nor is their role solely to recruit Smads to the promoter. FoxL2 overexpression can potentiate activin induction in gonadotropes and can confer activin responsiveness to FSHβ in heterologous cells where this promoter is normally refractory to activin induction. Although Smad3 requires the presence of FoxL2 sites to induce mouse FSHβ, even through its consensus Smad-binding element; the human promoter, which is induced by activin independently of Smad3, also requires FoxL2 sites for its induction by activin; thus the actions of FoxL2 are not exclusively through interactions with the Smad pathway. Thus, FoxL2 plays a key role in activin induction of the FSHβ gene, by binding to sites conserved across multiple species.
FoxL2 binding sites are necessary for activin induction of not only the Smad-dependent mouse FSHbeta gene but also the Smad-independent human FSHbeta gene.
Activin is a potent activator of the FSH β-gene, which encodes the subunit of the heterodimeric hormone that provides biological specificity, and is the limiting factor in synthesis of the mature hormone (1,2). The control of FSH levels occurs primarily through regulation of β-subunit transcription, which is critical for ovarian follicle development in both mice and humans (3,4). Recruitment of follicles for the following cycle and follicular growth is dependent on the secondary rise of FSH (2,5), which occurs separately from the preovulatory increase that coincides with the LH surge, in both rodents and humans (6,7). This secondary FSH rise is likely caused by an increase in bioavailable activin, due to a decrease in ovarian inhibin (8), and may involve synergistic interactions between activin and GnRH and/or progesterone, which specifically induce FSHβ (9,10).
Activin was originally identified in a positive feedback loop, secreted from the gonads to induce FSH secretion and FSHβ gene expression (11,12). However, the gonadotrope cell itself also expresses activin, which acts in an autocrine manner within the pituitary to regulate FSH production as well (13,14). The primary form of gonadal activin is activin A, whereas the pituitary gonadotrope primarily expresses activin B, although these forms can heterodimerize (11). Upon ligand binding, type II activin receptors, ActRIIA and ActRIIB, interact and phosphorylate a type I receptor, activin receptor-like kinase (ALK) (15). ALK4 type I receptor has been shown to have a higher affinity for ActRIIA bound to its ligand, activin A, whereas ALK7 selectively binds ActRIIB bound to activin B (16,17,18).
After development of the mouse gonadotrope LβT2 cell line that endogenously expresses FSH (19,20,21,22), several contributions have been made to the elucidation of the promoter elements that regulate transcription of the FSHβ gene. Transcription factors, such as pituitary homeobox 1 (23), LIM-homeodomain LHX3 (24), steroidogenic factor 1, and nuclear factor-Y (25), contribute to basal expression of the FSHβ gene. Sites resembling TPA-response element or cAMP response element sites that bind activator protein 1 or cAMP response element-binding protein contribute to the GnRH responsiveness of the ovine, mouse, rat, and human FSHβ genes (26,27,28,29), whereas steroid hormone regulation occurs through several hormone-response elements (30,31,32).
Because activin is the most potent inducer of the FSHβ gene, several reports have identified and analyzed activin-responsive elements in the FSHβ promoter. Activin receptor activation leads to phosphorylation of Sma- and Mad-related protein (Smad)2 and Smad3 transcription factors that heterodimerize with Smad4 to activate the transcription of target genes, acting either alone or in complexes with other proteins (33,34). For activin induction of FSHβ, Smad3 appears to be a limiting factor because its overexpression can induce a FSHβ reporter (35,36), and male mice deficient in Smad3 have lower levels of FSHβ mRNA (37). A consensus Smad-binding element (SBE) made up of the palindrome sequence GTCTAGAC is located at −267 in the mouse and rat FSHβ promoters and contributes to activin induction of rodent genes, but it is not conserved in other species (35,36,38). Because the Smad proteins have rather weak DNA-binding affinity (39,40), it is not surprising that Smads interact with other proteins to stabilize DNA binding and therefore may activate target genes through other activin-responsive elements, not only the consensus SBE. These Smad-interacting proteins not only provide higher affinity binding, but may also convey signal specificity, if they are tissue-restricted binding partners. Indeed, the pituitary-expressed transcription factor, paired-like homeodomain transcription factor-2 (Pitx2), complexes with Smad proteins, specifically Smad3, to induce the rat FSHβ promoter (41). Several other elements were identified in the FSHβ proximal promoter that are critical for activin induction in all species examined (32,42). The site at −120 of the mouse promoter (−134 in the ovine) binds a heterodimer of the homeodomain proteins Pbx and Prep, which can interact with Smads to tether them to the promoter after their activation (42), whereas the identity of the proteins binding the mouse −153 (−166 in the ovine) or the mouse −106 (−120 in the ovine) elements critical for activin induction are not known.
Given that activin responsiveness requires multiple regions in the FSHβ promoter, in addition to the previously identified sites (9,32,42), we sought to further analyze the regions that contain elements required for response to activin, its receptors, and Smad3. Our initial studies determine that a major portion of activin responsiveness requires a novel upstream region that does not contain full SBEs, indicating that activin may exert its effect through factors other than Smads. One element, required for activin response in this region, is bound by the FoxL2 forkhead protein, which plays a critical role in reproduction and is expressed in the pituitary in vivo. We determine that multiple FoxL2 sites, conserved among various species, are required for activin induction of the FSHβ gene and bind FoxL2. These FoxL2 sites are necessary for activin responsiveness of the FSHβ promoter because the response is lost with their mutation, despite the presence of other sites, including the consensus SBE. FoxL2 also confers activin/Smad responsiveness to the FSHβ gene in heterologous cells, which normally lack expression of FSHβ; however, the FoxL2 element is itself nonresponsive to activin on a heterologous promoter. Because FoxL2 sites are not sufficient for a response to activin, their role is not solely to tether Smads to the promoter. These results identify FoxL2 as a critical factor required for activin responsiveness of the FSHβ gene that acts through species-specific sets of promoter elements to allow induction by the activin signaling pathway.
Results
Activin A and B and constitutively active activin receptors induce FSHβ transcription
Because activin is a major inducer of FSHβ gene transcription, we examined whether both forms of activin, A and B, and their cognate receptors can induce FSHβ. When transiently transfected LβT2 cells were treated with activin A or activin B, both forms of activin induce FSHβ gene expression (Supplemental Fig. 1A published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), although activin B, the autocrine form, is more effective at lower concentrations. Because ALK4 type I receptor has been shown to have a higher affinity for activin A-bound ActRIIA, whereas ALK7 selectively binds activin B-bound ActRIIB (16,17,18), we next investigated whether type I activin receptors are sufficient to induce FSHβ and whether their activity localizes to distinct regions of the promoter. Single amino acid substitutions in either ALK4 or ALK7 allow these receptors to generate intracellular signals in the absence of their ligand-bound type II receptor partners (15,43). These constitutively active forms of the type I activin receptors, caALK4 and caALK7, were overexpressed in LβT2 cells. Expression of caALK4 and caALK7 resulted in 40-fold and 77-fold induction of the 1.5-kb mouse FSHβ promoter, respectively (Supplemental Fig. 1B and Fig. 1, A and B), demonstrating that constitutively active type I activin receptors can potently induce the promoter and are sufficient to mimic the effects of activin. Whereas we cannot be sure that activin A and B are both properly folded and have adequate biological activity, although both forms of activin were provided by the same supplier, neither can we be sure that, although they are in the same vector background, both caALK4 and caALK7 are expressed at the same level, due to the lack of specific antibodies for Western blotting; it is intriguing to speculate that locally produced or autocrine activin B is a more potent inducer of FSHβ.
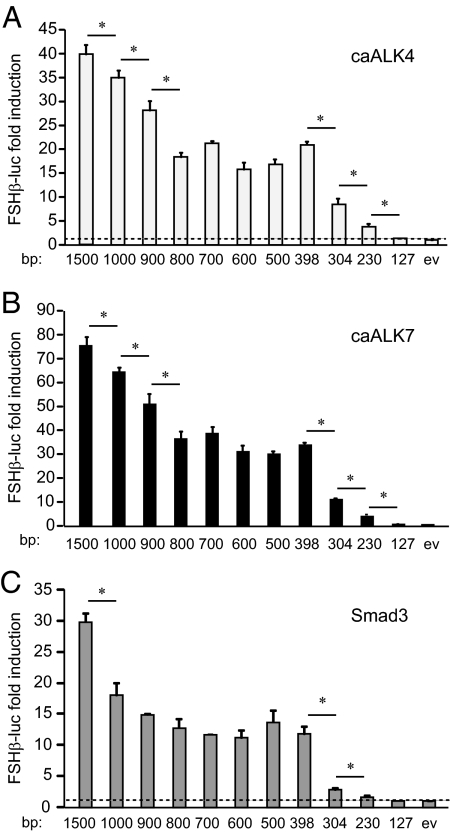
Figure 1.
Smad3 activity maps to the same regions as activin receptor activity. A–C, LβT2 cells were cotransfected with caALK4 (A), caALK7 (B), or Smad3 (C) and different lengths of the FSHβ promoter, indicated under each bar, for 48 h. The luciferase to β-galactosidase ratio from caALK- or Smad3-transfected cells was normalized to the ratio from cells transfected with empty vector (ev) control. An average of three experiments, performed in triplicate, is represented as fold induction of the same truncation over its control. *, Significant decrease in induction from the previous truncation with P < 0.05. The dashed line indicates no regulation, i.e. a ratio of 1.
Smad3 responsiveness correlates with constitutively active ALK activity
To identify which promoter elements convey caALK response, truncation analysis of the mouse FSHβ promoter was performed, in which different lengths of the promoter were transiently transfected into LβT2 cells together with caALK4 or caALK7. To determine fold induction and eliminate the influence of basal expression, luciferase levels (relative to β-galactosidase levels driven by a constitutive promoter as internal control) from caALK-transfected cells were further normalized to the luciferase levels from cells transfected with empty expression vector control for each truncation. Significant decreases in fold induction by either caALK4 or caALK7 occur between all truncations in the distal region upstream of −800. Further, dramatic decreases in fold induction occur when the regions between −398 and −304, and −304 and −230 are truncated (Fig. 1, A and B). The induction is completely lost with truncation of the region upstream of −127 bp from the start site of transcription, indicating that the most proximal region is not sufficient for activin responsiveness. The fact that the effects of ALK4 and ALK7 map to multiple regions is consistent with our previously published finding that activin maps to many regions of the mouse FSHβ promoter (9). The decrease in fold induction when the region from −304 to −230 is deleted is not surprising, because the previously identified full consensus SBE at −267 is present in this region. An additional small decrease occurs with the deletion of the region between −230 and −127, where the previously identified −153 site is located, that is critical for activin induction of the ovine and mouse genes, but the protein that binds it is unknown (32,42). However, the largest decrease in induction (66%) occurs when the region between −398 and −304 is deleted. This area represents a novel region required for activin induction of the FSHβ promoter and is the focus of our further investigation.
Upon ligand binding, activin receptors phosphorylate and activate receptor-associated Smads, Smad2 and Smad3, which then dimerize with Smad4 and translocate to the nucleus to induce gene expression. Overexpression of Smad3 is sufficient to induce the mouse FSHβ gene, and thus, the location of Smad3 responsiveness within the FSHβ promoter was determined on the same FSHβ promoter truncations used in the analysis of caALK4 and caALK7. Significant decreases in fold induction by Smad3 overexpression were identified between −1500 and −1000, −398 and −304, and −304 and −230, all of which overlap with sites of induction by caALK4 and caALK7 (Fig. 1C). The largest decrease in induction, from 12-fold to 3-fold, is observed when the region between −398 and −304 is truncated (Fig. 1C). There is also a significant attenuation of induction between −304 bp and −230 bp (from 3-fold to 1.7-fold), the region that contains the SBE site at −267. Surprisingly, Smad3 induction is significantly diminished with truncation of the −398 and −304 region, despite the fact that there appears to be no full-consensus SBE site within this region. The same region also confers the largest induction by caALK4 and 7. Because the region between −398 and −304 is a novel region required for activin, activin-receptor, and Smad3 response, we focused on it to identify critical elements required for induction.
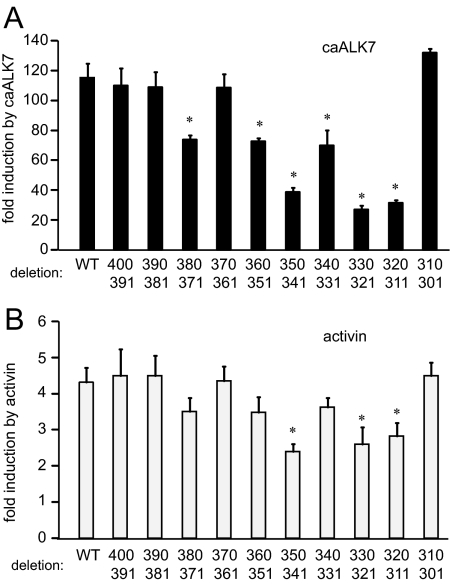
To further refine the areas critical for activin responsiveness and to map sites required for Smad3, ALK7, and activin response in the −398/−304 region, we constructed 10-bp internal deletions and used these in transient transfection assays with overexpression of ALK7 and Smad3, and with activin treatment. We used ALK7 in further experiments for the following reasons: the activity of both ALK4 and ALK7 map to the same regions and they are likely to activate the same pathways; fold induction by caALK7 is higher than fold induction by caALK4, and activin exerts its effects primarily on the autocrine or paracrine levels, making activin B and its receptor more relevant. Deletion of the −350/−341, −330/−321 or −320/−311 regions reduced fold induction by caALK7 and activin (Fig. 2, A and B). The deletions have the same effect on induction by overexpression of Smad3 (Supplemental Fig. 2). Although the −380/−371, −360/−351, and −340/−331 regions also played a role in ALK7 and Smad3 induction, which is possibly due to their proximity and interaction with the −350/−341 segment, there was no significant decrease observed in fold induction by activin. Therefore, the elements at −350/−341, 330/−321, and −320/−311 are strongly implicated in the induction of the FSHβ promoter by activin and activin-signaling components.
Figure 2.
Constitutively active ALK7 and activin activity are localized to the −350/−341, −330/−321, and −320/−311 regions. LβT2 cells were transfected with the 1-kb mouse FSHβ promoter with a 10-bp internal deletions, with the initial 5′-residue of the deletion indicated on the top row and the last 3′-most residue deleted, indicated in the bottom row, under the corresponding bar. Cells were also transfected with caALK7 (A), or treated with 10 ng/ml activin for 5 h (B). *, Significant decrease in fold induction from wild type (WT), P < 0.05.
Analysis of the −350/−341 region of the mouse FSHβ promoter reveals a FoxL2-binding site
To further investigate the regions required for activin induction, EMSAs were performed to determine whether proteins from gonadotrope-derived cells are capable of binding to these areas of the FSHβ promoter. Because these sites confer responsiveness to Smad3, we first assessed whether Smad protein can bind to these regions. However, direct Smad-DNA interactions could not be detected in EMSA with either nuclear extracts from control or activin-treated LβT2 cells or from Cos-1 cells with overexpressed Smad proteins, although Smads do bind our positive control, a probe encompassing the −267 SBE site (data not shown).
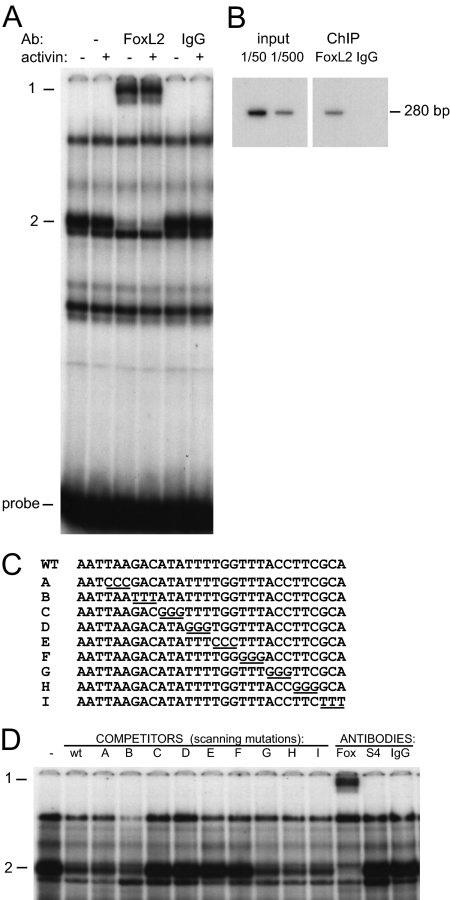
We then performed in silico analysis of the sequences within −350/−341, −330/−321, and −320/−311 using Internet programs for identification of transcription factor-binding sites and identified several putative transcription factor-binding sites for the upstream region. The downstream −330/−321 and −320/−311 regions do not show any known transcription factor-binding sites, although specific complexes from LβT2 cells bind to the probe encompassing this region (data not shown). Therefore, we focused on the −350/−341 region, and performed EMSA using a probe surrounding this region, incubating it with nuclear extracts and antibodies for the putative transcription factors identified by sequence analysis. Inclusion of a FoxL2 antibody resulted in the supershift of a specific band (Fig. 3A). The supershift (dash 1) by the FoxL2 antibody (FoxL2) of the complex indicated with dash 2, occurs in both control and activin-treated cells. The nonspecific antibody, IgG, serves as control. This demonstrates that the endogenous FoxL2 protein in LβT2 cells can bind to the −350/−341 region and that the FoxL2-containing complex doesn’t change after activin treatment. Furthermore, we did not observe a change in the level of FoxL2 protein present in LβT2 cells with activin treatment utilizing Western blotting (data not shown), indicating that activin does not induce FoxL2 protein itself.
Figure 3.
FoxL2 binds to the −350/−341 region of the mouse FSHβ promoter. Panel A, Nuclear extracts from LβT2 cells treated with activin or vehicle control were incubated with the probe, indicated as WT (wild type) in panel C, encompassing the −350/−341 element and an antibody (Ab) for FoxL2 or IgG control. Dash 1 indicates the supershift of the FoxL2 complex (indicated with dash 2) by the addition of anti-FoxL2. Panel B, ChIP of cross-linked chromatin from LβT2 cells using FoxL2 antibody, followed by PCR of the FSHβ promoter proximal region determined that endogenous mouse FoxL2 associates with FSHβ gene in vivo in LβT2 cells. Panel C, Unlabeled oligonucleotides (A–I) that were used in 200-fold excess in EMSA to compete with the radiolabeled probe (WT) for binding nuclear extracts from LβT2 cells. Residues (3 bp) from the WT sequence that are mutated in each competitor are underlined. Panel D, Unlabeled oligonucleotides A–I were used as competitors with radioactively labeled WT oligonucleotide as a probe and are presented above the corresponding lane. Oligonucleotides with mutations that prevent binding of the complex of interest are unable to compete with the WT probe. Antibodies are included in the last three binding reactions (instead of competitors) to determine the identity of the complex. The FoxL2 complex (dash 2) is supershifted (dash 1) with the addition of anti-FoxL2 antibody. Anti-Smad4 and nonimmune IgG are unable to cause a supershift.
To determine whether FoxL2 binds to the FSHβ promoter in vivo, we performed chromatin immunoprecipitation (ChIP) experiments using FoxL2 antibody to precipitate cross-linked, sonicated chromatin from LβT2 cells. After precipitation of the chromatin with FoxL2 antibodies and PCR with oligonucleotide primers specific for the FSHβ proximal promoter, we demonstrated that FoxL2 associates with the FSHβ gene (Fig. 3B).
Sequence analysis revealed a Forkhead site: GTAAACA (or TGTTTAC on the opposite strand, with only 1 bp deletion) in the area of interest presented as wild-type probe −360 to −331 in Fig. 3C. To determine which nucleotides within this larger element are necessary for FoxL2 binding, competition EMSA analysis was performed using a 200-fold excess of unlabeled oligonucleotides containing 3-bp scanning mutations as competitors (Fig. 3C). Residues that were mutated in the competitors to differ from those found in the wild-type probe are underlined. The unlabeled scanning mutation oligonucleotides (A through I, indicated above the corresponding lanes) were incubated with nuclear extracts from LβT2 cells, to compete with the radiolabeled wild-type probe (Fig. 3D). The failure of oligonucleotides to compete indicates that the mutated base pairs are needed for protein binding (dash 2). The supershift with an anti-FoxL2 antibody (Fox) confirms the identity of the protein (dash 1), whereas Smad4 antibody (S4) or control IgG fail to cause any changes in binding complexes. Competitors C–F are not able to compete for FoxL2 binding, indicating that the sequence necessary for FoxL2 binding includes 5′-ATATTTTGGTTT-3′, which is more extensive than the 7-bp site indicated by the on-line database (Fig. 3D).
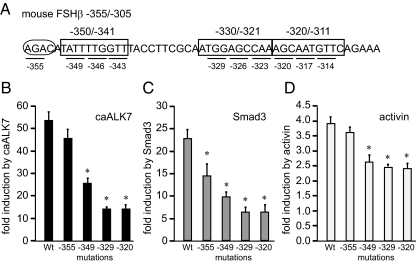
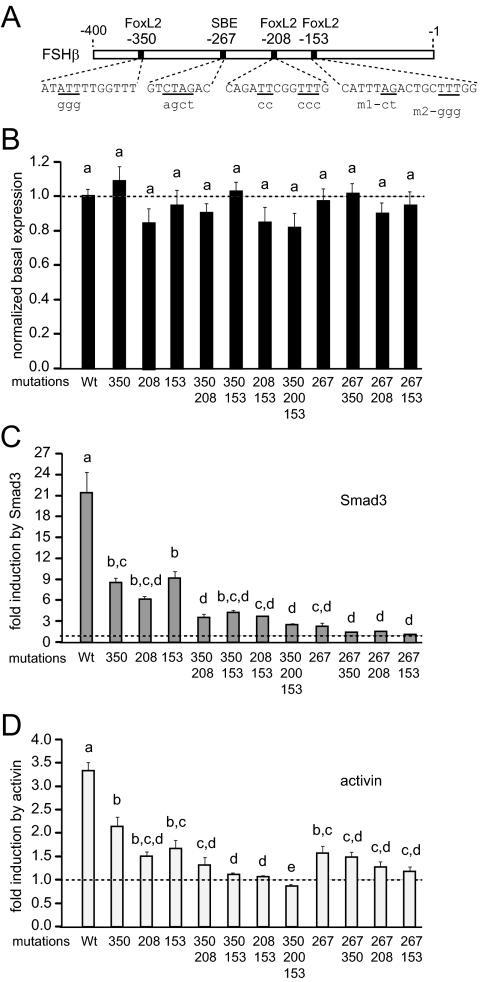
We performed site-directed mutagenesis to assess the functional significance of the three 10-bp regions that were identified as important for activin induction of the FSHβ gene and to delineate the base pairs that are critical for activin responsiveness. Throughout the −350/−341, −330/−321, and −320/−311 regions (boxed in Fig. 4A), 3-bp mutations were created as indicated with underlining, and numbered according to the position of the 5′ mutated base pair. We determined that each of the nine 3-bp mutations in the three 10-bp regions causes a decrease in caALK7, Smad3, and activin induction (Fig. 4, B–D). Because all three mutations within each of the 10-bp regions had the same effect, we present only one 3-bp mutation per region for simplicity. For example, mutation of three residues starting with −349 had the same decrease in fold induction as 3-bp mutations starting with −346 and −343. Induction by constitutively active ALK7 decreases 50–80% with any of the 3-bp mutations between −350/−341, −330/−321 and −320/−311 (Fig. 4B; 52-fold for wild type vs. 25-fold for mutations throughout the −350/−341 region, vs. 14-fold with mutations throughout the −330/−321 and −320/−311 regions). Similar decreases are observed in response to Smad3 induction (Fig. 4C; from 22.5-fold for wild type to 8.3-fold with mutations throughout the −350/−341 and 5.4-fold, with mutation throughout the −330/−321 and −320/−311 regions). Fold induction by activin also decreases significantly (Fig. 4D; 3.8-fold for wild type, vs. 2.6- and 2.4-fold with mutations of the −350/−341 and −330/−311 regions, respectively). Surprisingly, none of these mutations affects basal transcription of the FSHβ gene (data not shown), only the activin induction. Though the key transcription factor(s) binding in the −330/−321 and −320/−311 regions remain unidentified, these regions clearly play a role in activin induction of the FSHβ gene. It is also interesting that each of the six mutations in the −330/−321 and −320/−311 regions caused similar decreases in responsiveness, implying that there is a relatively large element that encompasses those 18 bp that may be bound by the unidentified protein(s). On the other hand, we were able to show that the −350/−341 region contains a functional FoxL2-binding site, and all 9 bp of the site are critical for FoxL2 binding and activin responsiveness.
Figure 4.
Mutations (3 bp) throughout the −350/−341, −330/−321, and −320/−311 regions decrease induction of the mouse FSHβ by caALK7, Smad3, and activin. A, Mutations (3 bp), indicated with underlines, throughout each of the 10-bp regions (boxed) and a mutation of the adjacent Smad half-site (in the oval), were transfected into LβT2 cells with caALK7 (B), Smad3 (C), or treated with activin (D). The label −349 beneath a bar represents mutated residues at the position −349, −348, and −347, for example. The −346 and −343 3-bp mutations within −350/−341 region were not presented because their effects were the same as with the −349 3-bp mutation. The same is true for mutations throughout the −330/−321 and −320/−311 10-bp regions. *, Statistically significant decrease in fold induction. Wt, Wild type.
Additionally, we created a 3-bp mutation in the Smad half-site, AGAC, indicated with an oval, at −355/−352. Although, there is a small decrease in the fold induction by Smad3 (Fig. 4C), mutation of the −355 Smad half-site does not reduce fold induction by activin or caALK7 (Fig. 4, B and D). This lack of an effect on activin responsiveness is surprising, because a Smad half-site adjacent to a FoxL2 site is necessary for activin responsiveness of the follistatin gene (44). In addition, as demonstrated by competition EMSA, the −355 Smad half-site is not necessary for FoxL2 binding either (Fig. 3D). Thus, FoxL2 binds its site and plays a role in activin responsiveness, without a functional juxtaposed Smad element.
FoxL2 potentiates activin induction of the FSHβ promoter
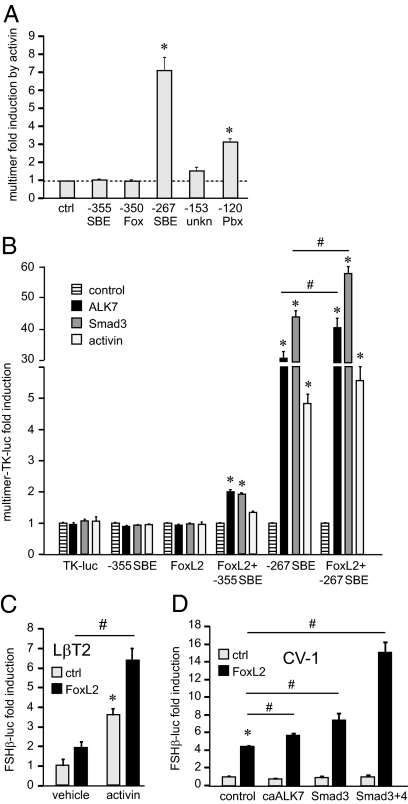
We have shown that FoxL2 regulates the FSHβ promoter through binding to a site at −350 bp from the transcriptional start site. To assess whether this site is sufficient for activin induction, we created multimers that consist of four tandem repeats of the −350 FoxL2/forkhead site (cATATTTTGGTTTa) linked to the heterologous thymidine kinase promoter. Furthermore, we created a multimer of the Smad half-site AGAC (aAGACat) found at −355, because a majority of activin- and TGFβ-responsive promoters contain only half-sites for Smad protein binding. The only promoters that we are aware of that contain full SBEs (GTCTAGAC) are mouse Smad7 (45) and rodent FSHβ (46). Multimers of the −350 FoxL2 site and the AGAC half of the SBE (−355 SBE) were compared with multimers of previously identified sites important for activin responsiveness of the mouse FSHβ promoter: the −267 full consensus SBE (aGTCTAGACt), the −120 site (ctGTCCGTCTaa) that binds Pbx/Prep to recruit Smads, and the −153 site (aTTTAGACTGCTTTg) that binds unknown protein(s) (32,42). The −267 SBE and the −120 Pbx site are each sufficient to confer activin responsiveness to the heterologous promoter, whereas the other sites are not (Fig. 5A). There was a trend in increased induction with the −153 multimer, but it didn’t reach statistical significance, whereas neither the FoxL2, nor the half-SBE are sufficient for responsiveness to activin, strongly indicating that the forkhead sites and Smad half-sites that are present in all activin- and TGFβ-responsive promoters require additional sequences or protein interactions for activin responsiveness.
Figure 5.
Overexpression of FoxL2 potentiates the effects of activin in LβT2 cells and confers activin responsiveness to the FSHβ gene in heterologous cells. A, LβT2 cells were transfected with a minimal heterologous thymidine kinase (TK) promoter (ctrl) or with four tandem copies (multimers) of the individual elements critical for activin responsiveness (indicated under each bar) linked to the TK promoter. The cells were treated with activin for 5 h and results presented as fold induction over vehicle control for each multimer. B, LβT2 cells were transfected with multimers consisting of four tandem repeats of the elements indicated below each group of bars, and contransfected with caALK7 or Smad3 or treated with activin for 5 h. *, Statistically significant induction of the multimer reporter compared with its control; #, significant difference in fold induction between two multimer reporters with the same treatment. C, LβT2 cells were transfected with the 1-kb mouse FSHβ reporter and treated with activin for 5 h. Normalized luciferase values are presented as fold induction from the vehicle-treated cells transfected with the empty vector control for FoxL2 overexpression. Data from empty vector control-transfected cells are represented by gray bars, whereas FoxL2-transfected cells are represented by black bars. *, Significant induction by activin; #, significant increase in fold induction by activin after transfection of FoxL2 by two-way ANOVA. D, CV-1 cells were transfected with empty vector control, caALK7, Smad3, or Smad3 and 4 with (black bars) or without FoxL2 (gray bars) expression vector. *, Significant induction by FoxL2; #, significant change in fold induction or synergy determined by two-way ANOVA after cotransfection of FoxL2 with caALK7, Smad3, or Smad3 and -4 (P < 0.05). ctrl, Control; unkn, unknown.
We additionally created multimers with combinations of the FoxL2 site with its natural adjacent Smad half-site at −355 or with −267 full SBE site, and tested them in transient transfection assays in LβT2 cotransfected with caALK7 or Smad3 or treated with activin. Because we showed that the −267 SBE is sufficient for activin responsiveness, not surprisingly, it is sufficient for robust ALK7 and Smad3 responsiveness as well. Including the FoxL2 site with its adjacent Smad half-site or the full SBE increases their responsiveness (Fig. 5B). Although the induction by activin fails to reach statistical significance, the −355 Smad half-site becomes responsive to ALK7 and Smad3 with the addition of the forkhead element, and the induction of the full −267 SBE by ALK7 and Smad3 is significantly increased with addition of the −350 forkhead site.
To investigate how FoxL2 proteins regulate the expression of the FSHβ promoter, a FoxL2 expression vector was transiently transfected into LβT2 cells with or without activin treatment. Transient transfection of FoxL2 expression vectors into LβT2 cells does not significantly increase basal FSHβ promoter activity as compared with control (Fig. 5C), because LβT2 cells already express high endogenous levels of FoxL2. However, transfection of FoxL2 in activin-treated cells increases induction of the FSHβ gene from 3.6-fold by activin alone to 6.8-fold with the combination, representing a significant increase as determined by two-way ANOVA. Thus, FoxL2 potentiates activin induction in LβT2 cells.
The CV-1 cell line, a heterologous fibroblast cell line that does not express endogenous FoxL2, was used in subsequent experiments to assess the effect of FoxL2 overexpression together with caALK7 or Smad proteins. caALK7, Smad3, or Smad3 and Smad4 overexpression in CV-1 cells did not induce the FSHβ promoter, whereas transfection of FoxL2 induces it 4.5-fold compared with empty vector control (Fig. 5D). Significantly, cotransfection of FoxL2 together with caALK7, Smad3, or Smad3 and Smad4 potentiates induction by FoxL2 alone (Fig. 5D, black bars). FSHβ promoter activity increased from 4.5- to 5.7-, 7.4-, or 15.1-fold when FoxL2 was cotransfected with caALK7, Smad3, or Smad3 and 4, respectively. Therefore, FoxL2 expression can potentiate the effects of activin receptor or Smad proteins on the FSHβ promoter. Furthermore, FoxL2 confers Smad and activin responsiveness of the FSHβ gene to heterologous cells.
Localization of FoxL2 induction reveals additional FoxL2-responsive regions
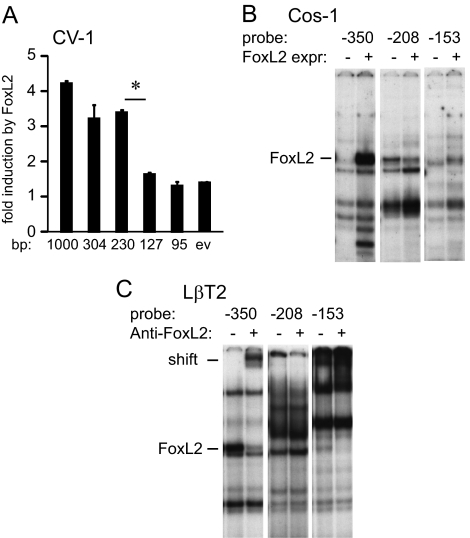
Because mutation of the −350 FoxL2 site reduced fold induction by FoxL2 (34% decrease) in CV-1 cells (data not shown), but did not completely prevent it, we postulated the existence of additional FoxL2 sites in the mouse FSHβ promoter. To determine whether there were additional FoxL2 sites, truncation analysis was performed in CV-1 cells with overexpressed FoxL2. Complete loss of induction occurs when the region from −230 bp to −127 bp is truncated, upon which the induction by FoxL2 is lost (Fig. 6A). We concentrated on this region because we hypothesized that it would contain additional FoxL2 sites. Sequence analysis of the −230/−127 region reveals two putative FoxL2-binding sites, −208 and −153. To test whether these putative sites can bind FoxL2 in vitro, EMSAs were performed with probes encompassing these regions. Radiolabeled probes were incubated with either nuclear extract from Cos-1 cells containing overexpressed FoxL2 (FoxL2 expr) and compared with cells transfected with vector control (Fig. 6B) or with nuclear extracts from LβT2 cells with or without inclusion of the FoxL2 antibody (anti-FoxL2) to identify any FoxL2-containing complexes (Fig. 6C). The FoxL2 overexpressed in Cos-1 cells exhibits binding, indicated with a dash, to a probe encompassing the −153 bp site, which is not present in the control lane, in addition to binding to the previously identified −350 site. FoxL2 from LβT2 cells also binds both the −153 and −350 probes and is supershifted upon addition of a FoxL2 antibody, again indicating that FoxL2 protein interacts with these sites. We were unable to determine whether FoxL2 binds the −208 site, due to a comigrating band, and attempts to resolve the complexes were unsuccessful. FoxL2 binding to the −153 site occurs at a lower affinity than binding to the −350 site, but it is clear that FoxL2 binds this element that we previously identified as critical for activin induction (32,42). Thus, multiple elements within the FSHβ promoter bind FoxL2.
Figure 6.
FoxL2-binding sites in the −230/−127 region of the mouse FSHβ promoter. A, CV-1 cells were transfected with different lengths of the mouse FSHβ promoter indicated under the corresponding bar, with the FoxL2 expression vector or empty vector (ev) control. Fold induction by FoxL2 overexpression over empty vector control for each truncation are represented. *, Significant decrease in fold induction with P < 0.05. B, Probes encompassing the FoxL2-binding elements were incubated with nuclear extracts from Cos-1 cells transfected with FoxL2 (FoxL2 expr.) or ev control (−). C, Probes encompassing putative FoxL2-binding elements were incubated with nuclear extracts from LβT2 with or without inclusion of the FoxL2 antibody (Anti-FoxL2).
To test whether the −208 and −153 FoxL2 sites are also essential for FSHβ promoter induction, site-directed mutagenesis was performed, and mutations were tested in transient transfections. Wild-type sequences are listed in uppercase letters, and mutated residues are presented below in lowercase letters (Fig. 7A). At the −153 site, we created two different mutations: m1 mutates the AGAC-putative Smad site that was studied previously when the importance of the −153 site was originally established, but binding of Smad proteins was not observed (42), and m2 mutates three T-residues to mimic the mutations that were created in the other FoxL2 sites. Both m1 and m2 had the same effects (data not shown), so only data from m2 are shown (noted as 153 in Fig. 7, B–D). A 4-bp mutation of the −267 full Smad consensus, an established full consensus Smad site important for activin responsiveness (35,36,38), was also included, to compare its effects directly with the newly identified FoxL2 sites. None of the mutations significantly affect basal expression of the promoter (Fig. 7B). The mutations at −208 and −153 significantly reduce induction of the mouse FSHβ promoter by Smad3 and activin treatment (Fig. 7, C and D), as well as by overexpression of caALK7 (Supplemental Fig. 3). Surprisingly, the effects of mutating even single FoxL2 sites on activin responsiveness are as great as the effects of mutating the −267 Smad element, a known player in activin induction of the FSHβ gene (Fig. 7D). Because the −267 element is a full Smad consensus, it is not surprising that its mutation has a larger effect on induction by Smad3, but it is surprising that the −350, −208, and −153 FoxL2 elements also play a role in induction by Smad3, especially in the light of the fact that FoxL2 sites do not confer activin induction to a heterologous promoter and that, contrary to the follistatin promoter, the Smad element juxtaposed to the FoxL2 site does not play a role in FoxL2 binding or activin induction. The −208 and −153 FoxL2 site mutations have a larger effect on activin induction than the −350 mutation (Fig. 7D), although these sites have lower affinity for FoxL2 binding than the −350 site (Fig. 6, B and C), implying that these sites have a greater role in activin responsiveness of the mouse FSHβ gene.
Figure 7.
The FoxL2 elements at −350, −208, and −153 are necessary for full FSHβ promoter response to caALK7 and activin. A, Location of the FoxL2 sites, identified by sequence analysis and confirmed by EMSA, on the mouse FSHβ promoter. Wild-type sequence is represented by uppercase letters, residues that were mutated are underlined, and mutations are listed in lowercase underneath. Two different mutations (m1 and m2) of the −153 site were created, but had the same effect; thus, only results with m2 are presented. B, The FoxL2 sites were mutated and the activity of mutant reporters compared with the activity of the wild-type reporter to assess the effect of mutations on basal gene expression. C and D, The effects of the mutations of the FoxL2 sites individually or in combination, and in comparison with the mutation of the −267 Smad response element, were analyzed in response to overexpression of Smad3 (C), or activin treatment (D). Bars not connected by the same letters are significantly different, P < 0.05.
We then created double and triple mutations of these sites to assess whether they can compensate for each other or interact. We determined that double mutations had additive effects in reducing the Smad3 and activin responsiveness of the promoter (Fig. 7, C and D). Double mutation of any pair of FoxL2 sites caused a complete loss of FSHβ induction by activin. Double mutation of the −267 Smad site and each of the FoxL2 sites (especially the −208, which has the largest effect on induction by caALK7, Smad3, and activin; see also Supplemental Fig. 3) was not significantly different than single mutation, indicating that without the Smad site, an individual FoxL2 mutation does not reduce responsiveness further, and vice versa. Thus, without FoxL2 sites, the FSHβ gene is not responsive to activin, despite the fact that FoxL2 mutations do not decrease basal expression and FoxL2 elements do not act as individual activin-response elements on a heterologous promoter, and despite the presence of the functional Smad sites.
The human FSHβ promoter also requires FoxL2 sites for activin induction
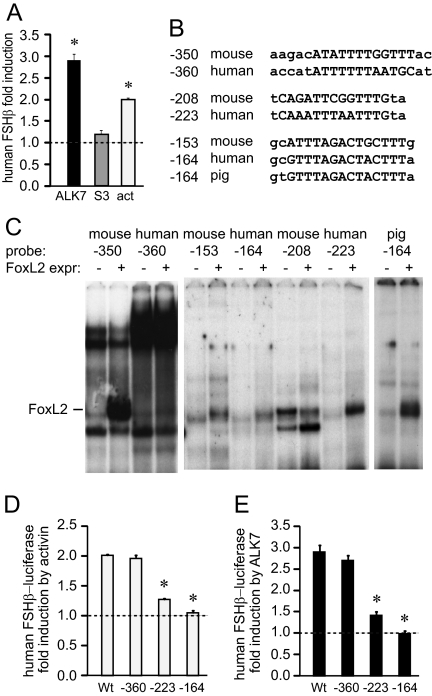
Sequence analysis of the human FSHβ promoter shows strong homology to the mouse FSHβ promoter in the proximal 350 bp from the transcriptional start site, except that the human promoter lacks the consensus SBE located at −267 of the rodent genes. Although the human FSHβ promoter does not respond to cotransfection with Smads (Fig. 8A and Ref. 47) due to the lack of the consensus SBE, and is less responsive to activin, some responsiveness to activin and its receptor is maintained (Fig. 8A), yet the elements responsible are not known. We analyzed the conservation of the homologous elements in the mouse and human promoters at the forkhead elements (Fig. 8B). The high-affinity FoxL2-binding site at −350 in the mouse promoter comprises the sequences shown in uppercase that were identified in Fig. 4D as necessary for binding. The additional 5 bp on the 5′-end, written in lowercase, are presented to show that this forkhead site in the mouse promoter is adjacent to the Smad half-site “AGAC”. However, mutation of the −355 Smad half-site affects neither activin responsiveness of the mouse FSHβ gene (Fig. 4D) nor the ability of FoxL2 to bind its site (Fig. 3D). An additional 2 bp on the 3′-end are presented because they align with the forkhead consensus TGTTTAG listed by the Consite and Jaspar programs. The human sequence has only 7 of 12 bp in common with the mouse sequence at this core FoxL2 element.
Figure 8.
FoxL2 elements at −223 and −164 of the human FSHβ gene that are homologous to −208 and −153 in the mouse are critical for activin (act) responsiveness of human FSHβ gene. A, The −1029 human FSHβ promoter linked to the luciferase reporter was tested in transient transfection for its responsiveness to caALK7 or Smad3 overexpression or 50 ng/ml activin treatment for 24 h. Results are presented as fold induction from control; *, statistical significance. B, Sequence alignment of identified FoxL2 elements in the mouse and human FSHβ genes. The pig −164 sequence was added after publication of the report that FoxL2 binds this site (47). C, Comparison of human and mouse elements in binding efficiency for FoxL2, which was overexpressed in Cos-1 cells. Probes encompassing putative FoxL2 elements are indicated above corresponding lanes. Presence of the FoxL2 expression vector (FoxL2 expr.), as opposed to the empty vector control, is indicated with (+), whereas a dash indicates the location of the FoxL2-containing complex. The pig −164 probe was added after publication of the report that FoxL2 binds this site (47), for comparison of binding affinities. D and E, Mutations of the −360, −223, and −163 elements in the human FSHβ promoter (mouse equivalent elements at −350, −208, and −153) were created and transfected into LβT2 cells with caALK7 (D) or treated with activin for 24 h (E) and compared with wild type. *, Significant decrease in fold induction from the wild-type reporter with P < 0.05 for the −223 and −164 mutations.
In contrast, the downstream FoxL2 sites, at −208 and −153, are highly conserved between the mouse and human FSHβ promoters, with 12 of 16 bp and 15 of 17 bp in common, respectively. While this manuscript was in preparation, Lamba et al. (48) showed that FoxL2 binds the porcine promoter at a site homologous to the mouse −153 site, and, thus, we also present the porcine sequence for the −153 homologous site for comparison.
Because elements that contribute to activin responsiveness of the human gene are not known and the human gene lacks the consensus SBE, it was important to examine whether FoxL2 can contribute to activin induction of the human FSHβ promoter. EMSAs were used to analyze whether FoxL2 proteins are capable of binding to the human FSHβ probes encompassing homologous regions to those identified in the mouse gene. Nuclear extracts from Cos-1 cells with overexpressed FoxL2 were used to compare complexes with nuclear extracts from cells transfected with vector control. FoxL2 neither binds the human site (human −360) homologous to the mouse element at −350 (Fig. 8C), nor does addition of a 2-bp mutation to create the homologous Smad half-site in the human sequence confer FoxL2 binding (data not shown); therefore, additional disparities must be responsible for differences in binding. The human −223 site, however, does bind FoxL2 with high affinity, and the human equivalent of the −153 site (human −164) binds FoxL2, with similar low affinity to the mouse −153 site. Thus, both the mouse and human FSHβ promoters have one high-affinity FoxL2-binding site, which in the mouse promoter is the −350 site and in the human is the −223 site, and each have another low-affinity binding site at −153 of the mouse sequence (−164 in human). We compared binding of the FoxL2 sites and support the previous report that the porcine equivalent of the mouse −153 site has a much higher affinity for FoxL2 than the mouse −153 element, but contrary to that report, we show that FoxL2 is able to bind the mouse and human −153 sites. Thus, the mouse and human −153 sites are lower affinity binding sites, when compared with the −350 site in the mouse or −223 in the human, or −153 in the porcine promoter, but both the mouse −153 and the homologous human −164 site bind FoxL2, nevertheless (Fig. 8C).
Because the homologous sites for −208 and −153 in the human promoter bind FoxL2 protein in EMSAs, it was important to test whether these newly discovered FoxL2 sites are required for activin responsiveness of the human FSHβ promoter. Thus, mutations were created in the equivalent of the −350, −208, and −153 sites in the human FSHβ promoter (−360, −223, and −164, respectively), and their responsiveness to activin (Fig. 8D) and caALK7 (Fig. 8E) induction of the human FSHβ gene was tested. Mutation of the −360 FoxL2 site does not have a significant effect on either activin or ALK7 induction of human FSHβ, consistent with a lack of FoxL2 binding to this site in the human gene. However, either the −164 or the −223 mutation can completely eliminate activin and ALK7 responsiveness of the human FSHβ promoter. Thus, the FoxL2 sites at −164 and −223 are required for activin responsiveness of the human FSHβ promoter and currently comprise the only elements known to be required for activin responsiveness thus far identified in the human FSHβ promoter.
Therefore, the FoxL2 transcription factor plays a critical role in activin induction of both the human and mouse FSHβ genes. The FoxL2 element does not confer activin responsiveness itself; however, it is strictly required for activin action on the FSHβ promoter. Contrary to previous reports, FoxL2 binds and functions through its site, without the necessity for the juxtaposed SBE and does not serve solely to tether Smad proteins to the promoter, as previously suggested, because FoxL2 sites are not sufficient for activin responsiveness. More importantly, both the Smad-dependent mouse gene and the Smad-independent human gene require FoxL2 for responsiveness. Although the exact position of the FoxL2-binding sites differs among species, FoxL2 exerts its effect through binding to multiple FoxL2 elements within the FSHβ promoter in each species.
Discussion
In this study, we identify FoxL2 as a critical participant in activin induction of the mouse and human FSHβ genes in pituitary cells. FoxL2 is a member of the forkhead family of transcription factors that shares a conserved DNA-binding domain (49). FoxL2 has important roles in reproductive function. Blepharophimosis Ptosis Epicanthus Inversus Syndrome is due to a human FoxL2 mutation and causes premature ovarian failure (50), whereas FoxL2 null mice exhibit disruption of granulosa cell differentiation and failure of oocyte growth (51). FoxL2 has cell-specific expression in the pituitary, where it is restricted to cells of the gonadotrope and thyrotrope lineages and colocalizes with FSH (44); thus, some of the reproductive effects of these mutations could be due to effects in gonadotropes as well.
Truncations of the mouse FSHβ promoter result in significant decreases in activin induction in a step-wise manner, indicating that there are multiple sites crucial for activin response throughout the 5′ 1.5 kb of regulatory sequence (9). Elements required for activin response of the mouse FSHβ promoter have been identified at −267, which contains a full consensus SBE at −153, which binds previously unknown proteins, and at −120, which binds Pbx/Prep homeodomain proteins that recruit Smads (32,42). But these elements do not fully account for activin induction of the FSHβ gene. In fact, the largest response to cotransfection with constitutively active activin receptors or Smad3 in LβT2 cells localizes to the −398/−304 region, where none of the previously identified sites are located. Fine resolution mapping and EMSA in this region reveals the presence of a binding site for FoxL2 at −350/−341, and cis mutations demonstrate its necessity for activin induction. In CV-1 cells, which do not contain FoxL2, the majority of FoxL2 responsiveness localizes between −230 and −127, which led us to identify two additional FoxL2 sites at −208 and −153. Interestingly, mutation of any of the three sites decreases induction, and combinations of mutations abrogate induction, indicating that all three sites are important for activin response. Previously, we had established a requirement for the −153 element in activin responsiveness, but the associated proteins remained unknown (42). Herein, we determined that the −153 site binds FoxL2 protein, although at weaker affinity than to the homologous site in the porcine promoter (48). The −208 FoxL2 element, which has an even larger effect, is a newly identified site required for activin induction of the murine FSHβ promoter.
Although there is sequence homology between the mouse and human promoters at the −350 site, FoxL2 does not bind the human promoter at this site, whereas it binds the mouse with relatively high affinity. Furthermore, mutations in the human promoter at the equivalent of the −350 site (−360) have no effect, whereas mutations of the −208 or −153 elements (homologous −223 and −164 in the human promoter) result in a significant decrease in activin induction, indicating that these sites are important for maintaining a full activin response. Upon further analysis of the −350 FoxL2 site and identification of the −153 and −208 sites, we postulate that two repetitive stretches of three thymidines (TTT) (Fig. 8B) are important for FoxL2 binding, and the human −350 site lacks the second 3′-TTT repeat. The requirement for the TTT repeats may explain a previous report that FoxL2 can homodimerize (48). The residues around TTT may contribute to binding affinity, giving the porcine −153-equivalent site higher affinity than the −153 site in the mouse or human promoters. Similarly, the human −223 (−208 equivalent in the mouse) has two sections of TTT, whereas the mouse has only two TT in the 5′-repeat, which may contribute to the higher affinity of the human −223 site. Unfortunately, the affinity of the mouse and human −153 sites was too low to determine the exact base pairs using scanning mutations as competitors in EMSA. However, scanning mutation analysis determined that residues ATATTTTGGTTT were necessary for FoxL2 binding to the −350 site in the mouse promoter and confirms this hypothesis. This is more extensive than the previously published 7-bp forkhead site.
Using FoxL2 antibody in ChIP, followed by PCR with primers spanning FSHβ proximal promoter, we determined that FoxL2 binds this region in vivo. Because all three FoxL2 sites, identified by EMSA, are in the proximal region of the promoter within 200 bp, we are not able to distinguish the sites with the ChIP assay; however we were able to compliment this in vivo assay with the binding we observed in EMSA in vitro, to establish FoxL2 interactions with the individual FoxL2 sites in the FSHβ gene.
FoxL2 has been shown to have roles in regulation of the follistatin, GnRH receptor, α-gonadotropin subunit (α-GSU), and porcine FSHβ genes in gonadotrope cells (44,48,52). In the follistatin gene, FoxL2 constitutively binds the forkhead element and is required for responsiveness to activin even in heterologous cells. FoxL2-mediated induction of the follistatin gene is dependant on the adjacent Smad half-site, and FoxL2 protein is a Smad3 binding partner (44). In GnRH receptor gene expression in the gonadotrope, FoxL2 acts as part of a large complex with Smad3 and Smad4 (52). FoxL2 also mediates activin induction of the porcine FSHβ gene through a conserved element corresponding to the mouse −153 site, although binding to the homologous sites in the mouse and human promoters was not detected in this report (48). The role of FoxL2 in porcine FSHβ activin induction is Smad dependant, and FoxL2 interacts with Smads, in addition to homodimerization with itself (48). Interaction with Smads has also been demonstrated for other forkhead family members, such as FoxH1 and FoxO (52,53,54).
In this report, we identify FoxL2 sites as key elements without which activin and Smads cannot exert induction of the murine FSHβ gene and a key component for activin induction of the human FSHβ gene as well, although it is not responsive to Smads (47). FoxL2 and Smad3 have been shown to interact to recruit Smad3 to the follistatin promoter (44). Similar to the role of FoxL2 in follistatin gene expression, we observed that FoxL2 is required for the effects of activin, Smads, and ALK7. Further, in CV-1 cells, FoxL2 plays an important role in facilitating activin induction of FSHβ transcription, because lack of FoxL2 in nongonadotrope cells causes the FSHβ promoter to be nonresponsive to ALK7 or Smad induction. However, contrary to follistatin (44) or porcine FSHβ (48) gene expression, the role of FoxL2 in mouse and human FSHβ expression and binding to the mouse FSHβ gene is not dependant on the adjacent Smad half-site. Furthermore, although the human FSHβ gene is not responsive to Smads (Fig. 8A and Ref. 47), two FoxL2 sites are critical for activin and caALK7 induction of this gene. Thus, FoxL2 may exert its function in activin responsiveness on the mouse FSHβ gene through interaction with activated Smad3 binding at a distant element such as the −267, or it may facilitate activin responsiveness via changing overall chromatin structure allowing Smad actions. In the case of the human FSHβ gene, FoxL2 may be activated by activin signaling without interaction with Smads to cause activin induction.
Although the FoxL2 sites are required for activin induction, they are not sufficient for activin or even Smad induction. Thus, these remarkable sites are all required for activin responsiveness, yet play no role in basal expression of FSHβ, nor can they confer activin response to a heterologous promoter without the addition of an SBE. Thus, they cannot act as activin-response elements, yet they are required to allow for activin action on the FSHβ promoter, because in their absence, even the −267 consensus SBE is incapable of responding to activin or indeed to Smad cotransfection. This is in contrast to the −120 Pbx/Prep element, which, although required for activin induction, can confer activin response to a heterologous promoter and can recruit Smads to the promoter. Furthermore, the intact FSHβ promoter containing a full consensus SBE still cannot respond to activin or Smad3 in cells that do not express FoxL2, and cotransfection of FoxL2 can restore this responsiveness. FoxL2 binding to the FSHβ promoter does not change with activin treatment as compared with control cells (Fig. 3A). Additionally, the level of FoxL2 protein in LβT2 cells does not change after activin treatment, as indicated by Western blotting (data not shown). FoxL2 may be activated by recruitment of activated Smads to the promoter, for the murine FSHβ promoter that is Smad responsive, but not to the FoxL2 sites themselves, or they would be sufficient for activin action on a heterologous promoter. Alternatively, there is evidence that in ovarian follicles, FoxL2 can be phosphorylated through interaction with LATS1 kinase (55), and this may affect its transcriptional activity. Future studies will determine whether this kinase is present in gonadotropes, and whether it is activated by activin treatment. Furthermore, when phosphorylation-specific antibodies for FoxL2 are available, it will become possible to assess whether FoxL2 is phosphorylated after activin treatment in gonadotrope cells.
We show that FoxL2 has a critical role in both mouse and human FSHβ gene regulation by activin and that mutations of FoxL2 sites render both genes nonresponsive to activin. In the mouse gene, the FoxL2 site may interact with the more distant full-consensus SBE at −267, because the responsiveness of that site was augmented with the addition of the FoxL2 site. On the contrary, in the human promoter, activin induction is Smad independent, and the FoxL2 sites are the only sites required for activin response identified to date. Interaction with Smads may account for the rapid induction of the FSHβ gene during the rodents’ 4-d estrous cycle and the rapid secondary increase that occurs only hours later than the primary surge. Lack of FoxL2 interaction with Smads may account for the slower response of the human promoter to activin signaling both in culture, where it requires 24 h of treatment, and in vivo during the 28-d menstrual cycle.
In summary, we determine that FoxL2 is required for activin regulation and identify multiple FoxL2-responsive elements required for FSHβ induction. In the mouse promoter, FoxL2 binds to the −350 site with high affinity and binds to the −153 site with lower affinity. In human, FoxL2 binds to the −223 site with high affinity and the −164 site with lower affinity. FoxL2 sites are necessary for activin responsiveness of both mouse and human genes, and addition of the FoxL2 protein confers activin responsiveness to FSHβ in heterologous cells. Thus, FoxL2 is a critical component of FSHβ induction by activin.
Materials and Methods
Cell culture and transient transfections
The expression vectors for Smad3 and Smad4 were kindly provided by Drs. J. Massague and R. Derynck, respectively. The constitutively active ALK4 and ALK7 plasmids were kindly provided by Drs. Heldin and Dijke. The FoxL2 expression vector was a gift from Dr. Veitia, and the −1028 bp human FSHβ promoter linked to a luciferase-reporter was kindly provided by Dr. D. Bernard. The mouse FSHβ-luciferase reporter vectors were published previously (9,27).
LβT2 cells, CV1, or Cos-1 cells were cultured at 37 C in DMEM (Cellgro, Mediatech, Inc., Herndon, VA) containing 10% fetal bovine serum (Omega Scientific, Inc., Tarzana, CA) and penicillin. Cells were split into 12-well plates 1 d before transfection and transfected using Fugene 6 reagent (Roche Molecular Biochemicals, Indianapolis, IN) in accordance with the manufacturer’s protocol. Wells were transfected with 500 ng of either the mouse or human FSHβ-luciferase reporter plasmids, 100 ng of the β-galactosidase reporter plasmid driven by the Herpes virus thymidine kinase promoter to serve as an internal control for transfection efficiency, and 200 ng of expression vectors: FoxL2, Smad3, Smad4, caALK7, caALK4, or control, as indicated in the figure legends. Cells were incubated in serum-free DMEM containing 0.1% BSA and antibiotics overnight before hormone treatment with 10 ng/ml activin (Calbiochem, La Jolla, CA). Subsequent to treatment, cells were washed with 1× PBS and lysed with 60 μl of a 0.1 m K-phosphate buffer at pH 7.8 containing 0.2% Triton X-100. A 96-well luminometer plate was loaded with 20 μl of each of the lysates, and luciferase activity was measured after injection of a buffer containing 100 mm Tris-HCl with pH 7.8, 15 mm MgSO4, 10 mm ATP, and 65 μm luciferin per well, using a Veritas Microplate Luminometer (Turner Biosystems, Sunnyvale, CA). The Galacto-Light Assay (Tropix, Bedford, MA) was performed according to the manufacturer’s protocol and used to measure galactosidase activity. All experiments were performed a minimum of three times and in triplicate within each experiment. Luciferase values were normalized to β-galactosidase and were expressed as fold induction relative to empty vector pGL3 luciferase reporter activity. Statistical significance was determined with ANOVA and significance was set at P ≤ 0.05 represented by an asterisk.
Mutagenesis
Mutagenesis was performed using the QuikChange Site- Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer’s protocol. All mutagenesis in the FSHβ promoter was conducted using the mouse 1-kb promoter and/or the 1028-bp human promoter. Exact mutations of the residues in the mouse promoter are presented with lower case letters in Fig. 8A, and homologous residues were mutated in the human promoter. Mutations were confirmed via dideoxyribonucleotide sequencing performed by the DNA Sequencing Shared Resource at the University of California, San Diego, Moores Cancer Center. Truncations consisting of the different lengths of the promoter linked to the luciferase reporter were created as previously described (9,22) and also confirmed by sequencing.
ChIP assay
Proteins in LβT2 cells were cross-linked to DNA by addition of 1% formaldehyde directly to the cell medium and, after obtaining the nuclear fraction, chromatin was sonicated to an average length of 500 bp in sonication buffer (50 mm HEPES, pH 7.9; 140 mm NaCl; 1 mm EDTA; 1% Triton X-100; 0.1% sodium deoxycholate; 0.1% sodium dodecyl sulfate). Protein-DNA complexes were bound overnight to FoxL2 antibodies, and precipitated with protein A beads (Amersham Pharmacia, Piscataway, NJ). After extensive washing [twice each with sonication buffer defined above, high-salt sonication buffer (500 mm NaCl with other components as defined above), lithium chloride buffer (20 mm Tris, pH 8; 250 mm LiCl; 1 mm EDTA; 0.1% Nonidet P-40; 0.1% sodium deoxycholate), and Tris-EDTA buffer, cross-linking was reversed by addition of 300 mm NaCl and incubation at 65 C, and proteins were digested by incubation with Proteinase K. DNA was phenol-chloroform extracted and ethanol precipitated, and the sequence of interest was amplified by PCR. Primers used in PCR were 5′-GGTGTGCTGCCATATCAGATTCGG-3′ and 5′-GCATCAAGTGCTGCTACTCACCTGTG-3′ and spanned the 280-bp sequence in the mouse FSHβ gene from −223 to +57. The specificity of the product was assessed by the presence of a single band of the expected size on an ethidium bromide-stained agarose gel. For visualization, the PCR product was labeled by including [α32P]dATP in the nucleotide mix used in PCR, and run on a 5% acrylamide gel in 0.5×Tris-borate, EDTA buffer. The gels were dried and subjected to autoradiography.
EMSA
Cos-1 cells were transfected with 5 μg FoxL2, Smad3, or Smad4 expression vectors using Fugene 6 reagent (Roche Molecular Biochemicals) in accordance with the manufacturer’s protocol. Nuclear extracts were obtained, 48 h after transfection of Cos-1 cells or 2 h after activin treatment of LβT2 cells, by swelling the cells with hypotonic buffer containing the following: 20 mm Tris, pH 7.4; 10 mm NaCl; 1 mm MgCl2; 1 mm phenylmethylsulfonyl fluoride; protease inhibitor cocktail (Sigma-Aldrich), 10 mm NaF; 0.5 mm EDTA; 0.1 mm EGTA. Cells were broken by passing through a 255/8 G needle, three times. Samples were centrifuged at 4000 rpm for 4 min, and the nuclear pellets were resuspended in hypertonic buffer containing: 20 mm HEPES, pH 7.8; 20% glycerol; 420 mm KCl; 1.5 mm MgCl2; 1 mm phenylmethylsulfonyl fluoride; protease inhibitor cocktail (Sigma-Aldrich); 10 mm NaF; 0.5 mm EDTA; and 0.1 mm EGTA. After incubation on ice for 20 min., samples were centrifuged at 10,000 rpm for 10 min, and the supernatants were aliquoted and frozen until use. Protein determination was performed using the Bradford reagent (Bio-Rad Laboratories, Inc., Hercules, CA). The following oligonucleotides were used as 30-bp probes, named according to the 5′-location of the site of interest that they encompass: mouse −350, AATTAAGACATATTTTGGTTTACCTTCGCA; mouse −208, CATATCAGATTCGGTTTGTACAGAAACCAT; mouse −153, CTCTGTGGCATTTAGACTGCTTTGGCGAGG; and homologous human sequences. Oligonucleotides were annealed and labeled with γ32P ATP using T4 Polynucleotide Kinase (New England Biolabs, Inc., Beverly, MA). Binding reactions contained 2 μg of nuclear proteins in a total volume of 20 μl containing the following: 10 mm HEPES, pH 7.8; 50 mm KCl; 0.5 mm MgCl2; 10% glycerol; 0.1% Nonidet P-40; 0.25 μg deoxyinosinic deoxycytidylic acid; 5 mm dithiothreitol; and 5 fmol of labeled probe. Competition and antibody shift assays were performed using 200-fold excess of unlabeled oligonucleotide or 1 μg antibody, respectively. Reactions were loaded onto a 5% nondenaturing polyacrylamide gel and ran in 0.25× Tris-borate-EDTA buffer. Gels were run at 250 V/cm2 constant voltage and dried. Autoradiography was performed to identify complexes.
Supplementary Material
Acknowledgments
We thank Drs. J. Massague (Memorial Sloan-Kettering Cancer Center) and R. Derynck (University of California, San Francisco, CA) for Smad3 and Smad4 expression vectors; Drs. Heldin and Dijke (Ludwig Institute for Cancer Research, Uppsala, Sweden) for the constitutively active ALK4 and ALK7 plasmids; Dr. Veitia (INSERM, Paris, France) for the generous gift of the FoxL2 expression vector; and Dr. Daniel Bernard (McGill University, Montreal, Canada) for the −1028-bp human FSHβ promoter linked to a luciferase-reporter. We also thank Drs. Kellie Breen Church and Rachel Larder (University of California, San Diego, CA) for reading the manuscript and offering thoughtful suggestions.
Footnotes
This work was supported by National Institutes of Health Grants R01 HD057549, R21 HD058752, and R03 HD054595 (to D.C.); and R01 HD020377, R01 DK044838, and National Institutes of Child Health and Human Development/National Institutes of Health cooperative agreement (U54 HD12303) as part of the Specialized Cooperative Centers Program in Reproduction Research (to P.L.M.). L.L. was supported by the Howell Foundation and The Endocrine Society Student Research Fellowships.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 16, 2010
Abbreviations: ActR, Activin receptor; ALK, activin receptor-like kinase; caALK, constitutively active ALK; ChIP, chromatin immunoprecipitation; SBE, Smad-binding element; Smad, Sma- and Mad-related protein.
References
- Kaiser UB, Jakubowiak A, Steinberger A, Chin WW 1997 Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology 138:1224–1231 [DOI] [PubMed] [Google Scholar]
- Papavasiliou SS, Zmeili S, Khoury S, Landefeld TD, Chin WW, Marshall JC 1986 Gonadotropin-releasing hormone differentially regulates expression of the genes for luteinizing hormone α and β subunits in male rats. Proc Natl Acad Sci USA 83:4026–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM 1997 Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204 [DOI] [PubMed] [Google Scholar]
- Lamminen T, Jokinen P, Jiang M, Pakarinen P, Simonsen H, Huhtaniemi I 2005 Human FSH β subunit gene is highly conserved. Mol Hum Reprod 11:601–605 [DOI] [PubMed] [Google Scholar]
- DePaolo LV, Hirshfield AN, Anderson LD, Barraclough CA, Channing CP 1979 Suppression of pituitary secretion of follicle-stimulating hormone by porcine follicular fluid during pro-oestrus and oestrus in the rat: effects on gonadotrophin and steroid secretion, follicular development and ovulation during the following cycle. J Endocrinol 83:355–368 [DOI] [PubMed] [Google Scholar]
- Ortolano GA, Haisenleder DJ, Dalkin AC, Iliff-Sizemore SA, Landefeld TD, Maurer RA, Marshall JC 1988 Follicle-stimulating hormone β subunit messenger ribonucleic acid concentrations during the rat estrous cycle. Endocrinology 123:2946–2948 [DOI] [PubMed] [Google Scholar]
- Christin-Maitre S, Taylor AE, Khoury RH, Hall JE, Martin KA, Smith PC, Albanese C, Jameson JL, Crowley Jr WF, Sluss PM 1996 Homologous in vitro bioassay for follicle-stimulating hormone (FSH) reveals increased FSH biological signal during the mid- to late luteal phase of the human menstrual cycle. J Clin Endocrinol Metab 81:2080–2088 [DOI] [PubMed] [Google Scholar]
- Hoak DC, Schwartz NB 1980 Blockade of recruitment of ovarian follicles by suppression of the secondary surge of follicle-stimulating hormone with porcine follicular field. Proc Natl Acad Sci USA 77:4953–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL 2007 p38 mitogen-activated kinase is critical for synergistic induction of the FSH β gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol 21:3071–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray VG, Mellon PL 2008 Synergistic induction of follicle-stimulating hormone β-subunit gene expression by gonadal steroid hormone receptors and Smad proteins. Endocrinology 149:1091–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta O, Guillemin R 1986 Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin. Nature 321:779–782 [DOI] [PubMed] [Google Scholar]
- Weiss J, Guendner MJ, Halvorson LM, Jameson JL 1995 Transcriptional activation of the follicle-stimulating hormone β-subunit gene by activin. Endocrinology 136:1885–1891 [DOI] [PubMed] [Google Scholar]
- Roberts V, Meunier H, Vaughan J, Rivier J, Rivier C, Vale W, Sawchenko P 1989 Production and regulation of inhibin subunits in pituitary gonadotropes. Endocrinology 124:552–554 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Lee BL, Carroll RS, Unabia G, Chin WW, Childs GV 1992 Follistatin gene expression in the pituitary: localization in the gonadotropes and folliculostellate cells in diestrous rats. Endocrinology 130:3048–3056 [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL, Montalvo E, Massagué J 1996 Activation of signalling by the activin receptor complex. Mol Cell Biol 16:1066–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikjian LM, Blount AL, Leal AM, Donaldson CJ, Fischer WH, Vale WW 2004 Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol Cell Endocrinol 225:29–36 [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Blount AL, Donaldson CJ, Vale WW 2006 Pituitary actions of ligands of the TGF-β family: activins and inhibins. Reproduction 132:207–215 [DOI] [PubMed] [Google Scholar]
- Harrison CA, Gray PC, Vale WW, Robertson DM 2005 Antagonists of activin signaling: mechanisms and potential biological applications. Trends Endocrinol Metab 16:73–78 [DOI] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL 1996 Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- Alarid ET, Holley S, Hayakawa M, Mellon PL 1998 Discrete stages of anterior pituitary differentiation recapitulated in immortalized cell lines. Mol Cell Endocrinol 140:25–30 [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL 2001 Cell-specific transcriptional regulation of FSHβ by activin and GnRH in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 [DOI] [PubMed] [Google Scholar]
- Graham KE, Nusser KD, Low MJ 1999 LβT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to activin A. J Endocrinol 162:R1–R5 [DOI] [PubMed] [Google Scholar]
- Zakaria MM, Jeong KH, Lacza C, Kaiser UB 2002 Pituitary homeobox 1 activates the rat FSHβ (rFSHβ) gene through both direct and indirect interactions with the rFSHβ gene promoter. Mol Endocrinol 16:1840–1852 [DOI] [PubMed] [Google Scholar]
- West BE, Parker GE, Savage JJ, Kiratipranon P, Toomey KS, Beach LR, Colvin SC, Sloop KW, Rhodes SJ 2004 Regulation of the follicle-stimulating hormone β gene by the LHX3 LIM-homeodomain transcription factor. Endocrinology 145:4866–4879 [DOI] [PubMed] [Google Scholar]
- Jacobs SB, Coss D, McGillivray SM, Mellon PL 2003 Nuclear factor-Y and steroidogenic factor-1 physically and functionally interact to contribute to cell-specific expression of the mouse follicle-stimulating hormone-β gene. Mol Endocrinol 17:1470–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Pedersen NR, Wu JC, Ghosh BR, Miller WL 1997 Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-β gene. Endocrinology 138:2621–2631 [DOI] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL 2004 A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem 279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Fortin J, Lamba P, Bonomi M, Persani L, Roberson MS, Bernard DJ 2008 Activator protein-1 and smad proteins synergistically regulate human follicle-stimulating hormone β-promoter activity. Endocrinology 149:5577–5591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccone NA, Lacza CT, Hou MY, Gregory SJ, Kam KY, Xu S, Kaiser UB 2008 A composite element that binds basic helix loop helix and basic leucine zipper transcription factors is important for gonadotropin-releasing hormone regulation of the follicle-stimulating hormone β gene. Mol Endocrinol 22:1908–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray VG, McGillivray SM, Mellon PL 2006 Androgens, progestins and glucocorticoids induce follicle-stimulating hormone β-subunit gene expression at the level of the gonadotrope. Mol Endocrinol 20:2062–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady TJ, Shayya R, Thackray VG, Ehrensberger L, Bailey JS, Mellon PL 2004 Androgen regulates FSHβ gene expression in an activin-dependent manner in immortalized gonadotropes. Mol Endocrinol 18:925–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillivray SM, Thackray VG, Coss D, Mellon PL 2007 Activin and glucocorticoids synergistically activate follicle-stimulating hormone β-subunit gene expression in the immortalized LβT2 gonadotrope cell line. Endocrinology 148:762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Wotton D 2000 Transcriptional control by the TGF-β/Smad signaling system. EMBO J 19:1745–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massagué J 2003 Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113:685–700 [DOI] [PubMed] [Google Scholar]
- Gregory SJ, Lacza CT, Detz AA, Xu S, Petrillo LA, Kaiser UB 2005 Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-β gene. Mol Endocrinol 19:237–254 [DOI] [PubMed] [Google Scholar]
- Bernard DJ 2004 Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone beta subunit in mouse gonadotrope cells. Mol Endocrinol 18:606–623 [DOI] [PubMed] [Google Scholar]
- Coss D, Thackray VG, Deng CX, Mellon PL 2005 Activin regulates luteinizing hormone β-subunit gene expression through smad-binding and homeobox elements. Mol Endocrinol 19:2610–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suszko MI, Balkin DM, Chen Y, Woodruff TK 2005 Smad3 mediates activin-induced transcription of follicle-stimulating hormone β-subunit gene. Mol Endocrinol 19:1849–1858 [DOI] [PubMed] [Google Scholar]
- Shi Y, Wang YF, Jayaraman L, Yang H, Massagué J, Pavletich NP 1998 Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-β signaling. Cell 94:585–594 [DOI] [PubMed] [Google Scholar]
- Massagué J 1998 TGF-β signal transduction. Annu Rev Biochem 67:753–791 [DOI] [PubMed] [Google Scholar]
- Suszko MI, Antenos M, Balkin DM, Woodruff TK 2008 Smad3 and Pitx2 cooperate in stimulation of FSHβ gene transcription. Mol Endocrinol 281:27–36 [DOI] [PubMed] [Google Scholar]
- Bailey JS, Rave-Harel N, McGillivray SM, Coss D, Mellon PL 2004 Activin regulation of the follicle-stimulating hormone β-subunit gene involves Smads and the TALE homeodomain proteins Pbx1 and Prep1. Mol Endocrinol 18:1158–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Ichijo H, Franzén P, Laiho M, Miyazono K, Heldin CH 1994 Characterization of type I receptors for transforming growth factor-β and activin. Science 264:101–104 [DOI] [PubMed] [Google Scholar]
- Blount AL, Schmidt K, Justice NJ, Vale WW, Fischer WH, Bilezikjian LM 2009 FoxL2 and Smad3 coordinately regulate follistatin gene transcription. J Biol Chem 284:7631–7645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin G, Ahgren A, ten Dijke P, Heldin CH, Heuchel R 2000 Efficient TGF-β induction of the Smad7 gene requires cooperation between AP-1, Sp1, and Smad proteins on the mouse Smad7 promoter. J Biol Chem 275:29023–29030 [DOI] [PubMed] [Google Scholar]
- Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK 2003 Regulation of the rat follicle-stimulating hormone β-subunit promoter by activin. Mol Endocrinol 17:318–332 [DOI] [PubMed] [Google Scholar]
- Lamba P, Santos MM, Philips DP, Bernard DJ 2006 Acute regulation of murine follicle-stimulating hormone β subunit transcription by activin A. J Mol Endocrinol 36:201–220 [DOI] [PubMed] [Google Scholar]
- Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ 2009 A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone β subunit transcription. Mol Endocrinol 23:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson P, Mahlapuu M 2002 Forkhead transcription factors: key players in development and metabolism. Dev Biol 250:1–23 [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G 2001 The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166 [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M 2004 The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131:933–942 [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Burns AT, Escudero KW, Duval DL, Nelson SE, Clay CM 2003 The gonadotropin releasing hormone (GnRH) receptor activating sequence (GRAS) is a composite regulatory element that interacts with multiple classes of transcription factors including Smads, AP-1 and a forkhead DNA binding protein. Mol Cell Endocrinol 206:93–111 [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL 2002 Signal transduction by the TGF-β superfamily. Science 296:1646–1647 [DOI] [PubMed] [Google Scholar]
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M 1997 Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature 389:85–89 [DOI] [PubMed] [Google Scholar]
- Pisarska MD, Barlow GM, Bentsi-Barnes IK, Kuo FT, LATS1 phosphorylates FOXL2 and regulates its transcriptional activity. Program of the 91st Annual Meeting of the Endocrine Society, Washington, DC, 2009 (Abstract P3-260) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.